Abstract
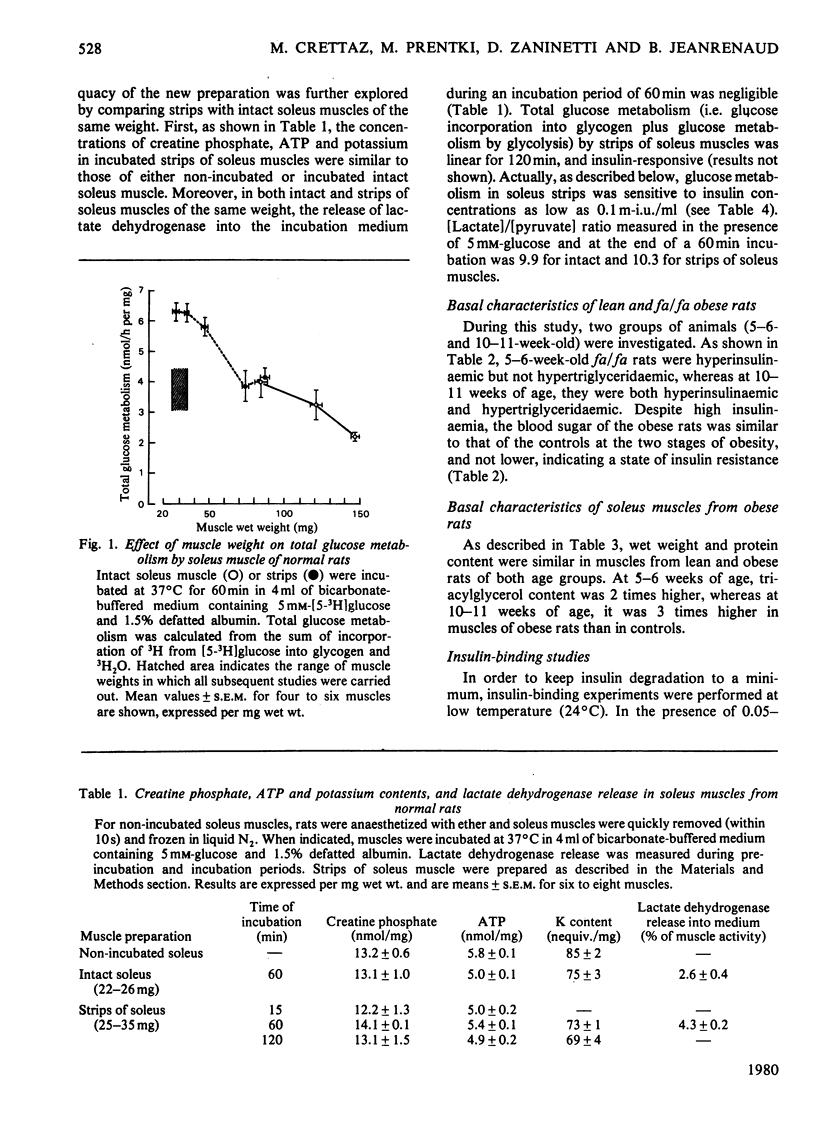
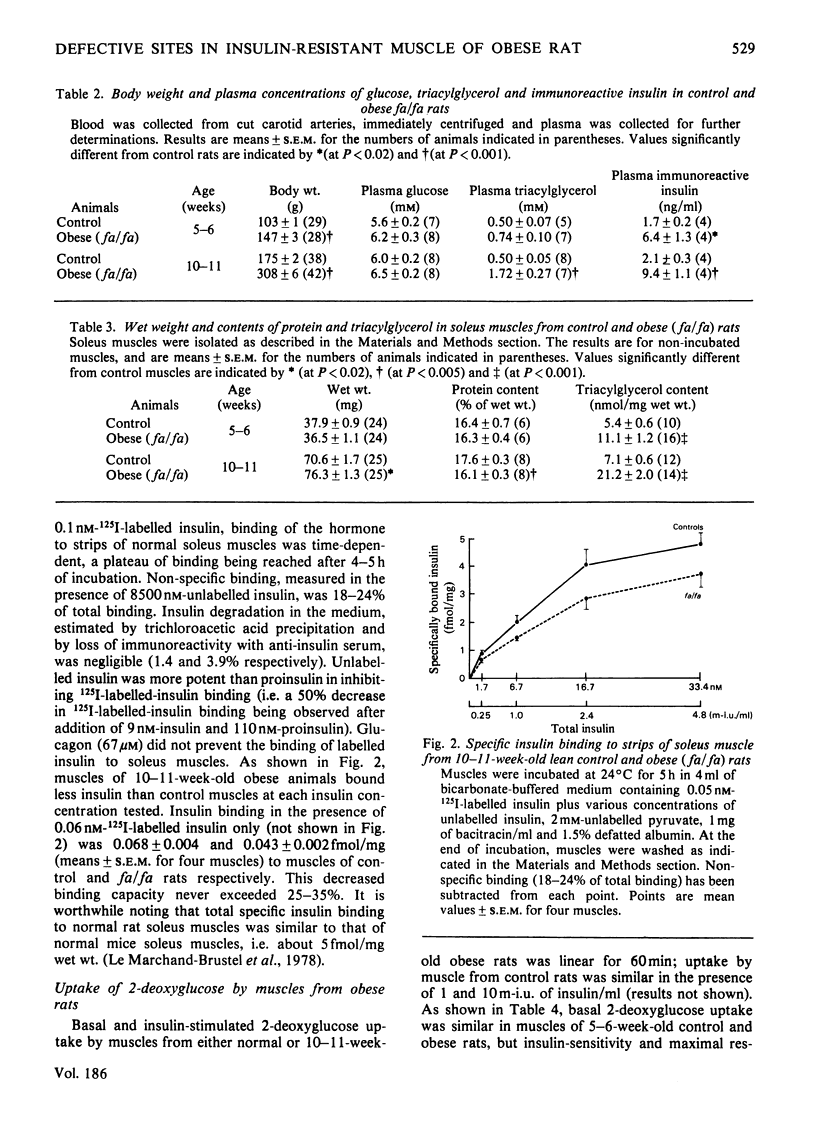
1. The effect of insulin upon glucose transport and metabolism in soleus muscles of genetically obese (fa/fa) and heterozygote lean Zucker rats was investigated at 5–6 weeks and 10–11 weeks of age. Weight-standardized strips of soleus muscles were used rather than the intact muscle in order to circumvent problems of diffusion of substrates. 2. In younger obese rats (5–6 weeks), plasma concentrations of immunoreactive insulin were twice those of controls, whereas their circulating triacylglycerol concentrations were normal. Insulin effects upon 2-deoxyglucose uptake and glucose metabolism by soleus muscles of these rats were characterized by both a decreased sensitivity and a decrease in the maximal response of this tissue to the hormone. 3. In older obese rats (10–11 weeks), circulating concentrations of insulin and triacylglycerols were both abnormally elevated. A decrease of 25–35% in insulin-binding capacity to muscles of obese rats was observed. The soleus muscles from the older obese animals also displayed decreased sensitivity and maximal response to insulin. However, at a low insulin concentration (0.1m-i.u./ml), 2-deoxyglucose uptake by muscles of older obese rats was stimulated, but such a concentration was ineffective in stimulating glucose incorporation into glycogen, and glucose metabolism by glycolysis. 4. Endogenous lipid utilization by muscle was calculated from the measurements of O2 consumption, and glucose oxidation to CO2. The rate of utilization of fatty acids was normal in muscles of younger obese animals, but increased in those of the older obese rats. Increased basal concentrations of citrate, glucose 6-phosphate and glycogen were found in muscles of older obese rats and may reflect intracellular inhibition of glucose metabolism as a result of increased lipid utilization. 5. Thus several abnormalities are responsible for insulin resistance of muscles from obese Zucker rats among which we have observed decreased insulin binding, decreased glucose transport and increased utilization of endogenous fatty acid which could inhibit glucose utilization.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assimacopoulos-Jeannet F., Jeanrenaud B. The hormonal and metabolic basis of experimental obesity. Clin Endocrinol Metab. 1976 Jul;5(2):337–365. doi: 10.1016/s0300-595x(76)80025-4. [DOI] [PubMed] [Google Scholar]
- Baldwin K. M., Klinkerfuss G. H., Terjung R. L., Molé P. A., Holloszy J. O. Respiratory capacity of white, red, and intermediate muscle: adaptative response to exercise. Am J Physiol. 1972 Feb;222(2):373–378. doi: 10.1152/ajplegacy.1972.222.2.373. [DOI] [PubMed] [Google Scholar]
- Becker S. G., Smollin P. F., Richardson D. K., Czech M. P. Insulin resistance in epitrochlearis muscles in the spontaneously obese rat. Horm Metab Res. 1978 May;10(3):204–208. doi: 10.1055/s-0028-1093435. [DOI] [PubMed] [Google Scholar]
- Bray G. A. The Zucker-fatty rat: a review. Fed Proc. 1977 Feb;36(2):148–153. [PubMed] [Google Scholar]
- Chan T. M., Exton J. H. A rapid method for the determination of glycogen content and radioactivity in small quantities of tissue or isolated hepatocytes. Anal Biochem. 1976 Mar;71(1):96–105. doi: 10.1016/0003-2697(76)90014-2. [DOI] [PubMed] [Google Scholar]
- Chaudry I. H., Gould M. K. Kinetics of glucose uptake in isolated soleus muscle. Biochim Biophys Acta. 1969 May 6;177(3):527–536. doi: 10.1016/0304-4165(69)90315-8. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Cuendet G. S., Loten E. G., Jeanrenaud B., Renold A. E. Decreased basal, noninsulin-stimulated glucose uptake and metabolism by skeletal soleus muscle isolated from obese-hyperglycemic (ob/ob) mice. J Clin Invest. 1976 Nov;58(5):1078–1088. doi: 10.1172/JCI108559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech M. P., Richardson D. K., Becker S. G., Walters C. G., Gitomer W., Heinrich J. Insulin response in skeletal muscle and fat cells of the genetically obese Zucker rat. Metabolism. 1978 Dec;27(12 Suppl 2):1967–1981. doi: 10.1016/s0026-0495(78)80013-4. [DOI] [PubMed] [Google Scholar]
- Czech M. P., Richardson D. K., Smith C. J. Biochemical basis of fat cell insulin resistance in obese rodents and man. Metabolism. 1977 Sep;26(9):1057–1078. doi: 10.1016/0026-0495(77)90024-5. [DOI] [PubMed] [Google Scholar]
- Forgue M. E., Freychet P. Insulin receptors in the heart muscle. Demonstration of specific binding sites and impairment of insulin binding in the plasma membrane of the obese hyperglycemic mouse. Diabetes. 1975 Aug;24(8):715–723. doi: 10.2337/diab.24.8.715. [DOI] [PubMed] [Google Scholar]
- Gliemann J., Gammeltoft S., Vinten J. Time course of insulin-receptor binding and insulin-induced lipogenesis in isolated rat fat cells. J Biol Chem. 1975 May 10;250(9):3368–3374. [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- KIPNIS D. M., CORI C. F. Studies of tissue permeability. VI. The penetration and phosphorylation of 2-deoxyglucose in the diaphram of diabetic rats. J Biol Chem. 1960 Nov;235:3070–3075. [PubMed] [Google Scholar]
- Kemmer F. W., Berger M., Herberg L., Gries F. A., Wirdeier A., Becker K. Glucose metabolism in perfused skeletal muscle. Demonstration of insulin resistance in the obese Zucker rat. Biochem J. 1979 Mar 15;178(3):733–741. doi: 10.1042/bj1780733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Olefsky J. M. Effect of experimental hyperinsulinemia on insulin binding and glucose transport in isolated rat adipocytes. Am J Physiol. 1978 Jul;235(1):E53–E62. doi: 10.1152/ajpendo.1978.235.1.E53. [DOI] [PubMed] [Google Scholar]
- Kono T., Barham F. W. The relationship between the insulin-binding capacity of fat cells and the cellular response to insulin. Studies with intact and trypsin-treated fat cells. J Biol Chem. 1971 Oct 25;246(20):6210–6216. [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Jeanrenaud B., Freychet P. Insulin binding and effects in isolated soleus muscle of lean and obese mice. Am J Physiol. 1978 Apr;234(4):E348–E358. doi: 10.1152/ajpendo.1978.234.4.E348. [DOI] [PubMed] [Google Scholar]
- Le Marchand Y., Singh A., Assimacopoulos-Jeannet F., Orci L., Rouiller C., Jeanrenaud B. A role for the microtubular system in the release of very low density lipoproteins by perfused mouse livers. J Biol Chem. 1973 Oct 10;248(19):6862–6870. [PubMed] [Google Scholar]
- Maldonato A., Trueheart P. A., Renold A. E., Sharp G. W. Effects of streptozotocin in vitro on proinsulin biosynthesis, insulin release and ATP content of isolated rat islets of Langerhans. Diabetologia. 1976 Oct;12(5):471–481. doi: 10.1007/BF01219511. [DOI] [PubMed] [Google Scholar]
- Malewiak M. I., Griglio S., Mackay S., Lemonnier D., Rosselin G. Nutritionally induced variations in insulinaemia, blood ketone bodies and plasma and liver triglycerides in genetically obese rats. Diabete Metab. 1977 Jun;3(2):81–89. [PubMed] [Google Scholar]
- Olefsky J. M. Mechanisms of decreased insulin responsiveness of large adipocytes. Endocrinology. 1977 Apr;100(4):1169–1177. doi: 10.1210/endo-100-4-1169. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. The effects of spontaneous obesity on insulin binding, glucose transport, and glucose oxidation of isolated rat adipocytes. J Clin Invest. 1976 Apr;57(4):842–851. doi: 10.1172/JCI108360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J., Bacon V. C., Baur S. Insulin receptors of skeletal muscle: specific insulin binding sites and demonstration of decreased numbers of sites in obese rats. Metabolism. 1976 Feb;25(2):179–191. doi: 10.1016/0026-0495(76)90048-2. [DOI] [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Garland P. B., Hales C. N., Newsholme E. A., Denton R. M., Pogson C. I. Interactions of metabolism and the physiological role of insulin. Recent Prog Horm Res. 1966;22:1–48. doi: 10.1016/b978-1-4831-9825-5.50004-x. [DOI] [PubMed] [Google Scholar]
- Rennie M. J., Winder W. W., Holloszy J. O. A sparing effect of increased plasma fatty acids on muscle and liver glycogen content in the exercising rat. Biochem J. 1976 Jun 15;156(3):647–655. doi: 10.1042/bj1560647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson D. K., Czech M. P. Primary role of decreased fatty acid synthesis in insulin resistance of large rat adipocytes. Am J Physiol. 1978 Feb;234(2):E182–E189. doi: 10.1152/ajpendo.1978.234.2.E182. [DOI] [PubMed] [Google Scholar]
- Soll A. H., Kahn C. R., Neville D. M., Jr Insulin binding to liver plasm membranes in the obese hyperglycemic (ob/ob) mouse. Demonstration of a decreased number of functionally normal receptors. J Biol Chem. 1975 Jun 25;250(12):4702–4707. [PubMed] [Google Scholar]


