Abstract
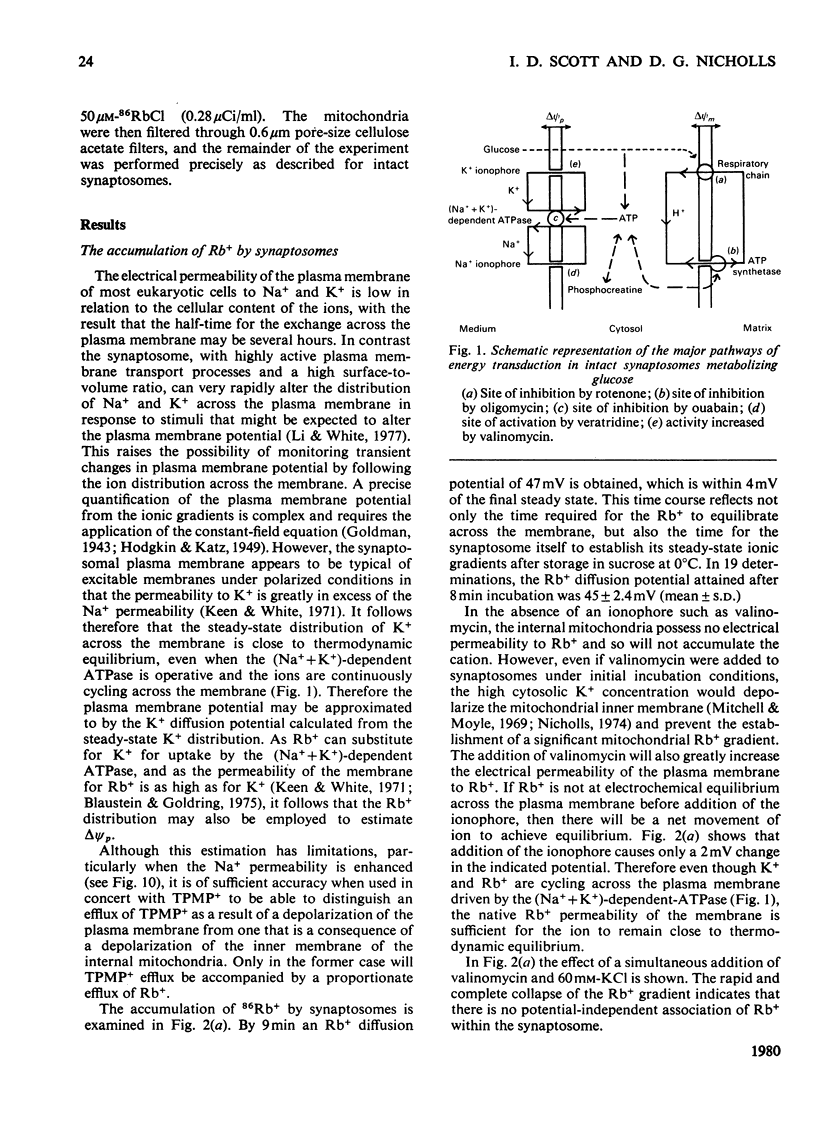
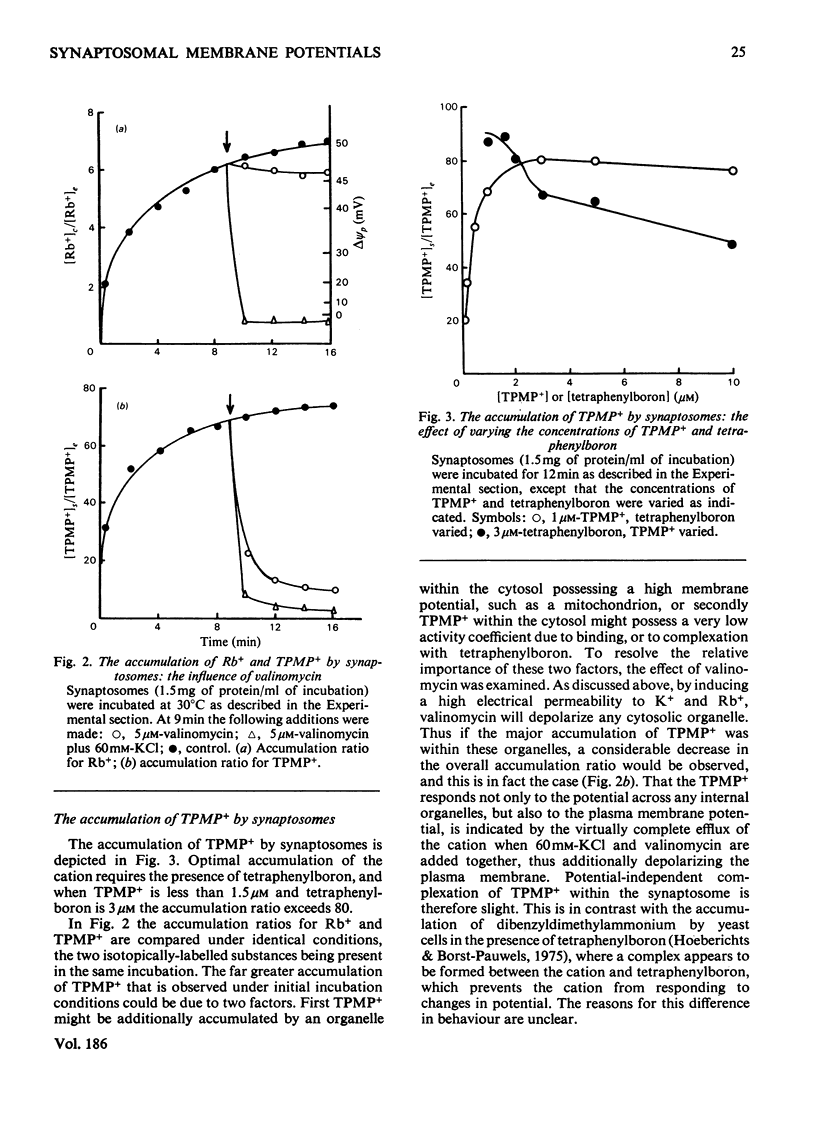
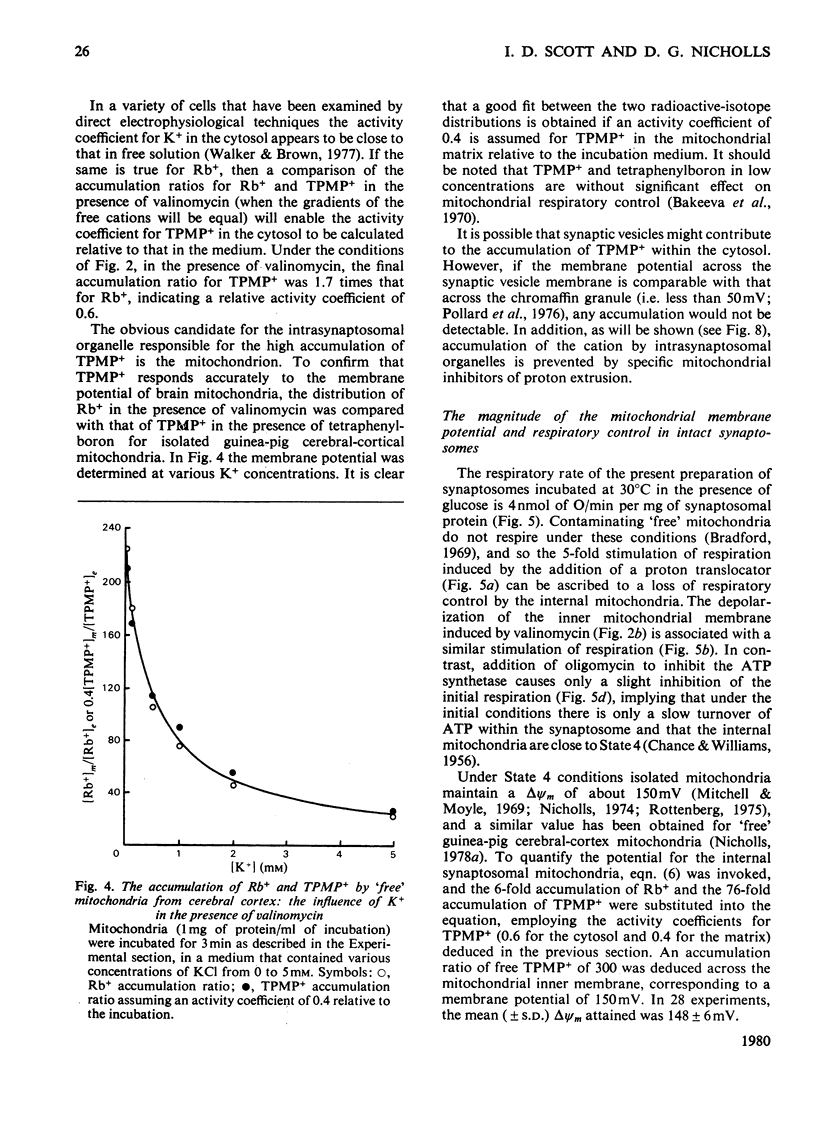
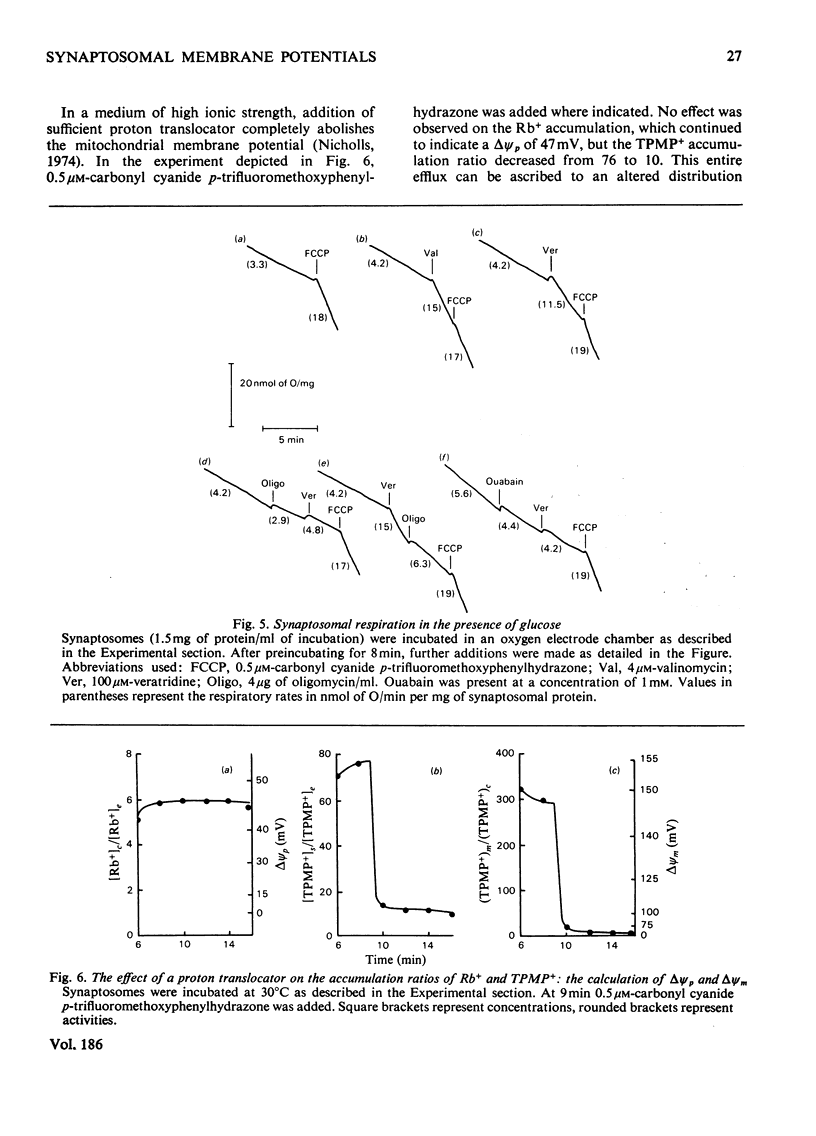
A method is described, based on the differential accumulation of Rb+ and methyltriphenylphosphonium, for the simultaneous estimation of the membrane potentials across the plasma membrane of isolated nerve endings (synaptosomes), and across the inner membrane of mitochondria within the synaptosomal cytoplasm. These determinations, together with measurements of respiratory rates, and ATP and phosphocreatine concentrations, are used to define the bioenergetic behaviour of isolated synaptosomes under a variety of conditions. Under control conditions, in the presence of glucose, the plasma and mitochondrial membrane potentials are respectively 45 and 148mV. Addition of a proton translocator induces a 5-fold increase in respiration, and abolishes the mitochondrial membrane potential. The addition of rotenone to inhibit respiration does not affect the plasma membrane potential, and only lowers the mitochondrial membrane potential to 128mV. Evidence is presented that ATP synthesis by anaerobic glycolysis is sufficient under these conditions to maintain ATP-dependent processes, including the reversal of the mitochondrial ATP synthetase. Addition of oligomycin under non-respiring conditions leads to a complete collapse of the mitochondrial potential. Even under control conditions the plasma membrane (Na+ + K+)-dependent ATPase is responsible for a significant proportion of the synaptosomal ATP turnover. Veratridine greatly increases respiration, and depolarizes the plasma membrane, but only slightly lowers the mitochondrial membrane potential. High K+ and ouabain also lower the plasma membrane potential without decreasing the mitochondrial membrane potential. In non-respiring synaptosomes, anaerobic glycolysis is incapable of maintaining cytosolic ATP during the increased turnover induced by veratridine, and the mitochondrial membrane potential collapses. It is concluded that the internal mitochondria must be considered in any study of synaptosomal transport.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A., Smith J. P., Hedrick N. Adenosinetriphosphatase and nucleotide metabolism in synaptosomes of rat brain. J Neurochem. 1970 Mar;17(3):391–401. doi: 10.1111/j.1471-4159.1970.tb02226.x. [DOI] [PubMed] [Google Scholar]
- Akerman K. E. Qualitative measurements of the mitochondrial membrane potential in situ in Ehrlich ascites tumour cells using the safranine method. Biochim Biophys Acta. 1979 May 9;546(2):341–347. doi: 10.1016/0005-2728(79)90051-3. [DOI] [PubMed] [Google Scholar]
- Akerman K. E., Wikström M. K. Safranine as a probe of the mitochondrial membrane potential. FEBS Lett. 1976 Oct 1;68(2):191–197. doi: 10.1016/0014-5793(76)80434-6. [DOI] [PubMed] [Google Scholar]
- Bachelard H. S. Glucose as a fuel for the brain. Biochem Soc Trans. 1978;6(3):520–524. doi: 10.1042/bst0060520. [DOI] [PubMed] [Google Scholar]
- Bakeeva L. E., Grinius L. L., Jasaitis A. A., Kuliene V. V., Levitsky D. O., Liberman E. A., Severina I. I., Skulachev V. P. Conversion of biomembrane-produced energy into electric form. II. Intact mitochondria. Biochim Biophys Acta. 1970 Aug 4;216(1):13–21. doi: 10.1016/0005-2728(70)90154-4. [DOI] [PubMed] [Google Scholar]
- Baldessarini R. J., Karobath M. Biochemical physiology of central synapses. Annu Rev Physiol. 1973;35:273–304. doi: 10.1146/annurev.ph.35.030173.001421. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Goldring J. M. Membrane potentials in pinched-off presynaptic nerve ternimals monitored with a fluorescent probe: evidence that synaptosomes have potassium diffusion potentials. J Physiol. 1975 Jun;247(3):589–615. doi: 10.1113/jphysiol.1975.sp010949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth R. F., Clark J. B. Studies on the mitochondrially bound form of rat brain creatine kinase. Biochem J. 1978 Jan 15;170(1):145–151. doi: 10.1042/bj1700145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford H. F., Bennett G. W., Thomas A. J. Depolarizing stimuli and the release of physiologically active amino acids from suspensions of mammalian synaptosomes. J Neurochem. 1973 Sep;21(3):495–505. doi: 10.1111/j.1471-4159.1973.tb05995.x. [DOI] [PubMed] [Google Scholar]
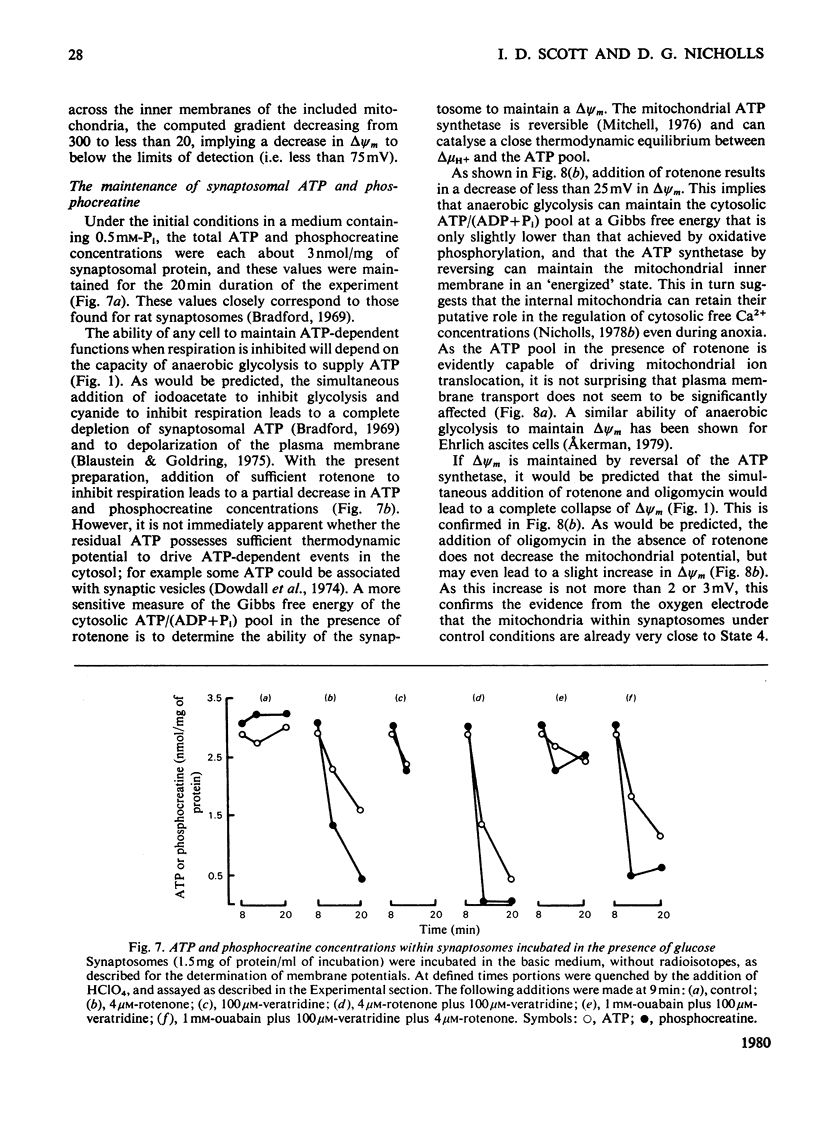
- Bradford H. F. Respiration in vitro of synaptosomes from mammalian cerebral cortex. J Neurochem. 1969 May;16(5):675–684. doi: 10.1111/j.1471-4159.1969.tb06444.x. [DOI] [PubMed] [Google Scholar]
- Burchkhardt G. Non-linear relationship between fluorescence and membrane potential. Biochim Biophys Acta. 1977 Jul 14;468(2):227–237. doi: 10.1016/0005-2736(77)90116-x. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Matthews D. A. Synaptic plasma membranes from rat brain synaptosomes: isolation and partial characterization. Biochim Biophys Acta. 1971 Dec 3;249(2):380–394. doi: 10.1016/0005-2736(71)90117-9. [DOI] [PubMed] [Google Scholar]
- Dowdall M. J., Boyne A. F., Whittaker V. P. Adenosine triphosphate. A constituent of cholinergic synaptic vesicles. Biochem J. 1974 Apr;140(1):1–12. doi: 10.1042/bj1400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
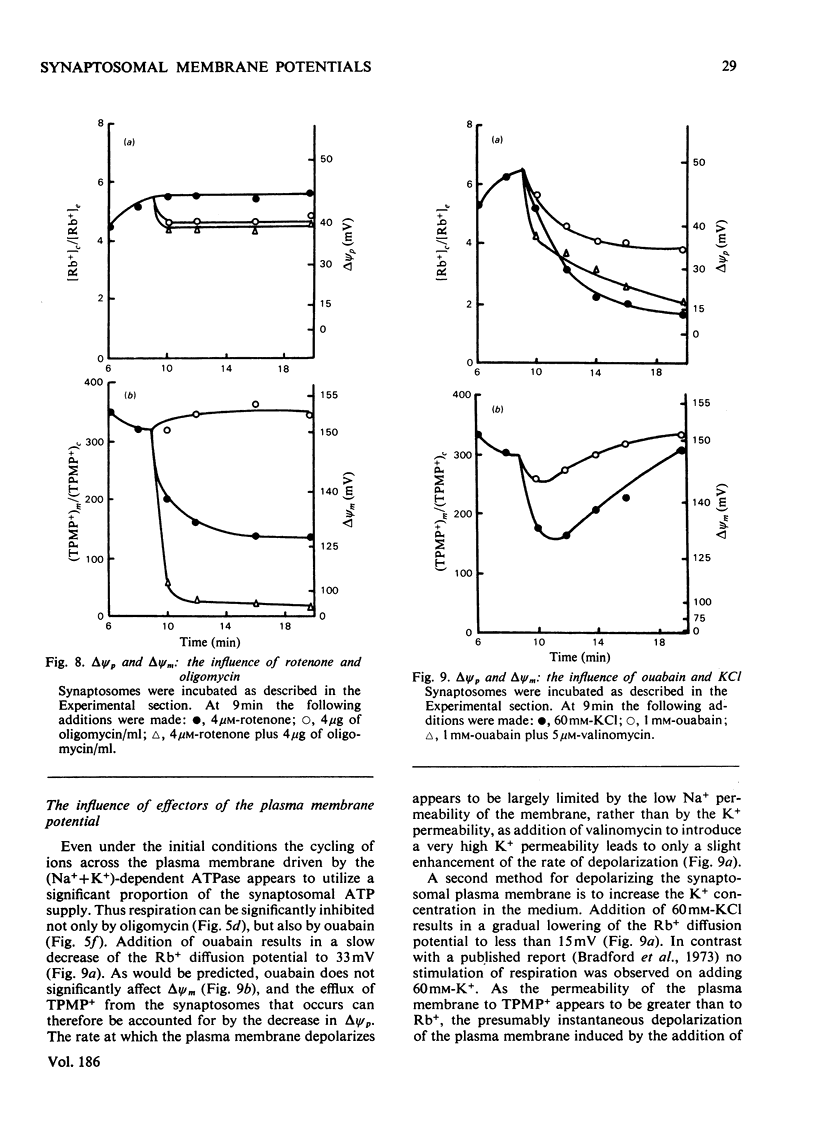
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeberichts J. A., Borst-Pauwels G. W. Effect of tetraphenylboron upon the uptake of the lipophilic cation dibenzyldimethylammonium by yeast cells. Biochim Biophys Acta. 1975 Dec 1;413(2):248–251. doi: 10.1016/0005-2736(75)90109-1. [DOI] [PubMed] [Google Scholar]
- Keen P., White T. D. The permeability of pinched-off nerve endings to sodium, potassium and chloride and the effects of gramicidin. J Neurochem. 1971 Jun;18(6):1097–1103. doi: 10.1111/j.1471-4159.1971.tb12038.x. [DOI] [PubMed] [Google Scholar]
- Lai J. C., Clark J. B. Preparation and properties of mitochondria derived from synaptosomes. Biochem J. 1976 Feb 15;154(2):423–432. doi: 10.1042/bj1540423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laris P. C., Pershadsingh H. A., Johnstone R. M. Monitoring membrane potentials in Ehrlich ascites tumor cells by means of a fluorescent dye. Biochim Biophys Acta. 1976 Jun 17;436(2):475–488. doi: 10.1016/0005-2736(76)90209-1. [DOI] [PubMed] [Google Scholar]
- Levi G., Raiteri M. Synaptosomal transport processes. Int Rev Neurobiol. 1976;19:51–74. doi: 10.1016/s0074-7742(08)60701-1. [DOI] [PubMed] [Google Scholar]
- Li P. P., White T. D. Rapid effects of veratridine, tetrodotoxin, gramicidin D, valinomycin and NaCN on the Na+, K+ and ATP contents of synaptosomes. J Neurochem. 1977 May;28(5):967–975. doi: 10.1111/j.1471-4159.1977.tb10658.x. [DOI] [PubMed] [Google Scholar]
- Marchbanks R. M. The chloride content, anion deficit and volume of synaptosomes. J Neurochem. 1975 Oct;25(4):463–470. doi: 10.1111/j.1471-4159.1975.tb04351.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Estimation of membrane potential and pH difference across the cristae membrane of rat liver mitochondria. Eur J Biochem. 1969 Feb;7(4):471–484. doi: 10.1111/j.1432-1033.1969.tb19633.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Vectorial chemistry and the molecular mechanics of chemiosmotic coupling: power transmission by proticity. Biochem Soc Trans. 1976;4(3):399–430. doi: 10.1042/bst0040399. [DOI] [PubMed] [Google Scholar]
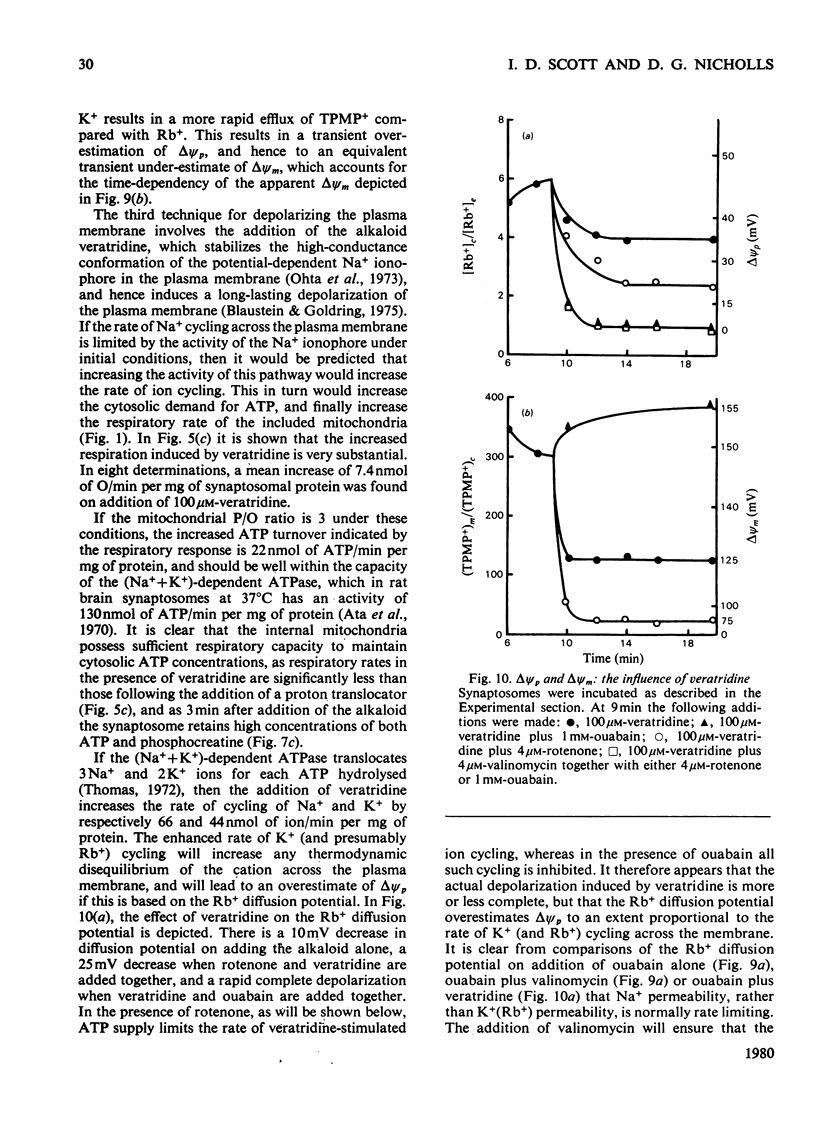
- Ng R. H., Howard B. D. Deenergization of nerve terminals by beta-bungarotoxin. Biochemistry. 1978 Nov 14;17(23):4978–4986. doi: 10.1021/bi00616a019. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G., Bernson V. S. Inter-relationships between proton electrochemical gradient, adenine-nucleotide phosphorylation potential and respiration, during substrate-level and oxidative phosphorylation by mitochondria from brown adipose tissue of cold-adapted guinea-pigs. Eur J Biochem. 1977 May 16;75(2):601–612. doi: 10.1111/j.1432-1033.1977.tb11560.x. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. Calcium transport and porton electrochemical potential gradient in mitochondria from guinea-pig cerebral cortex and rat heart. Biochem J. 1978 Mar 15;170(3):511–522. doi: 10.1042/bj1700511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D. G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem. 1974 Dec 16;50(1):305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. The regulation of extramitochondrial free calcium ion concentration by rat liver mitochondria. Biochem J. 1978 Nov 15;176(2):463–474. doi: 10.1042/bj1760463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas W. J., Clark J. B., Williamson J. R. Metabolism of rat brain mitochondria. Studies on the potassium ion-stimulated oxidation of pyruvate. Biochem J. 1971 Jun;123(1):83–95. doi: 10.1042/bj1230083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota M., Narahashi T., Keeler R. F. Effects of veratrum alkaloids on membrane potential and conductance of squid and crayfish giant axons. J Pharmacol Exp Ther. 1973 Jan;184(1):143–154. [PubMed] [Google Scholar]
- Pollard H. B., Zinder O., Hoffman P. G., Nikodejevic O. Regulation of the transmembrane potential of isolated chromaffin granules by ATP, ATP analogs, and external pH. J Biol Chem. 1976 Aug 10;251(15):4544–4550. [PubMed] [Google Scholar]
- Rottenberg H. The measurement of transmembrane electrochemical proton gradients. J Bioenerg. 1975 May;7(2):61–74. doi: 10.1007/BF01558427. [DOI] [PubMed] [Google Scholar]
- Scott I. D., Nicholls D. G. The estimation of the electrical potential across the inner membrane of mitochondria within intact synaptosomes [proceedings]. Biochem Soc Trans. 1979 Oct;7(5):969–970. doi: 10.1042/bst0070969. [DOI] [PubMed] [Google Scholar]
- Sen I., Cooper J. R. Similarities of beta-bungarotoxin and phospholipase A2 and their mechanism of action. J Neurochem. 1978 Jun;30(6):1369–1372. doi: 10.1111/j.1471-4159.1978.tb10468.x. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- Verity M. A. Cation modulation of synaptosomal respiration. J Neurochem. 1972 May;19(5):1305–1317. doi: 10.1111/j.1471-4159.1972.tb01456.x. [DOI] [PubMed] [Google Scholar]
- Walker J. L., Brown H. M. Intracellular ionic activity measurements in nerve and muscle. Physiol Rev. 1977 Oct;57(4):729–778. doi: 10.1152/physrev.1977.57.4.729. [DOI] [PubMed] [Google Scholar]


