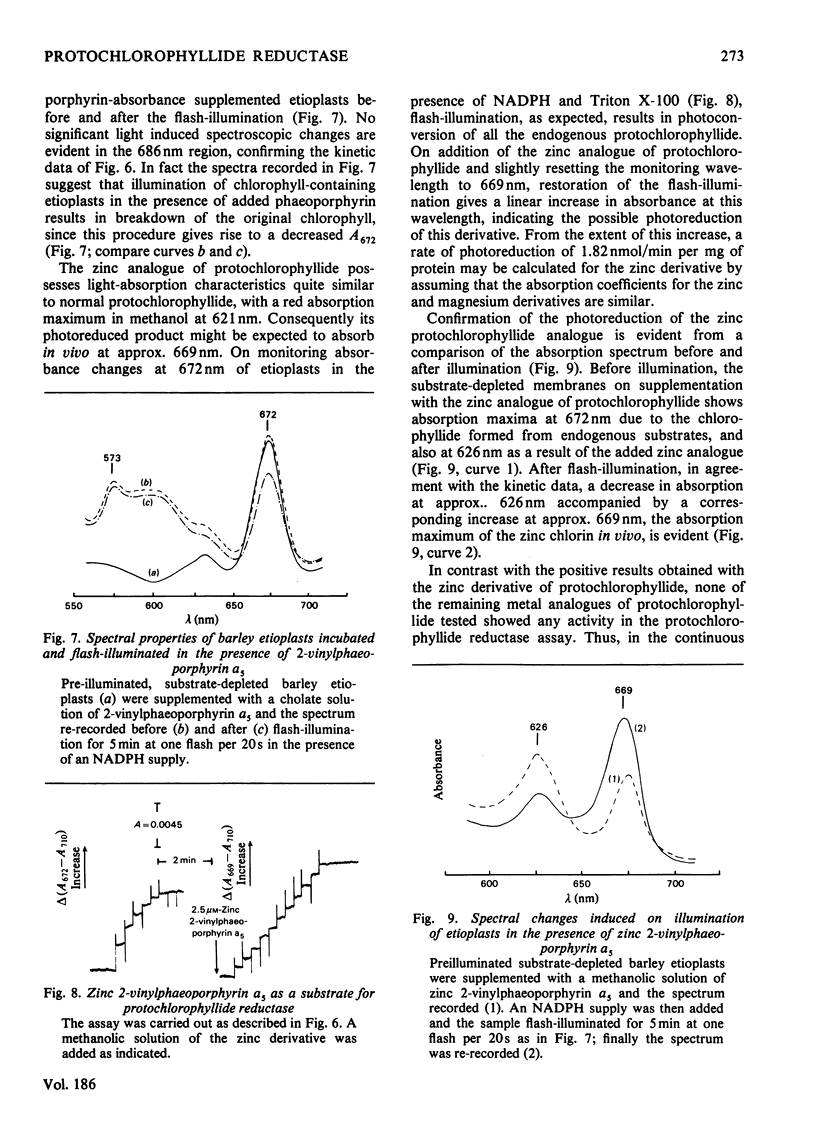
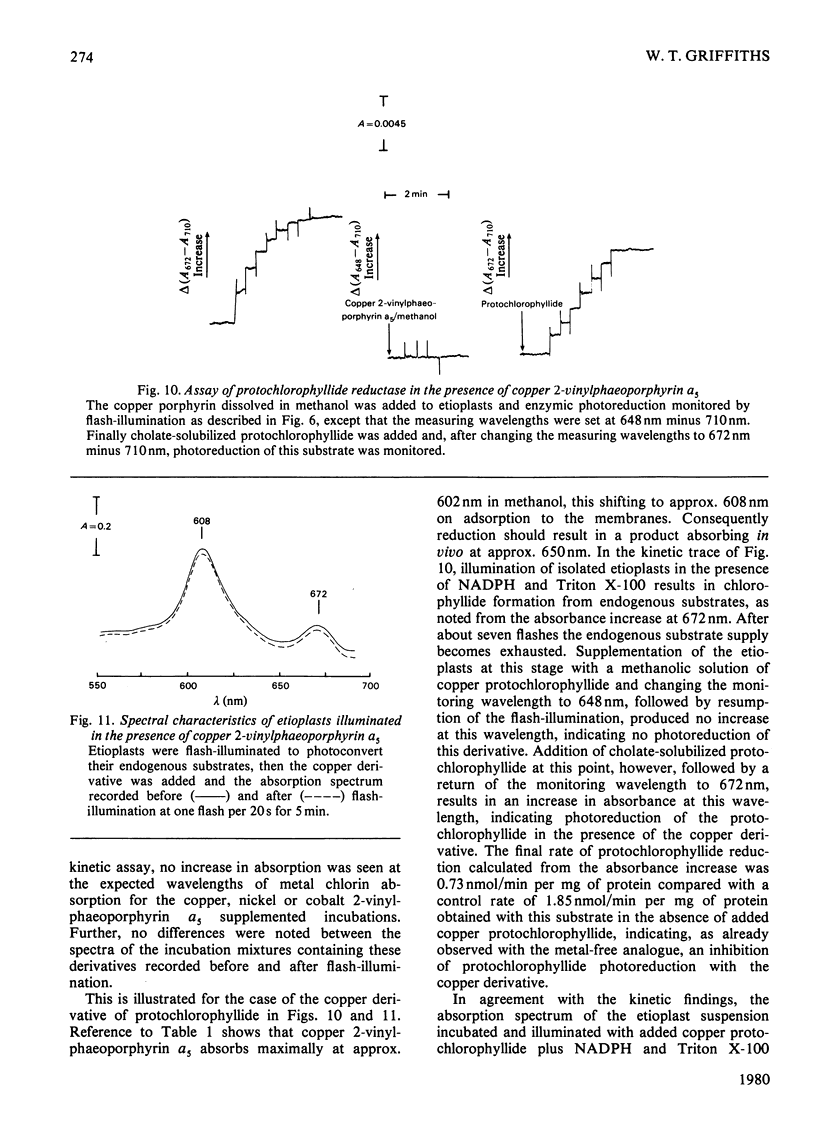
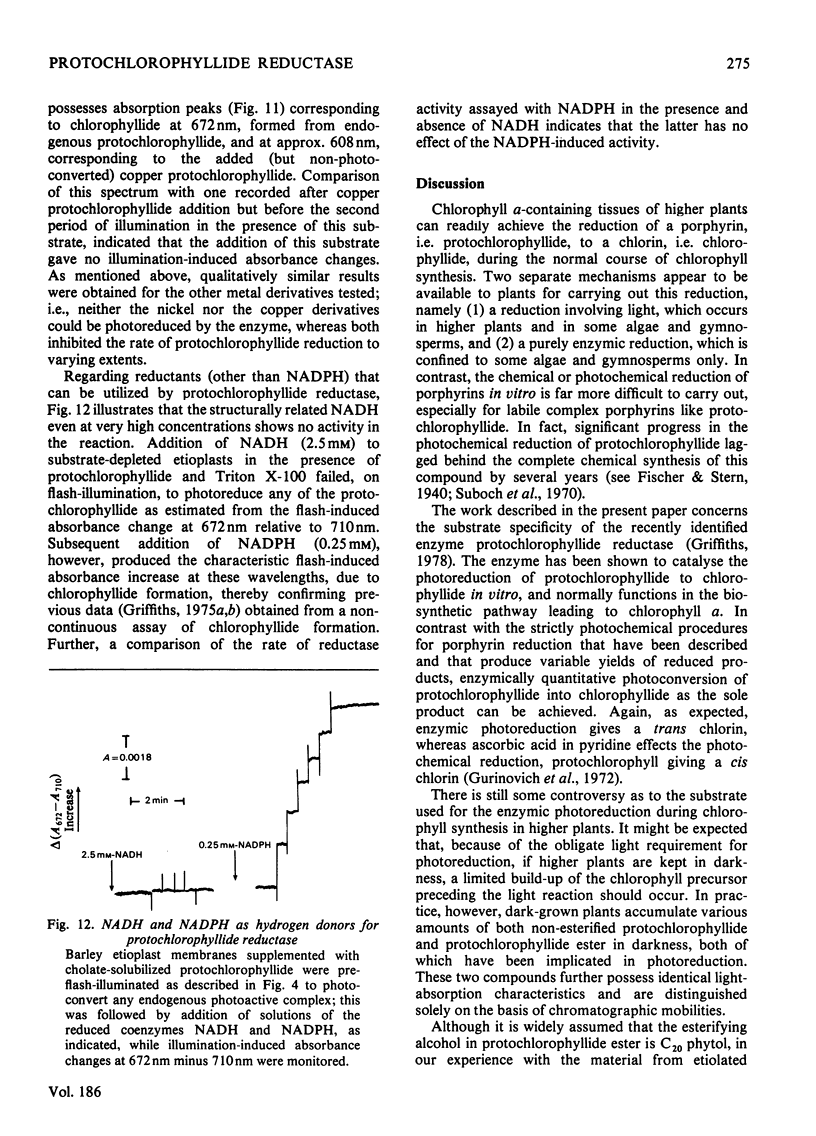
Abstract
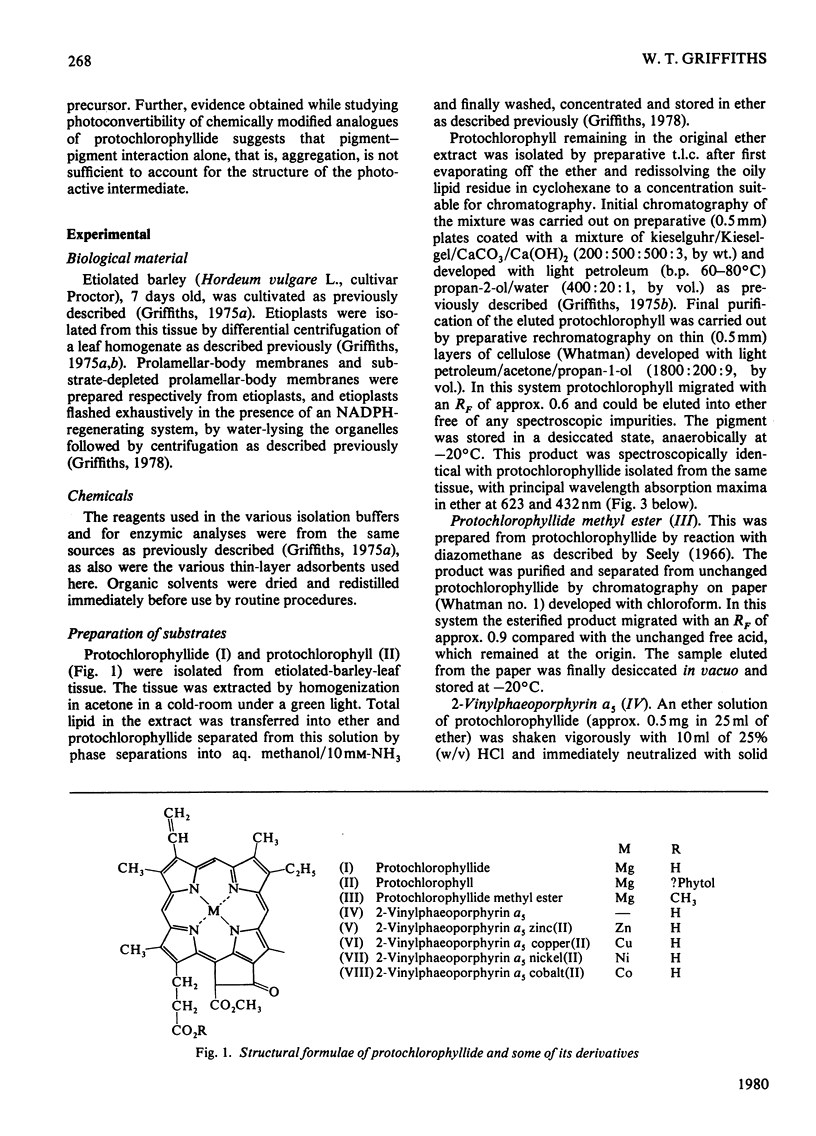
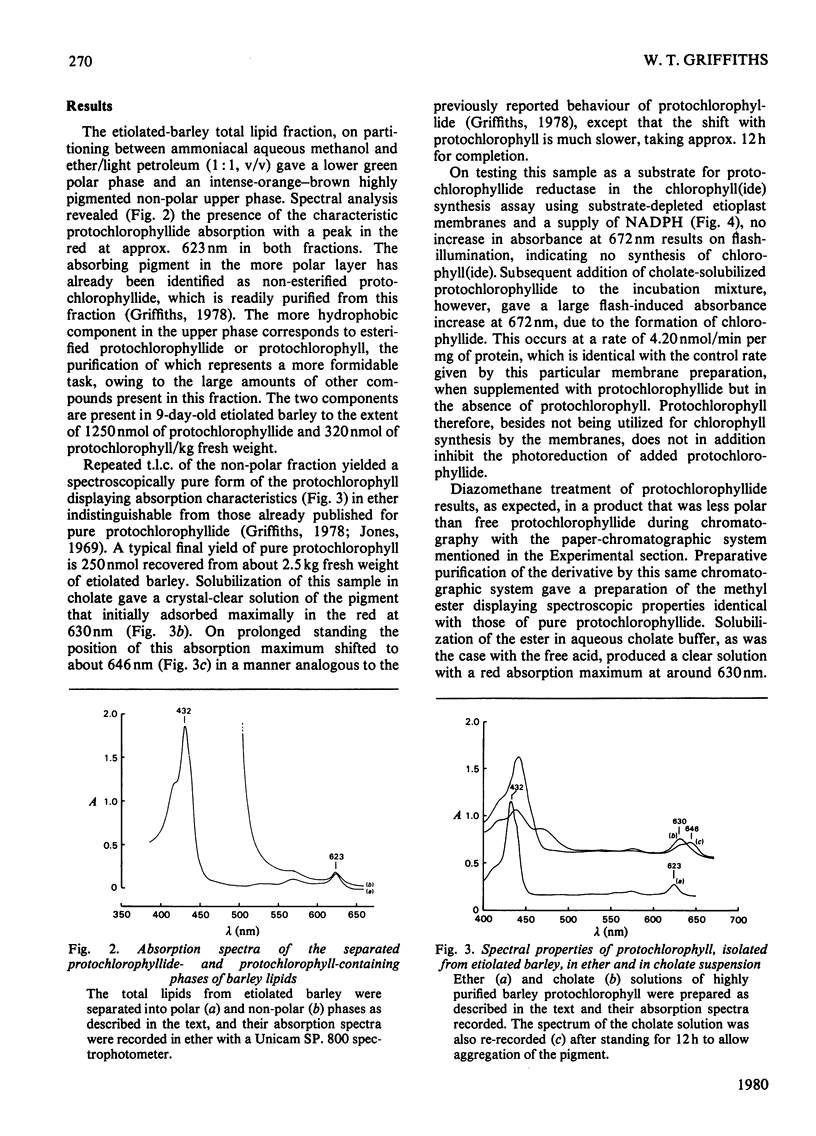
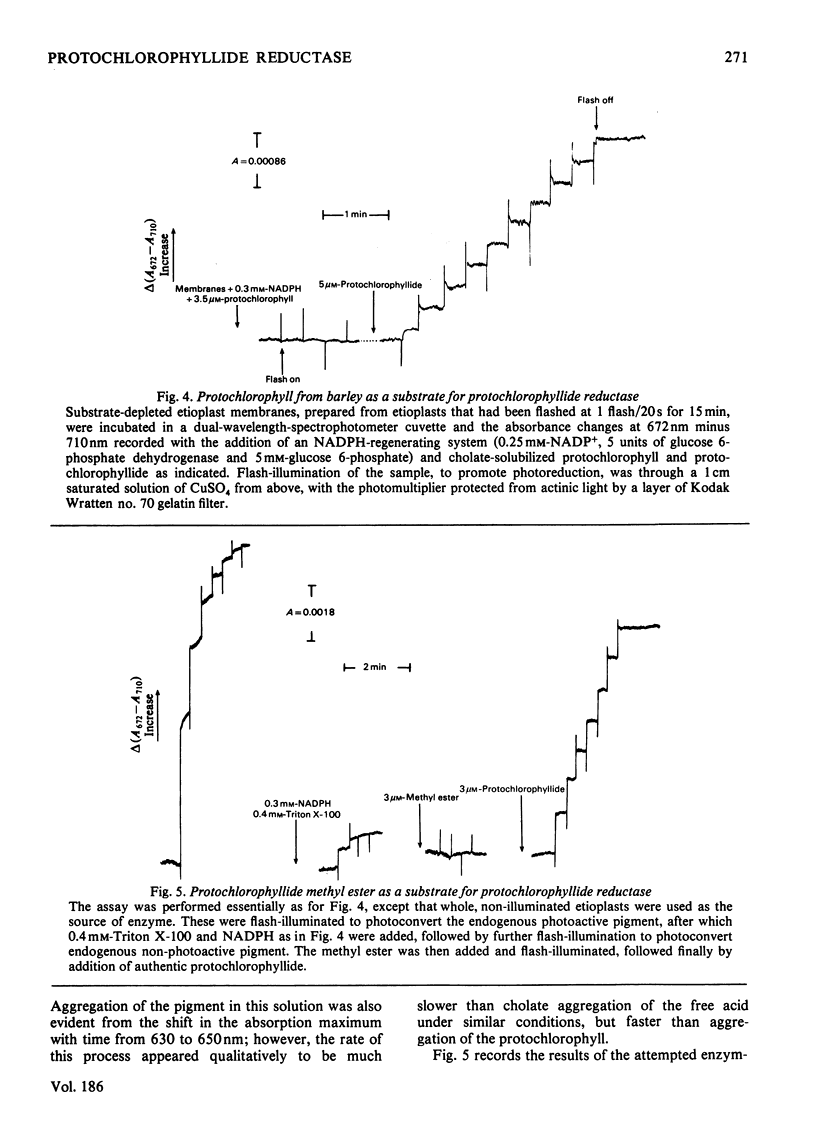
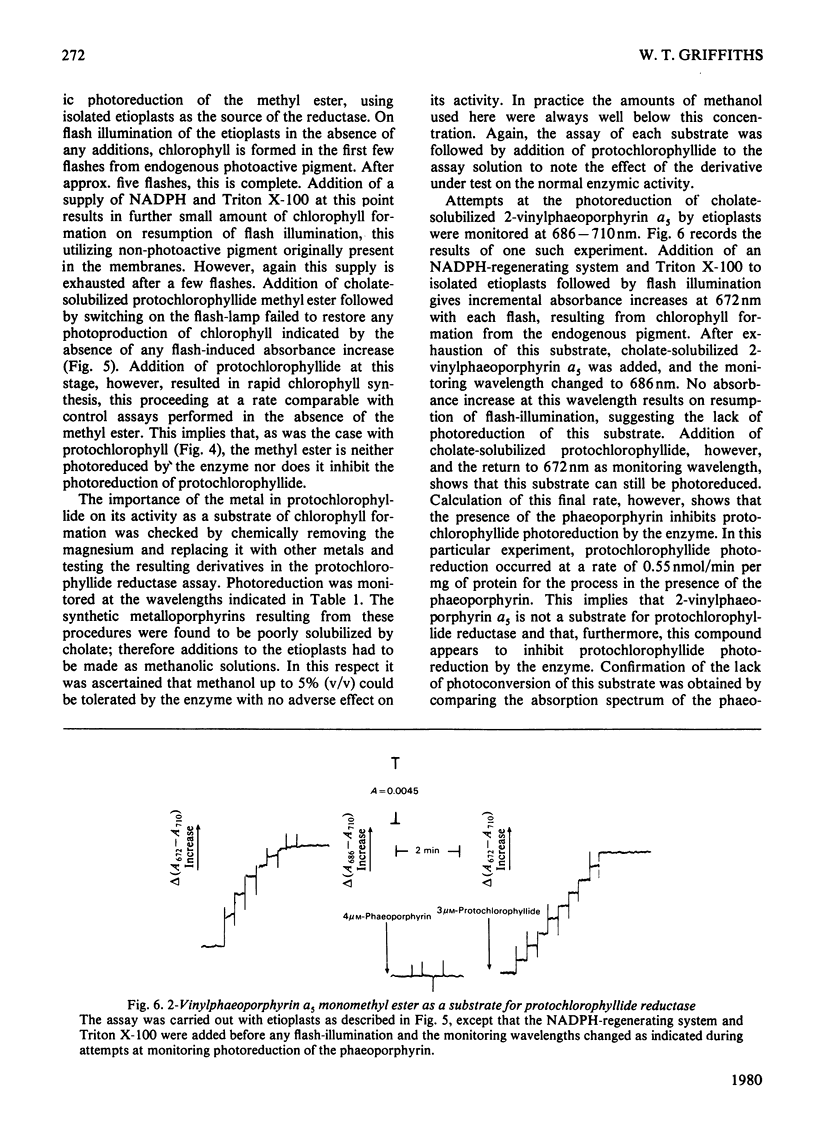
1. The substrate specificity of the enzyme protochlorophyllide reductase in barley (Hordeum vulgare) etioplasts was investigated. 2. It was shown that naturally occurring esterified protochlorophyllide and chemically prepared protochlorophyllide methyl ester are not substrates for the enzyme, suggesting an important role for the C-7 carboxylic acid group in binding of the porphyrin to the enzyme. 3. Removal of magnesium from the protochlorophyllide leads to inactivity of the compound as a substrate for the enzyme. However, activity can be restored by replacing the magnesium with zinc, whereas nickel, copper or cobalt failed to restore substrate activity. 4. Binding of the second substrate, NADPH, to the enzyme probably occurs through the 2'-phosphate group in the coenzyme.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen C. E., Schiff J. A. Events surrounding the early development of Euglena chloroplasts XI. Protochlorophyll(ide) and its photoconversion. Photochem Photobiol. 1976 Dec;24(6):555–566. doi: 10.1111/j.1751-1097.1976.tb06873.x. [DOI] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., TAIT G. H. STUDIES ON THE BIOSYNTHESIS OF PORPHYRIN AND BACTERIOCHLOROPHYLL BY RHODOPSEUDOMONAS SPHEROIDES. 4. S-ADENOSYLMETHIONINEMAGNESIUM PROTOPORPHYRIN METHYLTRANSFERASE. Biochem J. 1963 Aug;88:325–334. doi: 10.1042/bj0880325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassman M., Bogorad L. Studies on the regeneration of protochlorophyllide after brief illumination of etiolated bean leaves. Plant Physiol. 1967 Jun;42(6):781–784. doi: 10.1104/pp.42.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths W. T. Characterization of the terminal stages of chlorophyll (ide) synthesis in etioplast membrane preparations. Biochem J. 1975 Dec;152(3):623–635. doi: 10.1042/bj1520623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths W. T. Protochlorophyll and protochlorophyllide as precursors for chlorophyll synthesis in vitro. FEBS Lett. 1974 Dec 15;49(2):196–200. doi: 10.1016/0014-5793(74)80510-7. [DOI] [PubMed] [Google Scholar]
- Griffiths W. T. Reconstitution of chlorophyllide formation by isolated etioplast membranes. Biochem J. 1978 Sep 15;174(3):681–692. doi: 10.1042/bj1740681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones O. T. A protein-protochlorophyll complex obtained from inner seed coats of Cucurbita pepo. The resolution of its two pigment groups into true protochlorophyll and a pigment related to bacterial protochlorophyll. Biochem J. 1966 Oct;101(1):153–160. doi: 10.1042/bj1010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn A., Boardman N. K., Thorne S. W. Energy transfer between protochlorophyllide molecules: evidence for multiple chromophores in the photoactive protochlorophyllide-protein complex vivo and in vitro. J Mol Biol. 1970 Feb 28;48(1):85–101. doi: 10.1016/0022-2836(70)90220-2. [DOI] [PubMed] [Google Scholar]
- Lancer H. A., Cohen C. E., Schiff J. A. Changing ratios of phototransformable protochlorophyll and protochlorophyllide of bean seedlings developing in the dark. Plant Physiol. 1976 Mar;57(3):369–374. doi: 10.1104/pp.57.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis P., Sauer K. Circular dichroism studies on the structure and the photochemistry of protochlorophyllide and chlorophyllide holochrome. Biochim Biophys Acta. 1972 Jun 23;267(3):498–511. doi: 10.1016/0005-2728(72)90178-8. [DOI] [PubMed] [Google Scholar]
- Nielsen O. F. Protochlorophyll(ide) holochrome subunits from a mutant defective in the regulation of protochlorophyll(ide) synthesis. FEBS Lett. 1973 Dec 15;38(1):75–78. doi: 10.1016/0014-5793(73)80517-4. [DOI] [PubMed] [Google Scholar]
- SIRONVAL C., MICHEL-WOLWERTZ M. R., MADSEN A. ON THE NATURE AND POSSIBLE FUNCTIONS OF THE 673- AND 684-MU FORMS IN VIVO OF CHLOROPHYLL. Biochim Biophys Acta. 1965 Mar 29;94:344–354. doi: 10.1016/0926-6585(65)90043-9. [DOI] [PubMed] [Google Scholar]
- Seliskar C. J., Ke B. Protochlorophyllide aggregation in solution and associated spectral changes. Biochim Biophys Acta. 1968 Apr 2;153(3):685–691. doi: 10.1016/0005-2728(68)90195-3. [DOI] [PubMed] [Google Scholar]
- Tiede D. M., Prince R. C., Dutton P. L. EPR and optical spectroscopic properties of the electron carrier intermediate between the reaction center bacteriochlorophylls and the primary acceptor in Chromatium vinosum. Biochim Biophys Acta. 1976 Dec 6;449(3):447–467. doi: 10.1016/0005-2728(76)90155-9. [DOI] [PubMed] [Google Scholar]


