Abstract
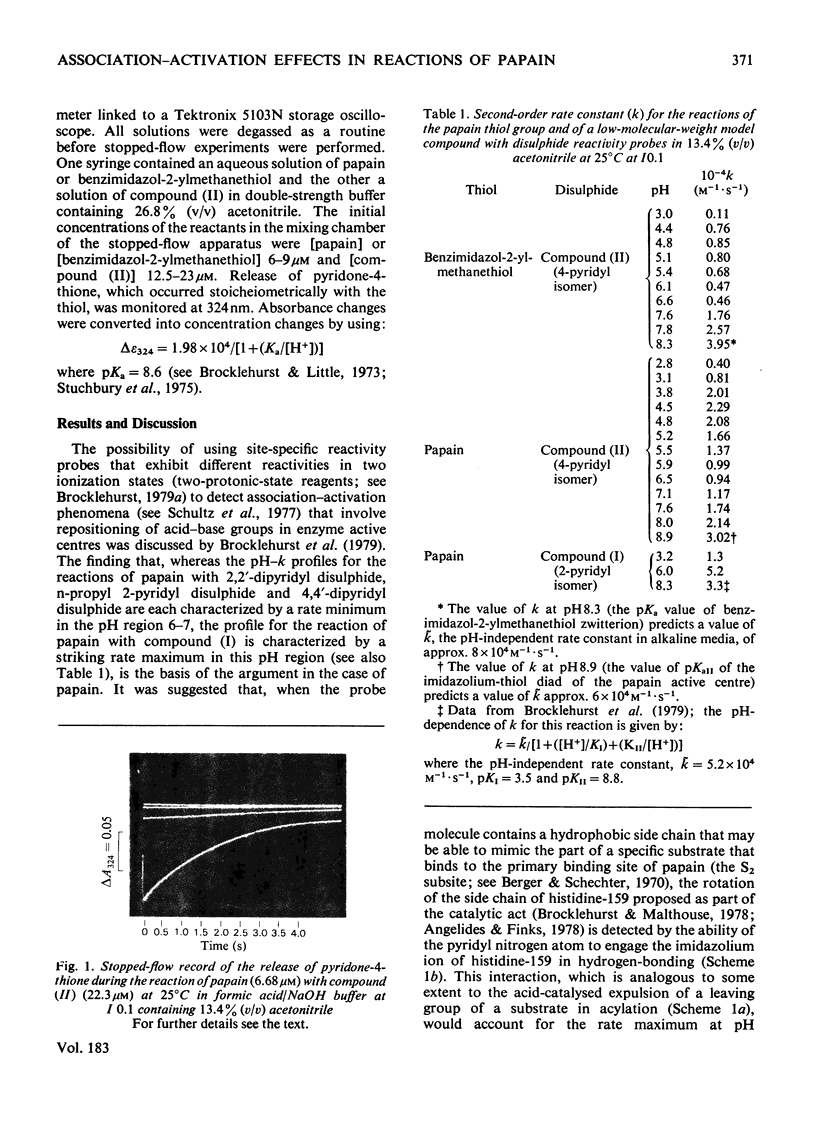
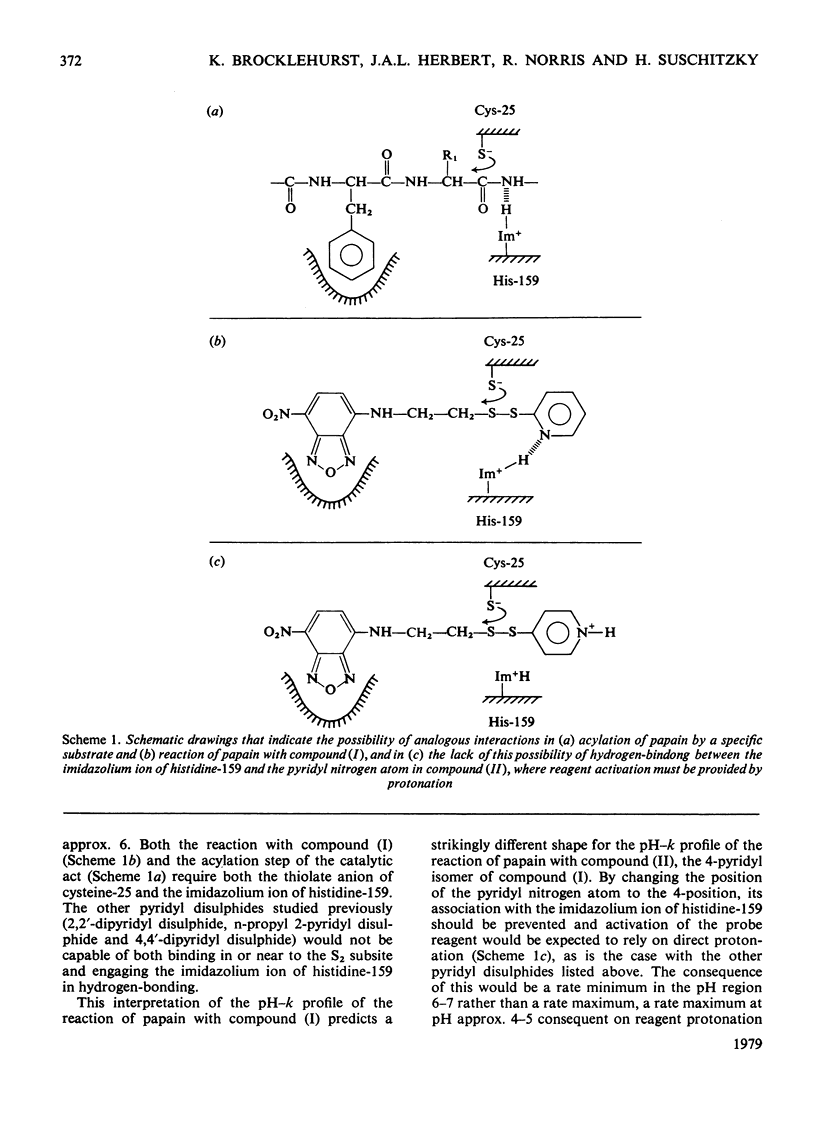
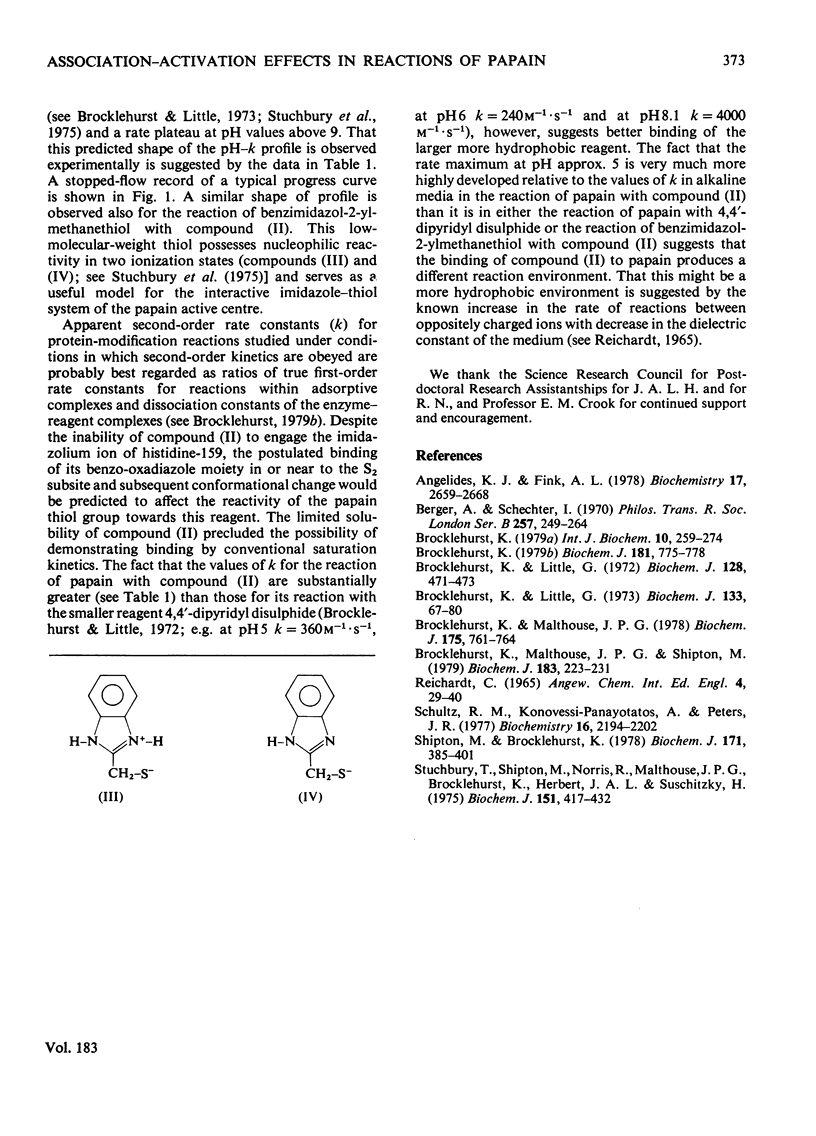
4-(N-Aminoethyl 4-pyridyl disulphide)-7-nitrobenzo-2-oxa-1,3-diazole was synthesized and evaluted as a two-protonic-state reactivity probe by kinetic study of its reactions with papain (EC 3.4.22.2) and with benzimidazol-2-ylmethanethiol. Evidence is presented to suggest that: (i) both this probe molecule and its 2-pyridyl isomer bind to papain; (ii) the binding is followed by a change in the environment of the thiol group of cysteine-25; (iii) the striking rate maximum in neutral media observed in the reaction of papain with the 2-pyridyl isomer but not with the 4-pyridyl isomer arises from association of the 2-pyridyl leaving group with the imidazolium ion of histidine-159.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelides K. J., Fink A. L. Cryoenzymology of papain: reaction mechanism with an ester substrate. Biochemistry. 1978 Jun 27;17(13):2659–2668. doi: 10.1021/bi00606a032. [DOI] [PubMed] [Google Scholar]
- Berger A., Schechter I. Mapping the active site of papain with the aid of peptide substrates and inhibitors. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):249–264. doi: 10.1098/rstb.1970.0024. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. Reactions of papain and of low-molecular-weight thiols with some aromatic disulphides. 2,2'-Dipyridyl disulphide as a convenient active-site titrant for papain even in the presence of other thiols. Biochem J. 1973 May;133(1):67–80. doi: 10.1042/bj1330067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. Reactivities of the various protonic states in the reactions of papain and of L-cysteine with 2,2'- and with 4,4'- dipyridyl disulphide: evidence for nucleophilic reactivity in the un-ionized thiol group of the cysteine-25 residue of papain occasioned by its interaction with the histidine-159-asparagine-175 hydrogen-bonded system. Biochem J. 1972 Jun;128(2):471–474. doi: 10.1042/bj1280471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Malthouse J. P. Mechanism of the reaction of papain with substrate-derived diazomethyl ketones. Implications for the difference in site specificity of halomethyl ketones for serine proteinases and cysteine proteinases and for stereoelectronic requirements in the papain catalytic mechanism. Biochem J. 1978 Nov 1;175(2):761–764. doi: 10.1042/bj1750761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Malthouse J. P., Shipton M. Evidence that binding to the s2-subsite of papain may be coupled with catalytically relevant structural change involving the cysteine-25-histidine-159 diad. Kinetics of the reaction of papain with a two-protonic-state reactivity probe containing a hydrophobic side chain. Biochem J. 1979 Nov 1;183(2):223–231. doi: 10.1042/bj1830223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K. Specific covalent modification of thiols: applications in the study of enzymes and other biomolecules. Int J Biochem. 1979;10(4):259–274. doi: 10.1016/0020-711x(79)90088-0. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K. The equilibrium assumption is valid for the kinetic treatment of most time-dependent protein-modification reactions. Biochem J. 1979 Sep 1;181(3):775–778. doi: 10.1042/bj1810775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R. M., Konovessi-Panayotatos A., Peters J. R. Thermodynamics of binding to native alpha-chymotrypsin and to forms of alpha-chymotrypsin in which catalytically essential residues are modified; a study of "productive" and "nonproductive" associations. Biochemistry. 1977 May 17;16(10):2194–2202. doi: 10.1021/bi00629a024. [DOI] [PubMed] [Google Scholar]
- Shipton M., Brochlehurst K. Characterization of the papain active centre by using two-protonic-state electrophiles as reactivity probes. Evidence for nucleophilic reactivity in the un-interrupted cysteine-25-histidine-159 interactive system. Biochem J. 1978 May 1;171(2):385–401. doi: 10.1042/bj1710385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuchbury T., Shipton M., Norris R., Malthouse J. P., Brocklehurst K., Herbert J. A., Suschitzky H. A reporter group delivery system with both absolute and selective specificity for thiol groups and an improved fluorescent probe containing the 7-nitrobenzo-2-oxa-1,3-diazole moiety. Biochem J. 1975 Nov;151(2):417–432. doi: 10.1042/bj1510417. [DOI] [PMC free article] [PubMed] [Google Scholar]