Abstract
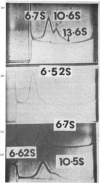
The self-association properties of bovine serum immunoglobulin G1 and colostral immunoglobulin G1 (IgG1) in 0.32 M-NaCl/0.01 M-Tris/HCl, pH 8.0, were investigated by analysing sedimentation data according to a monomer-dimer association model. The self-association was characterized by an equilibrium constant of 5.3 X 10(4) +/- 3.5 X 10(4) M-1 for serum IgG1 and 1.6 X 10(3) +/- 0.69 X 10(3) M-1 for colostral IgG1. The removal of the Fc portion of IgG1 by pepsin digestion abolished its property of self-aggregation. At high total protein concentrations of serum IgG1, low concentrations of the ostensible trimer species were observed. However, no self-aggregation was evident when 0.14 M-NaCl/0.01 M-sodium phosphate. pH 6.0, was used as a solvent, thus confirming results published previously [Tewari & Mukkur (1975) Immunochemistry 12, 925--930].
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fang W. D., Mukkur T. K. Physiochemical characterization of proteolytic cleavage fragments of bovine colostral immunoglobulin G1 (IgG1). Biochem J. 1976 Apr 1;155(1):25–30. doi: 10.1042/bj1550025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukkur T. K., Froese A. Isolation and characterization of IgM from bovine colostral whey. Immunochemistry. 1971 Mar;8(3):257–264. doi: 10.1016/0019-2791(71)90480-0. [DOI] [PubMed] [Google Scholar]
- Mukkur T. K. Valence and association constant of bovine colostral immunoglobulin M antibody (IgM). Immunochemistry. 1972 Nov;9(11):1049–1055. doi: 10.1016/0019-2791(72)90074-2. [DOI] [PubMed] [Google Scholar]
- Tewari U. J., Mukkur T. K. Isolation and physico-chemical characterization of bovine serum and colostral immunoglobulin G (IgG) subclasses. Immunochemistry. 1975 Dec;12(12):925–930. doi: 10.1016/0019-2791(75)90254-2. [DOI] [PubMed] [Google Scholar]