Abstract
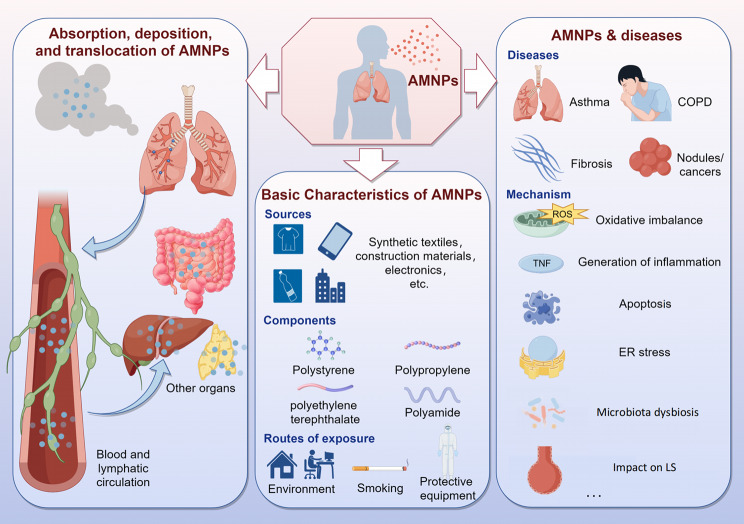
Airborne micro- and nanoplastics (AMNPs) are ubiquitously present in human living environments and pose significant threats to respiratory health. Currently, much research has been conducted on the relationship between micro- and nanoplastics (MNPs) and cardiovascular and gastrointestinal diseases, yet there is a clear lack of understanding regarding the link between AMNPs and respiratory diseases. Therefore, it is imperative to explore the relationship between the two. Recent extensive studies by numerous scholars on the characteristics of AMNPs and their relationship with respiratory diseases have robustly demonstrated that AMNPs from various sources significantly influence the onset and progression of respiratory conditions. Thus, investigating the intrinsic mechanisms involved and finding necessary preventive and therapeutic measures are crucial. In this review, we primarily describe the fundamental characteristics of AMNPs, their impact on the respiratory system, and the intrinsic toxic mechanisms that facilitate disease development. It is hoped that this article will provide new insights for further research and contribute to the advancement of human health.
Graphical Abstract
Keywords: Airborne micro- and nanoplastics, Characteristics, Threats, Respiratory diseases, Toxic mechanisms
Introduction
Plastic is a cheap and durable multifunctional material. It is made from polymers through industrial polymerization processes. By adding specific additives to these polymers or mixing them with other substances, such as carbon fibers, people can customize their physicochemical and mechanical properties to suit specific applications, bringing great convenience to human life [1]. Plastic can be classified based on the main polymers and chemical compositions of any side chains, including polyamide (PA), polyethylene terephthalate (PET), polypropylene (PP) and polystyrene (PS), etc [2]. It is expected that global production will increase from 390.7 million tons in 2021 to approximately 590 million tons in 2050 [3]. Currently, only 21% of plastic is recycled, leading to exponential growth in plastic waste generated over the past few decades [4]. When plastic enters the environment, external factors such wave erosion, ocean currents, UV radiation, weathering of the environment, air oxidation, and biodegradation continuously work to break it down into MNPs [5]. Any synthetic solid particles or polymer matrices with regular or irregular forms and sizes ranging from 1 μm to 5 mm that are insoluble in water are referred to as microplastics (MPs) [6]. And sub-micrometer (< 1 μm) plastic particles are referred to as nanoplastics (NPs) [7].
Increasing evidence suggests that AMNPs are widely present in many regions of the world and play an important role in the occurrence of diseases, which has raised concerns about their impacts on human health [8]. The respiratory system, as the main structure through which the human body directly contacts the external air, can be directly exposed to the threats of AMNPs [9]. Inhaled from the respiratory system and deposited in the upper respiratory tract (such as in the throat), AMNPs can potentially be swallowed, leading to oral absorption. Previous reviews have extensively analyzed the effects of orally ingested MNPs on human health [10]. Therefore, this article will not reiterate those findings but instead focus on MNPs deposited in the respiratory system. Currently, some well-defined toxic mechanisms of AMNPs on the respiratory system include: (1) oxidative stress imbalance [11]; (2) inflammation [12]; (3) endoplasmic reticulum (ER) stress [13], which can lead to or aggravate the occurrence and development of respiratory system diseases such as asthma, pulmonary fibrosis, chronic obstructive pulmonary disease (COPD) and tumors. The toxicity of AMNPs is closely related to their physical properties, including size, shape, and ζ-potential, which will be further discussed in the following text.
Due to the scarcity of research papers on the impact of AMNPs on the respiratory system, we conducted a literature search on PubMed and Web Of Science using the keywords “microplastics” AND “lung”, “polystyrene nanoparticles” AND “lung”, “nanoplastics” AND “lung”, “microplastics” AND “respiratory”, and “nanoplastics” AND “respiratory”. We didn’t restrict the search to a specific time period, aiming to provide a systematic and comprehensive review of the relationship between AMNPs and respiratory diseases. This review is intended to offer new insights that could inform future research and disease prevention strategies.
Basic characteristics of AMNPs
Similar to how plastics are found everywhere in human life, once AMNPs are released into the environment, they will spread to every part of the planet due to factors including wind, rivers, rainfall, snowfall, and biotic transport [14, 15]. Since the first detection of AMNPs in 2015, widespread records of AMNP transport have been reported worldwide, including in urban, rural, marine, and remote mountainous areas [16–19]. AMNPs can travel great distances and for extended periods of time in the atmosphere because of their small size and low density, which allows them to be suspended by wind or air turbulence [20].
Currently, the majority of laboratory scientific studies on the ecological effects of AMNPs use commercial standard plastic particles for experimental research. The physicochemical properties of these particles significantly differ from microplastics found in the environment. The generation of real microplastics in reality is influenced by various environmental factors, including the aging of materials such as polyethylene caused by ultraviolet light exposure [21]. Therefore, in future experiments, it is crucial to explore how to produce microplastics with characteristics that are more similar to those found in the real environment.
Sources and major harmful components of AMNPs
There are many sources for AMNPs. Because of their great elasticity and durability, synthetic textiles are frequently utilized. However, throughout the washing, drying, and use processes, MNPs are shed and released into the air, which makes them a significant source of atmospheric fiber AMNPs [22]. Furthermore, a significant amount of microfibers are released into the atmosphere during the industrial processing of polymer fibers used in the textile production process [23]. Additionally, the COVID-19 epidemic has led to a global upsurge in the manufacture and application of protective gear, including masks, clothes, and gloves composed of polymers like PS, PE, and PP [24]. Data suggests that during the 2019 coronavirus pandemic, 129 billion masks were used globally each month [25]. It is estimated that in 2020, over 1.37 trillion MPs were released into the environment from discarded masks alone [26], contributing to the AMNPs burden to some extent. Re-suspension of road dust and vehicle emissions are significant sources of MPs released into the atmosphere [27, 28]. Other important sources of AMNPs include industrial processes, agricultural practices (fertilizer bags and plastic films), human waste disposal (bio-solids, incineration, landfilling), and the physical and chemical degradation of large plastic materials (synthetic furniture and construction materials) [29]. Electronics, paints, adhesives, and cosmetics can all release MNPs into the atmosphere when they are used [30]. It can be said that wherever there are human activities, AMNPs are continuously generated.
MNPs release various components that pose threats to the environment and human health, including plastic additives, organic pollutants, heavy metals, pathogens, and toxins [31–34]. Plastic additives are intentionally added chemicals used during production to improve the performance of plastics, such as phthalate esters (PAEs). Most additives are not chemically bound to polymer chains, so they can be released into the surrounding environmental media during the use, handling, and recycling processes of plastics [35]. These plastic additives undoubtedly have adverse effects on human health. Studies have shown that potential mechanisms include neurotoxicity, inflammation, lipid metabolism disorders, and tumor occurrence [36, 37]. Due to their hydrophobic surfaces, MNPs are important carriers of environmental organic pollutants. The increased surface area/volume ratio of MNPs and their hydrophobicity enhance their affinity for various hydrophobic and persistent compounds. Organic pollutants can adsorb and attach to their surfaces through charge adsorption, allowing them to be transported and ultimately delivered to humans or other organisms through environmental media [38, 39]. Similarly, studies have also shown that AMNPs provide adsorption sites for metals such as Cu, Cd, Ni, Zn, and Pb, which can be absorbed by organisms after exposure in the environment [40, 41]. However, the study by Mohamed Nor et al. showed that the chemical contribution of AMNPs to the total chemical intake is negligible and can be disregarded [42].
AMNPs are a part of PM2.5/PM10. Recently, a study conducted in a coastal city in southeast Spain indicated that with a 95% interval confidence level, the average AMNPs-PM10 and AMNPs-PM2.5 concentrations were 2.09 ± 0.8 ng/m− 3 and 1.81 ± 0.6 ng/m− 3 respectively. Although the average AMNPs-PM10 concentration is slightly higher than the average AMNPs-PM2.5 concentration, analysis of variance tests showed no significant difference between these values (p > 0.05) [43]. However, another study conducted in a purely agricultural area found a concentration of 36 ng/m− 3 of AMNPs-PM10 [44]. As mentioned earlier, long-term exposure to AMNPs, which contain plastic additives, organic pollutants, and heavy metals, can have serious effects on the respiratory system. An observational cross-sectional study conducted in Chengdu, China recruited 20 participants from a plastic factory (high exposure to microplastics area) and a park (low exposure to microplastics area). Inclusion criteria included participants spending more than 20 h per day in the respective areas, continuous exposure for over 3 years, and less than 7 days of absenteeism per year. Microbial analysis of nasal secretions was performed using 16 S rDNA sequencing. The results showed a significantly higher detection rate of microplastics in the high-exposure area compared to the low-exposure area. High exposure increased the number of harmful microbial communities in the nasal cavity, such as Klebsiella and Spirochaetes, which are associated with respiratory diseases, while reducing the abundance of beneficial communities such as Bacteroidetes. Comprehensive analysis showed that extensive exposure to microplastics not only alters the nasal microbial community but also affects the symbiotic relationships between microbial communities in the nasal cavity [45]. Previous studies have predominantly focused on research on laboratory animals, and research on the effects of microplastics on human health is still in its early stages. Therefore, further studies in this area are urgently needed.
Compared to MNPs in other environmental media, research on the composition of AMNPs and their effects on the human respiratory system is still limited. Nevertheless, these findings provide some insights for future research on AMNPs and more exploration is needed.
Recognition of AMNPs
In previous studies, researchers have used various methods to collect and characterize MNPs, but they often find inconsistent results in the same environment [46]. Therefore, in the relevant research on AMNPs and lung diseases, people have also explored the effective recognition of AMNPs in the model, which will lay a solid foundation for subsequent research on the relationship between AMNPs and respiratory system diseases (Table 1).
Table 1.
Recognition of AMNPs
| Models | Sample size | The limitation of AMNPs | Method | References |
|---|---|---|---|---|
| Human lung tissue samples | 13 patients | ≤ 3 μm | muFTIR spectroscopy | [47] |
| Outdoor particle samples | Not clear | ≥ 4.7 μm | Raman spectroscopic imaging with different chemometric techniques | [48] |
| Thorax and alveoli | 136 patients | PM10 and PM2.5 | thermogravimetry-mass spectrometry | [43] |
| Lung tissue | Not clear | 1 μm and 5 μm | proteinase K, pepsin/pancreatin, and 10% potassium hydroxide (KOH) solution | [49] |
| BEAS−2B cells | Not clear | 10 µg/ml | a microfluidic-based model of human bronchial epithelial cells in vitro | [50] |
Jenner LC et al. analyzed digested human lung tissue samples (n = 13) in a lung tissue model using muFTIR spectroscopy (size limit of 3 μm) to identify and characterize AMNPs present in the tissue. The study’s findings validate inhalation as a route of exposure to ambient AMNPs, and this characteristic can now give information for realistic exposure scenarios in lab studies to ascertain how AMNPs affect human lung health [47]. Levermore et al. optimized existing Raman spectroscopic imaging with different chemometric techniques to identify MPs (≥ 2 μm) in ambient particulate matter. The applicability was demonstrated by identifying AMNPs larger than 4.7 μm in outdoor particle samples obtained at sampling points in London, UK. This semi-quantitative method will be able to obtain the exposure concentration of AMNPs, thus guiding future toxicological assessments [48]. A different study proposed a thermogravimetry-mass spectrometry method that needed minimal sample preparation. By using this technique to quantify gas-phase PS in PM10 and PM2.5, researchers discovered that the alveolar component of the air contains the majority of PS-MPs, which are linked to serious respiratory and cardiovascular conditions [43]. Geppner et al. demonstrated that a 10% KOH solution is most suitable for dissolving different organ samples, thus allowing efficient, economical, and non-destructive separation of MNPs [49].
Research models on the relationship between AMNPs and lung diseases also include artificially constructed models, and it is crucial to better achieve the objectives of in vitro experiments. In contrast to the changing cellular milieu found in vivo, most cell culture models are static. In order to assess cell uptake of NPs, Gupta et al. developed a microfluidic-based model of human bronchial epithelial cells in vitro, in which the cells are stationary but the food supply is dynamic. The study discovered that cell absorption of NPs is highly dependent on the culture conditions and shown that it is possible to culture human lung cells without the use of animals using microfluidic-based technologies. Consequently, low-density particle uptake may not be effectively reflected in typical cell culture, which could result in an underestimating of the particles’ cellular effects [50]. Due to the varying methods of culturing cells in vitro and exposing them to AMNPs, this can lead to different reactions compared to those observed in vivo. Furthermore, distinct responses may also be observed in monoculture and co-culture models in vitro. Currently, research on the aforementioned issues is relatively scarce, and further in-depth investigation is required.
The physical properties of AMNPs
The physical properties of AMNPs include size, shape, color, and charge. Among them, size, shape, and charge are closely related to the toxicity of AMNPs to specific organisms [51, 52](Table 2).
Table 2.
The physical properties of AMNPs
| Characteristics | Categories | Descriptions/Examples | References |
|---|---|---|---|
| Size | 15–250 μm |
①Smaller particles generally pose a greater toxicity risk. ②The density and aerodynamic characteristics of atmospheric MPs are connected to their size dispersion. |
[53, 54] |
| Color | Red, white, black and yellow |
①Color assessment can be influenced by experimenter bias. ②Color can serve as a useful parameter to assist in identifying potential sources and associated surface contaminants related to AMNPs. |
[56, 57] |
| Shape | Spheres, fibers, films, and pieces |
①Offers crucial hints on the source and polymer makeup. ②The original shape of AMNPs is affected by aging processes, residence duration in the environment, and decrease of surface performance. ③Fibrous particles and their bending stiffness can increase the toxicity of AMNPs. |
[59, 63, 64] |
| Potential | ζ-potential |
①Depends on their surface charge. ②Involve in the cytotoxicity of AMNPs. |
[65, 66] |
Smaller particles generally pose a greater toxicity risk because they are more easily transported within organisms and increase reactivity with cells and tissues by crossing epithelial barriers [53, 54]. The diameter of atmospheric MPs is typically found to range from 15 to 250 μm, which is significantly lower than the diameter of MPs seen in aquatic and soil/sediment environments [55]. The density and aerodynamic characteristics of atmospheric MPs are connected to their size dispersion. Generally, larger particles are more likely to settle. Some AMNPs that adsorb other substances (such as dust) become larger in size/mass, increasing the likelihood of settling under gravity [53]. Quantitative classification of the size and distribution of AMNPs is beneficial for assessing their risks to human health.
It has been reported that AMNPs have various colors, including red, white, black and yellow [56, 57]. However, weathering in the environment and preparation of laboratory samples can also modify the hue of AMNPs [58]. Additionally, color assessment may be influenced by experimenter bias because bright colors are more easily detected in visual inspections. Therefore, color inspection alone is usually insufficient to infer the type and source of MNPs. However, color can still serve as a useful parameter to assist in identifying potential sources and associated surface contaminants related to AMNPs.
MPs can take on a variety of shapes in the environment, such as spheres, fibers, films, and pieces. The form of AMNPs frequently offers crucial hints on the source and polymer makeup of the particles. However, the original shape of AMNPs is affected by aging processes, residence duration in the environment, and decrease of surface performance [59]. Furthermore, the shape of AMNPs can also be influenced by different regional economic and industrial development [60–62]. There is evidence to suggest that fibrous particles may increase the toxicity of AMNPs compared to spherical particles [63]. In addition to fiber length, their bending stiffness may also determine their toxicity, affecting whether they can curl and be engulfed [64]. Since the inhibitory engulfment effect mainly depends on the geometric shape of particles, it may be a highly correlated toxicological mechanism for all fibrous AMNPs.
Another important physical feature of AMNPs is ζ-potential. The ζ-potential of AMNPs depends on their surface charge, such as the charge generated by functional surface groups [65, 66]. Surface functionalization is a common method to adjust material properties in the manufacture of plastic products. Studies have shown that the interaction between particles and cells varies with the ζ-potential of the particles, involving in the cytotoxicity of AMNPs [65]. Therefore, we believe that this should always be taken into account in future studies on the in vitro and in vivo effects of MNP particles.
Routes of human exposure to AMNPs
Humans can be exposed to AMNPs in various settings, including indoors and outdoors. Based on estimates from relevant studies, the annual indoor exposure level is 24.3/m³, while the annual outdoor exposure level is 23.5/m³ [67]. Researches on the relationship between AMNP exposure and health are gradually increasing. Eberhard et al. published a systematic review using PRISMA guidelines to systematically search all unpublished papers, published peer-reviewed journal articles and grey literature focusing on AMNPs in indoor and outdoor air, and calculated the inhalation exposure of AMNPs for different age groups for the first time. The findings demonstrated that different age groups have varied levels of AMNP inhalation exposure. Preschoolers, middle school students, pregnant women, teenagers, and non-pregnant adults had the highest calculated dose values across all locations, followed by newborns [68]. This study provides some ideas and theoretical basis for the protection of different age groups from AMNPs exposure and also prompts researchers to consider the causes of indoor and outdoor AMNPs generation and people’s normal life exposure levels.
During the COVID-19 pandemic, respiratory equipment and masks are widely used for virus protection measures. These disposable protective devices are typically made of plastic fiber-based nonwoven fabrics. If used masks are reused or improperly disposed of, they can become an important source of MNPs, thereby contaminating the environment and increasing the risk of human exposure to inhaled MNPs and organic pollutants [69–71]. Quantitative analysis of MNPs by pyrolysis gas chromatography-mass spectrometry revealed that reusable masks have a greater potential for release [72]. Kubátová et al. conducted thermal stability testing of MNPs using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA), and found that MNPs from protective equipment sources have a significant impact on soil organisms [73].
The routes of AMNP exposure also include personal habits such as smoking, work environments, and living areas. A prospective study conducted in Zhuhai, China, combining population-based and experimental work found that the concentration of total MNPs (9.99 particles/L) in bronchoalveolar lavage fluid (BALF) of a smoking simulation model was higher than that of the control group of non-smokers. The experiment demonstrated that AMNPs can accumulate in the lower respiratory tract as a result of smoking [74, 75]. Jiang et al. used polarized light microscopy and a laser direct infrared imaging system to quantitatively examine MNPs after collecting sputum and nasal lavage fluid from messengers and office workers that results revealed questionable MNPs. Compared to couriers, office workers’ nasal lavage fluid had a noticeably higher concentration of MNPs [76]. Research has shown that shoe factory workers also have AMNPs in their lower respiratory tract in fibrous form, which is significantly associated with a higher incidence of respiratory system diseases [77]. Therefore, these studies indicate that regulatory authorities should take stronger protective measures for occupational exposure of workers in specific locations. In addition, different living areas have different characteristics and abundance of AMNPs due to industrial development, environmental climate, population density, and other factors, which undoubtedly pose a threat to the health of local residents [78, 79].
Absorption, deposition, clearance and in vivo translocation of AMNPs in the respiratory system
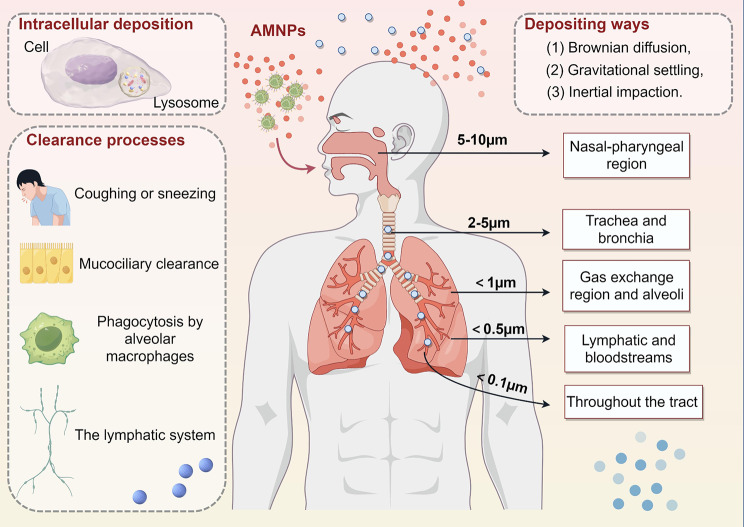
MNPs can be directly inhaled by humans through airborne transmission, and smaller-sized particles are more likely to deposit within the lung tissue [23]. It has been proposed that future research should focus on quantifying MNPs in the air with aerodynamic equivalent diameter (AED) less than 10 μm, as this is important for assessing the risks of AMNPs to human health [80]. The deposition of AMNPs in the lungs also triggers the body’s clearance mechanism [81](Fig. 1). The potential adverse effects on health mainly depend on the balance between the deposition rate and clearance rate in the respiratory system. Additionally, MNPs entering the body through the respiratory tract can also translocate to other tissues via lymph or blood circulation [82](Fig. 2).
Fig. 1.
Absorption, deposition, clearance of AMNPs in the respiratory system. (By Figdraw)
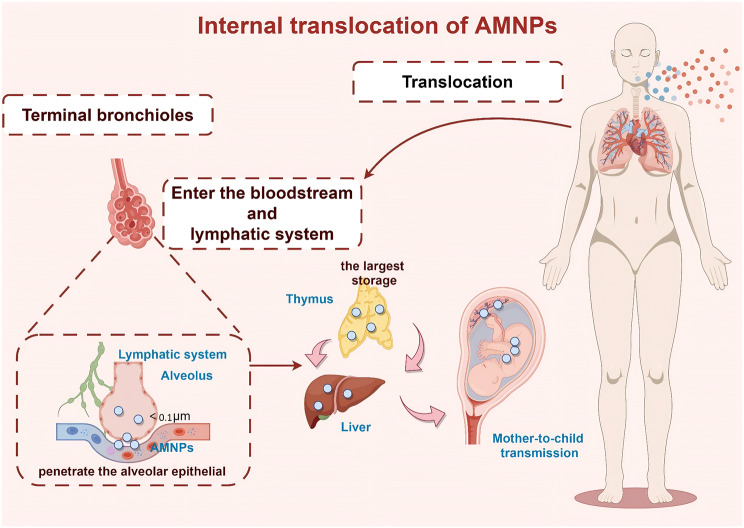
Fig. 2.
Internal translocation of AMNPs. (By Figdraw)
Absorption, deposition and clearance of AMNPs in the respiratory tract
MNPs are a part of the plastic pollution that can infiltrate the human body through the respiratory system and endanger health. In A549 cells and BEAS-2B cells, Liu et al. investigated the properties of endocytosis, distribution, and exocytosis of 50 nm and 100 nm PS-MNPs. It was discovered that MNP cellular uptake increased with exposure duration and dose, with A549 cells absorbing more MNPs than BEAS-2B cells. Furthermore, there were more 50 nm particles than 100 nm particles inside the cells. The lysosomes contained the majority of the ingested MNPs. In terms of exocytosis, it was harder to expel 100 nm particles than 50 nm ones. For the purpose of evaluating the safety of nanoplastics in the environment, these findings offer insightful information on the translocation of nanoplastics in lung cells [83]. Nevertheless, very little is now known regarding MNP absorption and exocytosis in human lung organ cells.
AMNPs are deposited in the respiratory system through three primary mechanisms: Brownian diffusion, gravitational settling, and inertial impaction [84]. The site of deposition in the respiratory tract is closely related to its AED, with AMNPs having lower AED more likely to penetrate the respiratory tract deeply [85]. Studies have shown that particles with AED of 5–10 μm can be inhaled and deposit on the upper respiratory tract wall through impaction, primarily in the nasal-pharyngeal region. Particles with AED of 2–5 μm may penetrate into the trachea and bronchial regions by diffusion and deposit in the lungs. In contrast, because of Brownian motion, particles with an AED smaller than 1 μm are more likely to lodge in the gas exchange region and alveoli, resulting in irreversible lung injury. Particles with an AED less than 0.5 μm are even capable of entering the lymphatic and bloodstreams and moving to different bodily tissues. Particles with AED smaller than 0.1 μm, due to their thermodynamic properties, can effectively deposit throughout the respiratory tract (from the upper respiratory tract to the alveoli) by diffusion [53, 86, 87].
The major clearance pathways of AMNPs in the respiratory tract include: (1) mechanical means, such coughing or sneezing; (2) mucociliary clearance entry into the pharynx, which causes swallowing or coughing; (3) phagocytosis by alveolar macrophages, which triggers immunological responses to eliminate undesired or unidentified cells; (4) clearance by the lymphatic system. Given that plastic fibers are difficult to remove due to their wide and irregular surface, particle shape may be a significant attribute [88].
Particle deposition patterns and respiratory tract diameters affect MNP deposition and clearance processes in addition to exposure duration, polymer kinds, and abundance of MNP exposure. Studying the MNPs’ deposition and clearance mechanisms in the human body under real-world exposure conditions is therefore critically important. Revealing the absorption, deposition, and clearance of AMNPs in the respiratory system will contribute to the specific mechanistic study of tissue toxicity caused by AMNPs. Subsequent research can focus on exploring treatments and protective measures for lung diseases caused by AMNPs based on these aspects.
Translocation of AMNPs in vivo
After entering the human body through the respiratory tract, AMNPs can undergo translocation within the body, thus it is impossible to overlook their eventual destination or possible build-up within the body. The fragile barrier of human lung tissue may allow MNPs smaller than 20 μm to penetrate and enter the bloodstream, migrating to other tissues and organs, causing damage to these organs or systems, including the cardiovascular system [89]. Studies have found that MPs can be found in both the alveoli and interstitium when fluid containing MPs is administered into the mouse airway through nasal infusion, suggesting that MPs can penetrate the alveolar epithelial barrier [90]. The thymus is the largest MNPs storage outside the lungs, which may also be due to the influence of lung-related lymphatic movement [91]. In addition, AMNPs can also undergo maternal or mother-to-child transmission [92]. In their investigation, Fournier et al. verified that the fetal placental unit may be vulnerable to negative consequences due to the transfer of plastic particles to the placenta and fetal tissues as a result of the mother’s lung exposure to MNPs formed from PS [93]. In another study, Wang et al. detected the presence of MNPs in lung tissue and also found changes in platelets, plateletcrit, fibrinogen, and direct bilirubin in the blood [94]. Although it is currently unclear whether the changes in these indicators are directly caused by MNPs entering the bloodstream or indirectly caused by the deposition of AMNPs in the lungs, these experimental results undoubtedly provide a theoretical basis for the harm caused by AMNPs to the body, which is crucial for understanding the toxicology of plastic particles and the origin of health and disease.
Potential driving factors of AMNPs toxicity
As mentioned earlier, the toxicity of AMNPs to the human body is related to several physical factors such as size, shape, and ζ-potential. Smaller, fibrous, or low ζ-potential MNPs with cationic properties have stronger toxic effects [95–97]. Environmental factors can also alter the chemical properties of MNPs, further changing their toxicity. Ultraviolet (UV) light is an inevitable environmental factor that can cause photoaging of AMNPs. Following photoaging treatment of MNPs, Hayek et al. examined the biological reactions of A549 cells to original and irradiated particles. High-resolution X-ray photoelectron spectroscopy and scanning electron microscopy revealed that photoaging enhanced the intensity of polar groups in the near-surface region and changed the surface shape of irradiated particles. In A549 cells, photoaged MNPs had more notable biological reactions than original MNPs, even at low doses (1–30 µg/ml) [98]. The mechanism of this occurrence may be attributed to the altered oxidative potential (OP) of photoaged MNPs, resulting in dynamic evolution of oxidative and reductive active species (including environmentally persistent free radicals (EPFRs), reactive oxygen species (ROS), peroxides, and conjugate carbonyl compounds), as well as related cellular toxicity and decreased cell viability [99]. Additionally, surface modification of AMNPs also plays a role. Shi et al. studied the cytotoxicity and genotoxicity of surface-modified MNPs (carboxyl- and amine-functionalized PS, original PS) in A549 cells and found that the modified MNPs exhibited stronger inhibition of cell viability [51]. Surface roughness is also considered a possible determining factor in the interaction between MNPs and cell membranes, as it can enhance physical damage to human cells [52]. The potential driving factors of MNPs’ toxicity are still poorly understood, and further exploration is needed in the future (Table 3).
Table 3.
Potential driving factors of AMNPs toxicity
| Characteristics | Mechanisms | Outcome | References |
|---|---|---|---|
| Size | Smaller particles are more easily transported within organisms and increase reactivity with cells and tissues by crossing epithelial barriers. | Toxicity-increased | [53, 54] |
| Shape | Fibrous particles’ length and their bending stiffness affect whether they can curl and be engulfed. | Toxicity-increased | [63, 64] |
| ζ-potential | A lower ζ-potential shows more particle–cell interactions and consequently decreased cell metabolism and proliferation | Toxicity-increased | [65, 66] |
| Photoaging | It can enhance the intensity of polar groups in the near-surface region and changed the surface shape of irradiated particles. | Toxicity-increased | [98] |
| It alter the OP, resulting in dynamic evolution of oxidative and reductive active species, as well as related cellular toxicity and decreased cell viability. | Toxicity-increased | [99] | |
| Surface modification | carboxyl-, amine-functionalized- and original PS-MNPs exhibite stronger inhibition of cell viability. | Toxicity-increased | [51] |
| Surface roughness | It can enhance physical damage to human cells . | Toxicity-increased | [52] |
AMNPs are one of the risk factors for respiratory diseases
After entering the human body through the respiratory tract, AMNPs deposit in lung tissues and can be detected in BALF and sputum [100, 101]. They are closely associated with the occurrence and development of various respiratory diseases, including asthma, pulmonary fibrosis, COPD, and tumors. Possible mechanisms include oxidative stress, inflammation, and imbalances in lung microbiota (Table 4). Studying the effects of MNPs on respiratory diseases and the role of particulate matter in healthy and diseased organisms will be beneficial for disease prevention and treatment and has significant clinical implications.
Table 4.
Possible mechanisms of AMNPs on respiratory health
| Characteristics | Sample type | AMNPs type |
The Sources of AMNPs | Shape | Size | Mechanism | References |
|---|---|---|---|---|---|---|---|
| Oxidative Imbalance | HPAEpiC and BEAS-2B cells | PS-MNPs | Commercially available beads | Not clear | 7.5, 15, and 30 µg/cm2 | Oxidative stress-induced mitochondrial damage can lead to ferroptosis | [121] |
| A549 cells | PTFE-MNPs | Ground PTFE raw powder materials with 450 μm average particle size using a homogenize | Not clear | 31.7 ± 5.6 μm and 6.0 ± 2.1 μm | Activate ERK signaling pathways, ultimately leading to the generation of ROS and oxidative stress | [122] | |
| HPAEpiC and BEAS-2B cells | PS-MNPs | Commercially available beads | Irregular geometric shape | 40 nm | Disrupt redox balance, decrease levels of tight junction proteins, reduce levels of matrix metalloproteinase 9 and surfactant protein A, thereby impairing lung repair capacity and causing tissue damage | [125] | |
| BEAS-2B cells | PS-MNPs | Commercially available beads | Spheres | 10 μm and 20 μm | Increased cell apoptosis, levels of MDA and iron content, decreased levels of key ferroxidases (GPX4 and FTH1), and mitochondrial changes | [13] | |
|
A549 cells, BEAS-2B cells and 5–8 weeks BALB/c mice |
PE-, PP-, PS-, PVC-MNPs | Commercially available beads | PE, PS AND PVC are regular spheres but have non-uniform size, while PP displays irregular geometric shape | 6.5 μm−1 mm, 6.5–100 μm, 3–100 μm, and 6.5–25 μm | Disrupt the cellular redox homeostasis and prompt cellular senescence | [115] | |
| A549 cells | PS-MNPs | Commercially available beads | Spheres | 1 and 5 μm | Reduce the integrity of the single-layer barrier and slowed down the regeneration in wound healing experiments | [98] | |
| A549 cells | PS-MNPs | Commercially available beads | Spheres | 50 nm | Induce significant increases in ROS production | [116] | |
| A549 cells | PET-MNPs | Commercially available beads | Spheres | ≤ 20 nm | Oxidative stress induces a decrease in the mitochondrial membrane potential | [118] | |
| Generation of inflammation | A549 cells | PTFE-MNPs | Ground PTFE raw powder materials with 450 μm average particle size using a homogenize | Not clear | 31.7 ± 5.6 μm and 6.0 ± 2.1 μm | Increase the secretion of IL−6 and TNF-α | [122] |
| A549 cells | PS-MNPs | Commercially available beads | Not clear | 25 and 75 nm | Increase the secretion of NF-κB, TNF-α and IL−8 | [132] | |
| Mice | PP-MNPs | Precipitate a solution dissolved at 200 °C on a hot plate with PP beads and xylene solvent with ethanol | irregular fragments with a spherical shape | 0.66 ± 0.27 μm | The NF-κB pathway regulated by p38 phosphorylation as a result of mitochondrial damage | [12] | |
| Six-week-old male and female (n = 40; 20 males and 20 females) specific pathogen-free (SPF) Sprague-Dawley rats | PS-MNPs | Commercially available beads | Spheres | 0.10 μm | The lung tissue exhibited an increase in TGF-β and TNF-α that is dependent on exposure concentration | [131] | |
| BEAS-2B cells |
PS-MNPs, NH2PS-MNPs, COOH-PS-MNPs |
Commercially available beads | Spheres | 100 nm | Increase the expression and secretion of the pro-inflammatory cytokine IL-β | [129] | |
| C57BL/6 mice, BALB/c and ICR mice | PE-, PP-, PS-MNPs | Commercially available beads | Irregular geometric shape | 6.40 ± 1.48 μm for PP, 17.53 ± 2.11 μm for PS and 21.27 ± 6.07 μm for PE | Induce lung inflammation through the TLR4 pathway and raise levels of IL−1β in BALF and elevated levels of NLRP3, ASC, and Caspase−1 in the lung tissue | [133] | |
| ICR mice | PET-MNPs | Microplastics were prepared from polyethylene beads (5 mm) | Irregular geometric shape | 27.0 ± 10.9 μm | Not clear | [127] | |
| A549 cells | PS-MNPs | Commercially available beads | Spheres | 104.77 ± 1.47 nm | Increase the secretion of pro-inflammatory cytokines | [128] | |
| A549 cells | Plastic-waste derived MNPs | Were recovered in the laboratory from industrially recycled plastic granules | Irregular shape, encompassing fiber-like formations, with noticeable surface irregularities and structural flaws at the submicron level | ≤ 50 μm | Increase the secretion of IL−6 and IL−8 | [126] | |
| ER stress | BEAS-2B cells |
PS-MNPs, NH2PS-MNPs, COOH-PS-MNPs |
Commercially available beads | Spheres | 100 nm | The protein levels associated with the PERK-EIF2α and ATF4-CHOP pathways increased | [128] |
| BEAS-2B cells | PS-MNPs | Commercially available beads | Spheres | 570 nm | Amino acids and TCA cycle intermediate metabolites | [119] | |
| BEAS-2B cells | PS-MNPs | Commercially available beads | Spheres | 10 μm and 20 μm | The elevation of IRE1α, PERK, XBP1S, and CHOP | [13] | |
| Apoptosis | A549 cells | PS-MNPs | Commercially available beads | Not clear | 25 nm and 70 nm | the expression of Bax/Bcl−2, DR5, caspase−3, caspase−8, caspase−9, and cytochrome c significantly upregulate, resulting in cell apoptosis | [132] |
| A549 cells | Bap@PS-MNPs | Ground styrene materials | Spheres | 410–470 nm | lead to a significant decrease in mitochondrial membrane potential and involvement of mitochondria in the apoptotic pathway | [138] | |
| Autophagy cell death | BEAS-2B cells |
PS-MNPs, NH2PS-MNPs, COOH-PS-MNPs |
Commercially available beads | Spheres | 100 nm | Inhibit the PI3K/Akt/mTOR pathway in cells. They in lysosomes and nuclear deformation can also be observed, resulting in autophagic cell death | [129] |
| Human primary nasal epithelial cells | PET-MNPs | Self-preparation within the laboratory | Irregular geometric shape | 62.38 nm ± 3.51 μm | Increases in the expression levels of LC3-II and p62 proteins, altering the autophagy pathway | [139] | |
| A549 cells | PS-MNPs | Commercially available beads | Not clear | 20 nm | Increased autophagic activity, but limit to autophagic capacity | [140] | |
| Impact on LS | LS extracted from porcine lungs | PS-MNPs | Commercially available beads | Spheres | 500–1000 nm | It can quicken the process of ascorbic acid to dehydroascorbic acid conversion, which causes hydrogen peroxide (HOOH) to be produced in simulated LS, which raises the level of hydroxyl radicals (·OH) | [142] |
| Simulated lung fluid | aged MNPs | Commercially available beads | Irregular geometric shape | 200 μm | Lipid peroxidation and protein degradation in LS | [143] | |
| BALB/c mice | PS-, PP-, PVC-MNPs | Self-preparation |
PP-MNPs : irregular block like structure; PS-, PET-MNPs: crinkled flocculent morphology |
PP-, PET-MNPs are all larger than 200 nm, while the PS-MNPs are around 52 nm | Particles adhered to the air-water interface and aggregated with the LS membrane | [144] | |
| Alveolar fluid | PS-, PET-, PP-, PE-, PVC-MNPs | Using melting-annealing approach to fabricate nanoplastics of different materials | Spheres | 10 nm and 5 nm | Once AMNPs have reached the alveolar air-water interface, they can disrupt the ultrastructure and fluidity of LS, which can interfere with LS’s regular biophysical function and lead to LS membrane collapse | [81] | |
| Microbiota dysbiosis | ICR male mice | AMNPs | Self-preparation within the laboratory | Not clear | 5 μm and 99 nm | The most closely linked to MP were nasal Staphylococcus and pulmonary Roseomonas, while the most closely linked to NP were nasal Prevotella and pulmonary unclassified bacteria. Both MP and NP were associated with nasal Staphylococcus, pulmonary Roseomonas, pulmonary Aggregatibacter, and pulmonary Bacillus | [145] |
| 20 subjects from a Plastic Factory and the other 20 from Huanhuaxi Park | AMNPs | Realistic MNPS | Not clear | Not clear | Increase the abundance of nasal microbiota and decrease the abundance of beneficial microorganisms. It can also increase the abundance of intestinal microbiota | [146] | |
| 20 subjects from a Plastic Factory and the other 20 from Huanhuaxi Park | AMNPs | Realistic MNPS | Not clear | Not clear | Not only cause changes in the major microbial communities in the intestines and nose, but also alter the symbiotic relationship between intestinal and nasal microbiota | [147] | |
| Inhibition of cell proliferation | A549 cells | PS-MNPs | Commercially available beads | Spheres | 1 μm and 10 μm | Decrease in cell proliferation | [148] |
| A549 cells | PS-MNPs | Commercially available beads | Not clear | 25 nm and 70 nm | Induce cell cycle arrest and have a substantial impact on cell viability | [132] | |
| Promotion of aging | MLE12 | PS-MNPs | Commercially available beads | Spheres | 100 nm | circ_kif26b binds to miR-346-3p and co-regulates the target gene, p21 of miR-346-3p | [148] |
|
A549 cells, BEAS-2B cells and 5–8 weeks BALB/c mice |
PVC-MNPs | Commercially available beads | PE, PS AND PVC are regular spheres but have non-uniform size, while PP displays irregular geometric shape | 6.5 μm−1 mm, 6.5–100 μm, 3–100 μm, and 6.5–25 μm | Increase the levels of ROS in A549 cells and the aging features induced by them can be largely reversed by antioxidant treatment. Increase in systemic inflammation levels can cause the accumulation of senescent cells | [115] | |
| Metabolism | HaCaT cells, A549cells and RAW 264.7 | ulfate-modified MNPs | Commercially available beads | Irregular geometric shape | 210 ± 4.63 nm | Human macrophages exposed to them can stimulate the accumulation of LDs in the cytoplasm, and the accumulated LDs are further transported and accumulated in lysosomes, leading to lysosomal damage and further differentiation of macrophages into foam cells | [150] |
| BEAS−2B cells and RAW264.7 | PS-MNPs | Commercially available beads | Spheres | 100 nm | Cause cell damage by regulating prostaglandin B1 and other metabolites via the cGAS-STING pathway | [151] | |
| Induction of ferroptosis | BEAS-2B cells | PS-MNPs | Commercially available beads | Spheres | 100 and 200 nm | The HIF-1α/HO-1 signaling pathway plays an important role in regulating ferroptosis in lung injury | [152] |
| Genetic toxicity | A549 cells | PET-MNPs | Was produced by grinding food containers | Spheres | 136 nm | Increase the level of DNA strand breaks | [153] |
| Impairment of lysosomal function | A549 cells | PS-MNPs | Commercially available beads | Not clear | 10 μm | Lysosomal dysfunction | [154] |
| Damage to epithelial airway cell | Human and murine alveolar and airway-type organoids as well as air–liquid interface cultures derived from primary lung epithelial progenitor cells | PS-MNPs | Self-preparation within the laboratory | Fibers | 15 × 52 μm | Damage developing and/or repairing airways | [157] |
| Primary rat alveolar epithelial monolayers | PS-MNPs | Commercially available beads | Not clear | 20, 100, 120 nm | Disrupt the characteristics of the alveolar epithelial barrier and involve changes in cellular transport pathways | [159] | |
| Immune responses | Not clear | AMNPs | Not clear | Not clear | Not clear | Insufficient response (immune suppression, increased susceptibility to infection and disease) and excessive response (pathological inflammation, allergies, autoimmune disorders) | [156] |
| Sulfonamide resistance | KM mice | PET-MNPs | Locally purchased mineral water bottles were cut and mechanically broke into small particles with the aid of a kitchen blender | Not clear | 63.4 μm | Increase the relative abundance of multidrug genes | [158] |
AMNPs are closely related to respiratory diseases
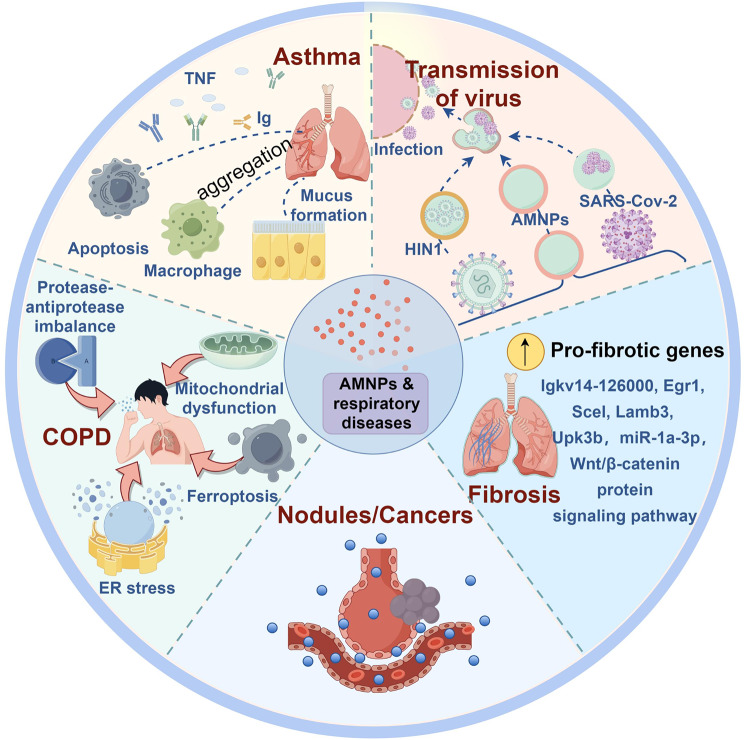
AMNPs can cause or worsen the occurrence and development of various respiratory diseases (Fig. 3). Asthma, a common respiratory allergic disease, may be negatively affected by AMNPs. When Guo et al. examined how exposure to MPs affected the physiology of the asthmatic mouse lung, they discovered that MPs exposure led to a considerable increase in macrophage aggregation, inflammatory cell infiltration, and mucus formation. A set of transmembrane B-cell antigen molecular mechanisms were activated, resulting in the regulation of cellular stress and programmed cell death in the asthma model. Additionally, it altered gene clusters related to cellular stress response, immunological response, and programmed cell death. These mechanisms were stimulated by tumor necrosis factor and immunoglobulin production [90]. Exposure to AMNPs also increases the risk of COPD, possibly by causing protease-antiprotease imbalance, mitochondrial dysfunction, ferroptosis, and ER stress, resulting in respiratory function decline and promoting the development of COPD [102, 103]. In addition, inhalation of AMNPs can cause changes in mouse lung genes, promoting the expression of pro-fibrotic genes, including Igkv14-126000, Egr1, Scel, Lamb3, and Upk3b [104]. miR-1a-3p can also inhibit the formation of F-actin by targeting the cell skeleton-regulating twinfilin-1 protein, leading to MNPs-induced lung fibrosis damage [105]. Epithelial-mesenchymal transition, oxidative stress, and Wnt/β-catenin protein signaling pathway activation also play important roles in the induction of pulmonary fibrosis [106, 107]. Exposure to MNPs may cause or exacerbate lung nodules [108]. Inhaling AMNPs can also indirectly affect the health of the gastrointestinal system [109]. It can also serve as a medium for the transmission of respiratory infectious diseases, including COVID-19 and H1N1 [110–112]. AMNPs are carcinogenic, which may be related to their endocrine-disrupting effects [113, 114]. The etiology of respiratory system diseases is diverse, and according to the current researches, it’s not yet possible to identify specific diseases related to AMNPs. Further research on the underlying mechanisms of AMNPs causing various respiratory diseases is of crucial importance to human health.
Fig. 3.
AMNPs and respiratory diseases. (By Figdraw)
Possible mechanisms of AMNPs on respiratory health
Oxidative imbalance
One of the processes behind the development of lung disorders like asthma, COPD, and acute respiratory distress syndrome (ARDS) is oxidative stress brought on by oxidative imbalance. Recent studies have shown that AMNPs can also induce this process in the lungs [98, 115–119]. Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene ontology (GO) analyses have suggested that oxidative imbalance is involved in the lung-damaging effects of AMNPs, which has been confirmed by ROS staining [120]. MNPs have the ability to infiltrate cells and alter the potential of the mitochondrial membrane, impede mitochondrial respiration, and produce excessive amounts of ROS [11, 118]. Yang et al. found that oxidative stress-induced mitochondrial damage can lead to ferroptosis, with autophagy-dependent ferritinophagy acting as a crucial intermediate step that causes ferritin degradation and release of iron ions. Furthermore, the use of iron chelator-1 can inhibit lung ferroptosis, alleviate lung and systemic toxicity, and reverse AMNP-induced lung injury in mice [121]. Polytetrafluoroethylene MNPs (PTFE-MNPs) can activate MAPK signaling pathways, particularly the ERK pathway, ultimately leading to the generation of ROS and oxidative stress [122]. Due to particle degradation and instability, superoxide dismutase (SOD) is the main enzyme triggering cellular toxicity [123]. In an in vitro experiment using two types of human lung epithelial cells (HPAEpiC and BEAS-2B), MNPs were found to significantly reduce cell viability in a dose-dependent manner. Functional analysis showed that MNPs can disrupt redox balance, decrease levels of tight junction proteins, reduce levels of matrix metalloproteinase 9 and surfactant protein A, thereby impairing lung repair capacity and causing tissue damage [124]. Oxidative stress induced by PS-NPs is characterized by increased cell apoptosis, levels of malondialdehyde (MDA) and iron content, decreased levels of key ferroxidases (GPX4 and FTH1), and mitochondrial changes [13].
Generation of inflammation
Inflammation is a common manifestation in the development of human diseases. AMNPs can lead to the generation of lung inflammation [125–128]. PTFE-MPs not only cause oxidative imbalance, but also increase the secretion of interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α) in A549 lung epithelial cell lines, inducing the occurrence of inflammation [122]. Other factors involved in this process include interleukin-1β (IL-1β), interleukin-8 (IL-8), transforming growth factor β (TGF-β), nuclear factor κB (NF-κB) and p38 [12, 129–132]. A study evaluating the toxicity of PS-MNPs in mice found that toll-like receptor 4 (TLR4) protein levels increased, while TLR1, TLR5, and TLR6 protein levels remained unchanged. In addition, there was a significant increase in phosphorylation of NF-κB and NF-κα, as well as increased expression of NLRP3 inflammasomes, indicating that PS-MNPs can induce lung inflammation through the TLR4 pathway [133]. Studies have shown that the expression of these inflammation-related proteins increases in a concentration-dependent manner [131].
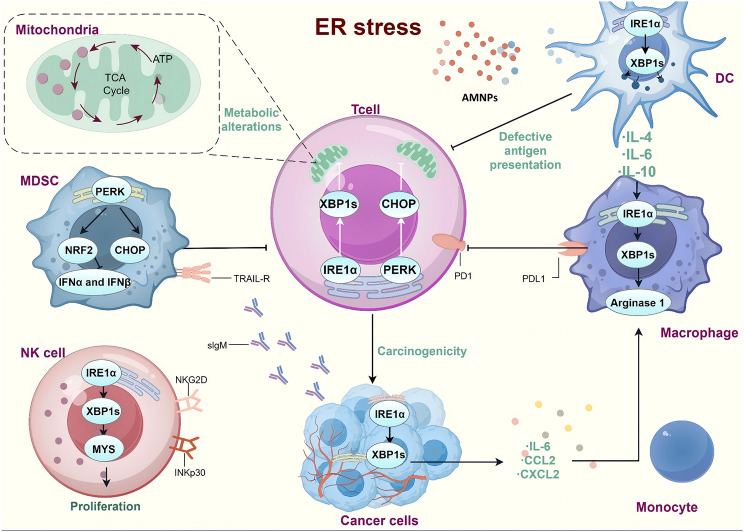
ER stress
In response to situations like improper protein folding and aggregation and abnormal calcium ion balance in the endoplasmic reticulum, cells’ protective stress response known as ER stress triggers signaling pathways such as endoplasmic reticulum overload response, the unfolded protein response, and caspase-12 mediated apoptotic pathway [134]. In an experiment on the toxicity of PS-MPs, it was found that the protein levels associated with the protein kinase R-like endoplasmic reticulum kinase-eukaryotic translation initiation factor 2 subunit α (PERK-EIF2α) and activating transcription factor-C/EBP-homologous protein (ATF4-CHOP) pathways increased, mediating ER stress [129]. Other stress proteins include inositol-requiring enzyme 1α (IRE1α), x-box binding protein 1 spliced (XBP1S) and ATF6 [13, 135]. Lim et al. found that nanoparticle exposure showed metabolic changes related to autophagy and ER stress, such as an increase in intermediates of amino acid and tricarboxylic acid cycle (TCA), which play a key role in regulating cell resistance to cytotoxic effects. Autophagy is reciprocally regulated to couple transcriptional reprogramming and metabolism, as demonstrated by metabolomic analysis, the ER stress pathway, and quantitative real-time polymerase chain reaction (qRT-PCR) analysis [119](Fig. 4).
Fig. 4.
ER stress triggered by AMNPs. (By Figdraw)
Apoptosis and autophagy cell death
Apoptosis and autophagy cell death are forms of programmed cell death. Apoptosis is a process in which cells form apoptotic bodies and are eventually engulfed by neighboring cells or phagocytes. Autophagic cell death is characterized by the presence of a large number of autophagosomes and autolysosomes in the cytoplasm, which are ultimately degraded by lysosomes within the cells. It is a self-protective mechanism of cells for the degradation and recycling of intracellular biomolecules (e.g., misfolded proteins) and dysfunctional organelles. It plays an important role in the regulation of cell growth and maintenance of intracellular homeostasis [136]. PS-MPs can inhibit the PI3K/Akt/mTOR pathway in cells. They in lysosomes and nuclear deformation can also be observed, resulting in autophagic cell death [129]. In lung tissue exposed to PS-MPs, the expression of Bax/Bcl-2 and caspase family proteins increases, the phosphorylation levels of the MAPK signaling pathway (p38, ERK, and JNK) increase, and the expression of apoptotic proteins such as DR5, caspase-3, caspase-8, caspase-9, and cytochrome c significantly upregulate, leading to a significant decrease in mitochondrial membrane potential and involvement of mitochondria in the apoptotic pathway, resulting in cell apoptosis [132, 137, 138]. Human primary nasal epithelial cells (HNEpCs) exposed to PET-NPs showed significant increases in the expression levels of microtubule-associated protein 1 light chain 3-II (LC3-II) and p62 proteins, altering the autophagy pathway in HNEpCs [139]. A549 cells exposed to AMNPs showed increased autophagic activity, but there is a limit to autophagic capacity [140].
Impact on lung surfactant (LS)
LS lowers surface tension in the alveoli by combining proteins and lipids [141]. It is yet unknown how MNPs affect the interfacial characteristics of LS and how this affects the production of reactive ROS. Research has revealed that the phase behavior, surface tension, and membrane structure of LS are all changed by the presence of MNPs. Adsorption tests revealed that PS adsorbed more strongly to the phospholipid component than to the protein in a mixed solution of PS and LS. Moreover, PS can quicken the process of ascorbic acid to dehydroascorbic acid conversion, which causes hydrogen peroxide (HOOH) to be produced in simulated lung fluid (which contains LS), which raises the level of hydroxyl radicals (·OH) [142]. Another study looked at the possible processes underlying the oxidative damage caused by aged MNPs in simulated lung fluid and the relationship between inhalable old MNPs and LS in vitro. The findings demonstrated that aged MNPs considerably decreased liquid paraffin’s capacity to foam and raised its surface tension. Additionally, they raised surface tension and improved the adsorption capacity to phospholipids; on the other hand, the structural alterations in proteins and their adsorption on MNPs may have contributed to the change in foaming ability. Reduced hydrodynamic diameter in aged MNPs may interact with bodily fluid biomolecules and intensify health risks. Additionally, they produced persistent free radicals, which raised the risk of respiratory illnesses by causing the production of ROS such superoxide radicals (O2•-), HOOH, and hydroxyl radicals (OH). These ROS caused lipid peroxidation and protein degradation in LS [143]. Mice that were exposed intranasally to AMNPs from foam boxes likewise experienced the greatest degree of lung damage and inflammation. MNP particles adhered to the air-water interface and aggregated with the LS membrane, as demonstrated by atomic force microscopy. These findings imply that, although though incidentally generated nanoparticles make just a little portion of the mass fraction, by aggregating at the alveolar-capillary interface, they may have a significant impact on determining nanobiointeractions and the pulmonary toxicity of AMNPs [144]. Once AMNPs have reached the alveolar air-water interface, they can disrupt the ultrastructure and fluidity of LS, which can interfere with LS’s regular biophysical function and lead to LS membrane collapse [81]. These findings offer fresh perspectives on the molecular destiny and toxicity of AMNPs in the human respiratory system.
Microbiota dysbiosis
It has been confirmed that dysbiosis of the human microbiota plays a significant role in the occurrence and development of many diseases. Zha et al. investigated the effects of MP and NP in the air on the nasal and pulmonary microbiota of mice by performing a number of bioinformatics and statistical analysis. The results showed that both MP and NP can cause dysbiosis of the nasal microbiota, with MP having a greater impact on the pulmonary microbiota. Both MP and NP were linked to a number of nasal and pulmonary bacterial communities. The most closely linked to MP were nasal Staphylococcus and pulmonary Roseomonas, while the most closely linked to NP were nasal Prevotella and pulmonary unclassified bacteria. Both MP and NP were associated with nasal Staphylococcus, pulmonary Roseomonas, pulmonary Aggregatibacter, and pulmonary Bacillus, which are putative indicators of airway dysbiosis brought on by micro/nanoplastics. In the MP group, SAR11_Clade_II and SAR11_Clade_Ia were correlated with nasal and pulmonary microbiota, while such bacteria were not detected in the NP group [145]. An observational study conducted in Sichuan, China found that high exposure to AMNPs may increase the abundance of nasal microbiota, such as Klebsiella and Treponema, which are positively correlated with respiratory diseases, and decrease the abundance of beneficial microorganisms, such as Bacteroides. At the same time, it can also increase the abundance of intestinal microbiota, which is positively correlated with gastrointestinal diseases, such as Lactobacillus, Streptococcus, and Shewanella, and decrease the quantity of beneficial and healthy intestinal microbiota, such as Bifidobacterium, Geobacillus, Clostridium, and Faecalibacterium. A comprehensive analysis showed that extensive exposure to microplastics may not only cause changes in the major microbial communities in the intestines and nose, but also alter the symbiotic relationship between intestinal and nasal microbiota [45, 146]. The effects of AMNPs on the airway microbiota are still poorly understood and require further investigation in the future.
Inhibition of cell proliferation and promotion of aging
As a model for environmental contaminants, PS-MPs with sizes of 1 μm and 10 μm were introduced to cultured human lung epithelial A549 cells. Trypan blue staining and calcein-AM staining for cell viability maintenance rate showed a significant decrease in cell proliferation for both sizes [147]. PS-NPs induced cell cycle arrest and had a substantial impact on cell viability [132]. After treatment with PS-MPs, the mouse lung epithelial cell line MLE12 showed increased senescent cells in lung tissue as indicated by β-galactosidase staining, elevated levels of senescence-associated secretory phenotype (SASP) in BALF determined by ELISA, and increased levels of senescence markers p21, p16, and p27. circ_kif26b is a circular structure of non-coding RNA (ncRNA) that is conserved in humans, rats, and mice. In both MLE12 cells treated with PS-MPs and rat lung tissue exposed to PS-MPs, its expression is elevated. circ_kif26b binds to miR-346-3p and co-regulates the target gene, p21 of miR-346-3p, according to luciferase reporter gene tests. In MLE12 cells, circ_kif26b knockdown or miR-346-3p overexpression reduces PS-MPs-induced senescence and the release of SASP cytokines IL-6 and IL-8 [148]. Polyvinyl chloride (PVC)-MNPs can increase the levels of ROS in A549 cells, and the aging features induced by them can be largely reversed by antioxidant treatment. Importantly, tracheal instillation of PVC-MNPs can effectively impair the physiological function of mice, induce an increase in systemic inflammation levels, and cause the accumulation of senescent cells [115]. These experimental results provide new insights into the potential mechanisms of AMNPs in the development of pulmonary diseases.
Others
AMNPs toxic mechanisms on the respiratory system include effects on metabolism, induction of ferroptosis, genetic toxicity, impairment of lysosomal function, and damage to epithelial airway cell differentiation. Experimental evidence has shown that oral ingestion of PS-NPs can cause hematological damage and lipid metabolism disorders [149]. Human macrophages exposed to PS-NPs (100 g/mL) can stimulate the accumulation of lipid droplets (LDs) in the cytoplasm, and the accumulated LDs are further transported and accumulated in lysosomes, leading to lysosomal damage and further differentiation of macrophages into foam cells [150]. Lei et al. demonstrated through non-targeted metabolomics and protein imprinting that PS-MNPs can cause cell damage by regulating prostaglandin B1 and other metabolites via the cGAS-STING pathway. Prostaglandin B1 supplementation reduced immunological activation and metabolic abnormalities brought on by exposure to PS-MNPs [151]. RNA-seq revealed that differentially expressed genes in BEAS-2B cells exposed to PS-MNPs were enriched in lipid metabolism and iron ion binding processes, with significant changes in the expression levels of high iron proteins, and further confirmed that the HIF-1α/HO-1 signaling pathway plays an important role in regulating ferroptosis in lung injury caused by PS-MNPs exposure [152]. In addition, exposure to PET-MNPs can increase the level of DNA strand breaks, leading to genetic toxicity [153]. The interaction between AMNPs and A549 cells also involves lysosomal dysfunction [154]. Chronic inhalation of AMNPs in rats leads to pulmonary overload, resulting in fibrosis and cancer in the lungs [155]. AMNPs pollutants can also have an impact on immune responses, including insufficient response (immune suppression, increased susceptibility to infection and disease) and excessive response (pathological inflammation, allergies, autoimmune disorders) [156]. The ingredients in MNPs may also damage developing and/or repairing airways [157]. Currently, it has been found that ingested MNPs may exacerbate sulfonamide resistance and increase the relative abundance of multidrug genes [158], but there have been no reports on the impact of lung antibiotic resistance. Yacobi et al. found that exposure to specific AMNPs compositions, shapes, and/or surface charges can disrupt the characteristics of the alveolar epithelial barrier and involve changes in cellular transport pathways [159].
The toxicity of nanoplastics on aquatic and marine organisms has received extensive attention, while studies on its toxicity evaluation in mammals are relatively limited. The above results are only a partial exploration of the toxicity of AMNPs on the respiratory system, and further research in this area is still needed. These findings are of great significance for the toxicological research and ecosystem protection of PS-MPs, and provide new perspectives on the toxicological mechanisms of AMNPs.
Discussion
With the development of human society, people are almost inevitably exposed to various sources of MNPs, which are one of the important threats to human life and health. Numerous research on the negative effects of AMNPs on human health have been conducted in recent years. Existing published data partly confirm the harm of AMNPs to the human respiratory system. However, the specific toxic mechanism is still unclear. There are also some challenges in the research on the prevention and treatment of AMNPs. (1) Firstly, there is a lack of data on the respiratory system exposure of susceptible groups, including infants, pregnant women, and the elderly. (2) In addition, as mentioned earlier, the indoor exposure to AMNPs is even higher than the outdoor exposure. However, data on indoor AMNPs are limited. It is necessary to study the existence, exposure, and health effects of indoor AMNPs in order to clarify possible health consequences and strengthen the necessity of taking mitigation measures. (3) There are some methodological obstacles in the detection, characterization, and quantification of AMNPs in lung tissue. The scientific community needs sensitive, high-throughput techniques and standardized methods to determine AMNPs. (4) So far, there is still insufficient research on the incidence and health effects of AMNPs in humans, especially in the respiratory system. (5) Currently, most of the MNPs used in experiments are commercially available, with relatively uniform characteristics such as shape, size, and composition. These polymers cannot represent the real world situation. (6) The research on AMNPs is currently mainly focused on in vitro and animal experiments, with relatively less research in the population. Further research is needed in the future.
Conclusion
In summary, the role of AMNPs in respiratory diseases is increasingly attracting attention and they are ubiquitous in human life. People can be exposed to them through masks, tobacco, and occupational contact, and they subsequently deposit in the respiratory tract and even circulate throughout the body through blood circulation. The harm to the respiratory system is closely related to the balance between the deposition rate and clearance rate in the body. Existing studies have shown that AMNPs can be toxic to the body through mechanisms such as oxidative stress imbalance, ER stress, and inflammation, leading to the occurrence and development of respiratory diseases such as asthma, COPD, pulmonary fibrosis, and tumors. In addition, the toxicity of AMNPs is closely related to their physical properties, including size, shape, and ζ-potential. Although research on the relationship between AMNPs and respiratory diseases has been ongoing in recent years, there are still significant knowledge gaps that require further exploration by researchers to contribute to human health.
Acknowledgements
We use Figdraw (https://www.figdraw.com/#/) to create our figures.
Abbreviations
- AMNPs
Airborne micro- and nanoplastics
- MNPs
Micro- and nanoplastics
- PA
Polyamide
- PET
Polyethylene terephthalate
- PP
Polypropylene
- PS
Polystyrene
- MPs
Microplastics
- NPs
Nanoplastics
- ER
Endoplasmic reticulum
- COPD
Chronic obstructive pulmonary disease
- NAC
N-acetylcysteine
- PAEs
Phthalate esters
- DSC
Differential scanning calorimetry
- TGA,
Thermogravimetric analysis
- BALF
Bronchoalveolar lavage fluid
- AED
Aerodynamic equivalent diameter
- UV
Ultraviolet
- EPFRs
Environmentally persistent free radicals
- ROS
Reactive oxygen species
- ARDS
Acute respiratory distress syndrome
- KEGG
Kyoto encyclopedia of genes and genomes
- GO
Gene ontology
- PTFE-MNP
Psolytetrafluoroethylene MNPs
- SOD
Superoxide dismutase
- IL-6
Interleukin-6
- TNF-α
Tumor necrosis factor α
- IL-1β
Interleukin-1β
- IL-8
Interleukin-8
- TGF-β
Transforming growth factor β
- NF-κB
Nuclear factor κB
- TLR4
Toll-like receptor 4
- PERK-EIF2α
Protein kinase R-like endoplasmic reticulum kinase-eukaryotic translation initiation factor 2 subunit α
- ATF4-CHOP
Activating transcription factor-C/EBP-homologous protein
- IRE1α
Inositol-requiring enzyme 1α
- XBP1S
X-box binding protein 1 spliced
- TCA
Tricarboxylic acid cycle
- qRT-PCR
Quantitative real-time polymerase chain reaction
- HNEpCs
Human primary nasal epithelial cells
- LC3-II
Microtubule-associated protein 1 light chain 3-II
- LS
Lung surfactant
- SASP
Senescence-associated secretory phenotype
- ncRNA
Non-coding RNA
- PVC
Polyvinyl chloride
- LDs
Lipid droplets
Author contributions
Zixuan Gou and Haonan Wu wrote the main manuscript text and Shanyu Li prepared figures. Ziyu Liu and Ying Zhang revised the manuscript. All authors reviewed the manuscript.
Funding
This work was supported by grants from Natural Science Foundation of Jilin Province (No. YDZJ202201ZYTS008), Special Project for Health Scientific Research Talents of Jilin Province (No. JLSWSRCZX2023-108) and Jilin Province health science and technology ability improvement program (2017J037).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zixuan Gou and Haonan Wu contributed equally to this work.
Contributor Information
Ziyu Liu, Email: liuziyu@jlu.edu.cn.
Ying Zhang, Email: yingzhang@jlu.edu.cn.
References
- 1.Kik K, Bukowska B, Sicinska P. Polystyrene nanoparticles: sources, occurrence in the environment, distribution in tissues, accumulation and toxicity to various organisms. Environ Pollut. 2020;262:114297. [DOI] [PubMed] [Google Scholar]
- 2.Borriello L et al. Microplastics, a global issue: human exposure through Environmental and Dietary sources. Foods, 2023. 12(18). [DOI] [PMC free article] [PubMed]
- 3.Adediran GA, et al. Fate and behaviour of Microplastics (> 25microm) within the water distribution network, from water treatment works to service reservoirs and customer taps. Water Res. 2024;255:121508. [DOI] [PubMed] [Google Scholar]
- 4.Rochman CM, Hoellein T. The global odyssey of plastic pollution. Science. 2020;368(6496):1184–5. [DOI] [PubMed] [Google Scholar]
- 5.Jansen MAK, et al. Plastics in the environment in the context of UV radiation, climate change and the Montreal Protocol: UNEP Environmental Effects Assessment Panel, Update 2023. Photochem Photobiol Sci. 2024;23(4):629–50. [DOI] [PubMed] [Google Scholar]
- 6.Mahmood M, et al. Toxicological assessment of dietary exposure of polyethylene microplastics on growth, nutrient digestibility, carcass and gut histology of Nile Tilapia (Oreochromis niloticus) fingerlings. Ecotoxicology. 2024;33(3):296–304. [DOI] [PubMed] [Google Scholar]
- 7.Hul G et al. Influence of concentration, Surface Charge, and Natural Water Components on the transport and adsorption of Polystyrene nanoplastics in Sand columns. Nanomaterials (Basel), 2024. 14(6). [DOI] [PMC free article] [PubMed]
- 8.Marfella R, et al. Microplastics and nanoplastics in Atheromas and Cardiovascular events. N Engl J Med. 2024;390(10):900–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markus MA, et al. Tracking of Inhaled Near-Infrared fluorescent nanoparticles in lungs of SKH-1 mice with allergic airway inflammation. ACS Nano. 2015;9(12):11642–57. [DOI] [PubMed] [Google Scholar]
- 10.Zhu L, et al. Tissue accumulation of microplastics and potential health risks in human. Sci Total Environ. 2024;915:170004. [DOI] [PubMed] [Google Scholar]
- 11.Lin S, et al. Metabolomics Reveal Nanoplastic-Induced mitochondrial damage in Human Liver and Lung cells. Environ Sci Technol. 2022;56(17):12483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woo JH, et al. Polypropylene nanoplastic exposure leads to lung inflammation through p38-mediated NF-kappaB pathway due to mitochondrial damage. Part Fibre Toxicol. 2023;20(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Q, et al. Polystyrene nanoplastics-induced lung apoptosis and ferroptosis via ROS-dependent endoplasmic reticulum stress. Sci Total Environ. 2024;912:169260. [DOI] [PubMed] [Google Scholar]
- 14.Schell T, Rico A, Vighi M. Occurrence, Fate and fluxes of Plastics and Microplastics in Terrestrial and Freshwater ecosystems. Rev Environ Contam Toxicol. 2020;250:1–43. [DOI] [PubMed] [Google Scholar]
- 15.Guo X, Wang J. The chemical behaviors of microplastics in marine environment: a review. Mar Pollut Bull. 2019;142:1–14. [DOI] [PubMed] [Google Scholar]
- 16.Kau D, et al. Fine micro- and nanoplastics concentrations in particulate matter samples from the high alpine site Sonnblick, Austria. Chemosphere. 2024;352:141410. [DOI] [PubMed] [Google Scholar]
- 17.Morioka T, et al. Quantification of microplastic by particle size down to 1.1 mum in surface road dust in an urban city, Japan. Environ Pollut. 2023;334:122198. [DOI] [PubMed] [Google Scholar]
- 18.Padha S, et al. Microplastic pollution in mountain terrains and foothills: a review on source, extraction, and distribution of microplastics in remote areas. Environ Res. 2022;207:112232. [DOI] [PubMed] [Google Scholar]
- 19.Dris R, et al. Microplastic contamination in an urban area: a case study in Greater Paris. Environ Chem. 2015;12(5):592–9. [Google Scholar]
- 20.Niu S, et al. Quantifying the Chemical composition and real-time Mass Loading of Nanoplastic particles in the atmosphere using Aerosol Mass Spectrometry. Environ Sci Technol. 2024;58(7):3363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang T, Ma Y, Ji R. Aging processes of Polyethylene Mulch films and Preparation of Microplastics with Environmental characteristics. Bull Environ Contam Toxicol. 2021;107(4):736–40. [DOI] [PubMed] [Google Scholar]
- 22.Salvador Cesa F, Turra A, Baruque-Ramos J. Corrigendum to Synthetic fibers as microplastics in the marine environment: A review from textile perspective with a focus on domestic washings [Sci. Total Environ. 598 (2017) 1116–1129]. Sci Total Environ, 2017. 603–604: p. 836. [DOI] [PubMed]
- 23.Zarus GM, et al. Worker studies suggest unique liver carcinogenicity potential of polyvinyl chloride microplastics. Am J Ind Med. 2023;66(12):1033–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shruti VC et al. Reusable masks for COVID-19: A missing piece of the microplastic problem during the global health crisis. Mar Pollut Bull, 2020. 161(Pt B): p. 111777. [DOI] [PMC free article] [PubMed]
- 25.Berthelot S, et al. Postpandemic evaluation of the Eco-efficiency of Personal Protective Equipment against COVID-19 in Emergency Departments: proposal for a mixed methods study. JMIR Res Protoc. 2023;12:e50682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun JJ, et al. Release of Microplastics from Discarded Surgical masks and their adverse impacts on the Marine Copepod Tigriopus japonicus. Volume 8. Environmental Science & Technology Letters; 2021. pp. 1065–70. 12.
- 27.Gossmann I, Halbach M, Scholz-Bottcher BM. Car and truck tire wear particles in complex environmental samples - a quantitative comparison with traditional microplastic polymer mass loads. Sci Total Environ. 2021;773:145667. [DOI] [PubMed] [Google Scholar]
- 28.Kole PJ et al. Wear and tear of tyres: a Stealthy source of Microplastics in the Environment. Int J Environ Res Public Health, 2017. 14(10). [DOI] [PMC free article] [PubMed]
- 29.Cai L, et al. Correction to: characteristic of microplastics in the atmospheric fallout from Dongguan City, China: preliminary research and first evidence. Environ Sci Pollut Res Int. 2019;26(35):36074–5. [DOI] [PubMed] [Google Scholar]
- 30.Zhong L, et al. Recent advances towards micro(nano)plastics research in wetland ecosystems: a systematic review on sources, removal, and ecological impacts. J Hazard Mater. 2023;452:131341. [DOI] [PubMed] [Google Scholar]
- 31.Bakan B, et al. Science-based evidence on pathways and effects of human exposure to micro- and nanoplastics. Arh Hig Rada Toksikol. 2024;75(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Hassel L, et al. Dynamics of leaching of POPs and additives from plastic in a Procellariiform gastric model: Diet- and polymer-dependent effects and implications for long-term exposure. PLoS ONE. 2024;19(3):e0299860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clerigo F et al. Cytotoxicity Assessment of Nanoplastics and plasticizers exposure in in Vitro Lung Cell Culture Systems-A systematic review. Toxics, 2022. 10(7). [DOI] [PMC free article] [PubMed]
- 34.Kirstein IV, et al. Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Mar Environ Res. 2016;120:1–8. [DOI] [PubMed] [Google Scholar]
- 35.Cao Y, et al. Microplastics: a major source of phthalate esters in aquatic environments. J Hazard Mater. 2022;432:128731. [DOI] [PubMed] [Google Scholar]
- 36.Romarate RA 2, et al. Breathing plastics in Metro Manila, Philippines: presence of suspended atmospheric microplastics in ambient air. Environ Sci Pollut Res Int. 2023;30(18):53662–73. [DOI] [PubMed] [Google Scholar]
- 37.Jeong J, Choi J. Development of AOP relevant to microplastics based on toxicity mechanisms of chemical additives using ToxCast and deep learning models combined approach. Environ Int. 2020;137:105557. [DOI] [PubMed] [Google Scholar]
- 38.Wang Q, et al. Effects of bisphenol A and nanoscale and microscale polystyrene plastic exposure on particle uptake and toxicity in human Caco-2 cells. Chemosphere. 2020;254:126788. [DOI] [PubMed] [Google Scholar]
- 39.Wright SL, Kelly FJ. Plastic and human health: a Micro Issue? Environ Sci Technol. 2017;51(12):6634–47. [DOI] [PubMed] [Google Scholar]
- 40.Munier B, Bendell LI. Macro and micro plastics sorb and desorb metals and act as a point source of trace metals to coastal ecosystems. PLoS ONE. 2018;13(2):e0191759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holmes LA, Turner A, Thompson RC. Adsorption of trace metals to plastic resin pellets in the marine environment. Environ Pollut. 2012;160(1):42–8. [DOI] [PubMed] [Google Scholar]
- 42.Mohamed Nor NH, et al. Lifetime Accumulation of Microplastic in children and adults. Environ Sci Technol. 2021;55(8):5084–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costa-Gomez I, et al. A novel application of thermogravimetry-mass spectrometry for polystyrene quantification in the PM(10) and PM(2.5) fractions of airborne microplastics. Sci Total Environ. 2023;856(Pt 2):159041. [DOI] [PubMed] [Google Scholar]
- 44.Peñalver R et al. Assessing the level of airborne polystyrene microplastics using thermogravimetry-mass spectrometry: results for an agricultural area. Sci Total Environ, 2021. 787.
- 45.Zhang X, et al. Effect of microplastics on nasal and intestinal microbiota of the high-exposure population. Front Public Health. 2022;10:1005535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyers N, et al. Microplastic detection and identification by Nile red staining: towards a semi-automated, cost- and time-effective technique. Sci Total Environ. 2022;823:153441. [DOI] [PubMed] [Google Scholar]
- 47.Jenner LC, et al. Detection of microplastics in human lung tissue using muFTIR spectroscopy. Sci Total Environ. 2022;831:154907. [DOI] [PubMed] [Google Scholar]
- 48.Levermore JM, et al. Detection of Microplastics in Ambient Particulate Matter using Raman Spectral Imaging and Chemometric Analysis. Anal Chem. 2020;92(13):8732–40. [DOI] [PubMed] [Google Scholar]
- 49.Geppner L et al. Testing of Different Digestion Solutions on Tissue Samples and the effects of used Potassium Hydroxide Solution on Polystyrene microspheres. Toxics, 2023. 11(9). [DOI] [PMC free article] [PubMed]
- 50.Gupta G, et al. Development of Microfluidic, serum-free bronchial epithelial cells-on-a-Chip to facilitate a more realistic in vitro testing of nanoplastics. Front Toxicol. 2021;3:735331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi X, et al. Cytotoxicity and genotoxicity of Polystyrene Micro- and nanoplastics with different size and surface modification in A549 cells. Int J Nanomed. 2022;17:4509–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wieland S, et al. From properties to toxicity: comparing microplastics to other airborne microparticles. J Hazard Mater. 2022;428:128151. [DOI] [PubMed] [Google Scholar]
- 53.Luo D, et al. Micro- and nano-plastics in the atmosphere: a review of occurrence, properties and human health risks. J Hazard Mater. 2024;465:133412. [DOI] [PubMed] [Google Scholar]
- 54.Niu X, et al. Characterization of chemical components and cytotoxicity effects of indoor and outdoor fine particulate matter (PM(2.5)) in Xi’an, China. Environ Sci Pollut Res Int. 2019;26(31):31913–23. [DOI] [PubMed] [Google Scholar]
- 55.Auta HS, Emenike CU, Fauziah SH. Distribution and importance of microplastics in the marine environment: a review of the sources, fate, effects, and potential solutions. Environ Int. 2017;102:165–76. [DOI] [PubMed] [Google Scholar]
- 56.Parolini M et al. Microplastic Contamination in Snow from Western Italian Alps. Int J Environ Res Public Health, 2021. 18(2). [DOI] [PMC free article] [PubMed]
- 57.Akhbarizadeh R, et al. Suspended fine particulate matter (PM(2.5)), microplastics (MPs), and polycyclic aromatic hydrocarbons (PAHs) in air: their possible relationships and health implications. Environ Res. 2021;192:110339. [DOI] [PubMed] [Google Scholar]
- 58.Nuelle MT, et al. A new analytical approach for monitoring microplastics in marine sediments. Environ Pollut. 2014;184:161–9. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, et al. Occurrence characteristics and Ecotoxic effects of Microplastics in Environmental Media: a Mini Review. Appl Biochem Biotechnol; 2023. [DOI] [PubMed]
- 60.Liu P, et al. Microplastic atmospheric dustfall pollution in urban environment: evidence from the types, distribution, and probable sources in Beijing, China. Sci Total Environ. 2022;838(Pt 1):155989. [DOI] [PubMed] [Google Scholar]
- 61.Dong H, et al. Microplastics in a Remote Lake Basin of the Tibetan Plateau: impacts of Atmospheric Transport and Glacial Melting. Environ Sci Technol. 2021;55(19):12951–60. [DOI] [PubMed] [Google Scholar]
- 62.Dris R, et al. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ Pollut. 2017;221:453–8. [DOI] [PubMed] [Google Scholar]
- 63.Bianchi MG, et al. Length-dependent toxicity of TiO(2) nanofibers: mitigation via shortening. Nanotoxicology. 2020;14(4):433–52. [DOI] [PubMed] [Google Scholar]
- 64.Lehmann SG, et al. Crumpling of silver nanowires by endolysosomes strongly reduces toxicity. Proc Natl Acad Sci U S A. 2019;116(30):14893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramsperger A, et al. Supposedly identical microplastic particles substantially differ in their material properties influencing particle-cell interactions and cellular responses. J Hazard Mater. 2022;425:127961. [DOI] [PubMed] [Google Scholar]
- 66.Meides N, et al. Reconstructing the environmental degradation of polystyrene by Accelerated Weathering. Environ Sci Technol. 2021;55(12):7930–8. [DOI] [PubMed] [Google Scholar]
- 67.Zarus GM, et al. A review of data for quantifying human exposures to micro and nanoplastics and potential health risks. Sci Total Environ. 2021;756:144010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eberhard T et al. Systematic review of microplastics and nanoplastics in indoor and outdoor air: identifying a framework and data needs for quantifying human inhalation exposures. J Expo Sci Environ Epidemiol, 2024. [DOI] [PMC free article] [PubMed]
- 69.Tesfaldet YT, Ndeh NT. Public face masks wearing during the COVID-19 pandemic: a comprehensive analysis is needed for potential implications. J Hazard Mater Adv. 2022;7:100125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao J et al. Face Mask: as a source or Protector of Human exposure to Microplastics and Phthalate plasticizers? Toxics, 2023. 11(2). [DOI] [PMC free article] [PubMed]
- 71.Liu Z, et al. Generation of environmental persistent free radicals (EPFRs) enhances ecotoxicological effects of the disposable face mask waste with the COVID-19 pandemic. Environ Pollut. 2022;301:119019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang X, Wang WX. Phthalate acid esters contribute to the cytotoxicity of mask leachate: cell-based assay for toxicity assessment. J Hazard Mater. 2023;459:132093. [DOI] [PubMed] [Google Scholar]
- 73.Kubatova H, et al. Toxicity testing of nonwovens used for production of respiratory protective equipment. Cent Eur J Public Health. 2023;31(1):74–80. [DOI] [PubMed] [Google Scholar]
- 74.Lu W, et al. New evidence of Microplastics in the Lower Respiratory Tract: inhalation through smoking. Environ Sci Technol. 2023;57(23):8496–505. [DOI] [PubMed] [Google Scholar]
- 75.Baeza-Martinez C, et al. Tobacco as a Source of Microplastics and Respiratory Health. Arch Bronconeumol. 2022;58(12):845. [DOI] [PubMed] [Google Scholar]
- 76.Jiang Y, et al. Exposure to microplastics in the upper respiratory tract of indoor and outdoor workers. Chemosphere. 2022;307(Pt 3):136067. [DOI] [PubMed] [Google Scholar]
- 77.Baeza-Martinez C, et al. Environmental microplastics in the Lower Airway of Shoe Manufacturing Workers. Open Respir Arch. 2022;4(4):100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baeza-Martinez C, et al. First evidence of microplastics isolated in European citizens’ lower airway. J Hazard Mater. 2022;438:129439. [DOI] [PubMed] [Google Scholar]
- 79.Abbasi S, et al. Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County, Iran. Environ Pollut. 2019;244:153–64. [DOI] [PubMed] [Google Scholar]
- 80.Amato-Lourenco LF, et al. An emerging class of air pollutants: potential effects of microplastics to respiratory human health? Sci Total Environ. 2020;749:141676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li L, et al. Molecular modeling of nanoplastic transformations in alveolar fluid and impacts on the lung surfactant film. J Hazard Mater. 2022;427:127872. [DOI] [PubMed] [Google Scholar]
- 82.Eyles JE, et al. Microsphere translocation and immunopotentiation in systemic tissues following intranasal administration. Vaccine. 2001;19(32):4732–42. [DOI] [PubMed] [Google Scholar]
- 83.Liu YY et al. Endocytosis, distribution, and exocytosis of polystyrene nanoparticles in human lung cells. Nanomaterials (Basel), 2022. 13(1). [DOI] [PMC free article] [PubMed]
- 84.Muchao FP, Filho LV. Advances in inhalation therapy in pediatrics. J Pediatr (Rio J). 2010;86(5):367–76. [DOI] [PubMed] [Google Scholar]
- 85.Zhang J, Wang L, Kannan K. Microplastics in house dust from 12 countries and associated human exposure. Environ Int. 2020;134:105314. [DOI] [PubMed] [Google Scholar]
- 86.Prata JC. Airborne microplastics: consequences to human health? Environ Pollut. 2018;234:115–26. [DOI] [PubMed] [Google Scholar]
- 87.Geiser M, Kreyling WG. Deposition and biokinetics of inhaled nanoparticles. Part Fibre Toxicol. 2010;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Enright HA, et al. Tracking retention and transport of ultrafine polystyrene in an asthmatic mouse model using positron emission tomography. Exp Lung Res. 2013;39(7):304–13. [DOI] [PubMed] [Google Scholar]
- 89.Winiarska E, Jutel M, Zemelka-Wiacek M. The potential impact of nano- and microplastics on human health: understanding human health risks. Environ Res. 2024;251(Pt 2):118535. [DOI] [PubMed] [Google Scholar]
- 90.Lu K, et al. Detrimental effects of microplastic exposure on normal and asthmatic pulmonary physiology. J Hazard Mater. 2021;416:126069. [DOI] [PubMed] [Google Scholar]
- 91.Sarlo K, et al. Tissue distribution of 20 nm, 100 nm and 1000 nm fluorescent polystyrene latex nanospheres following acute systemic or acute and repeat airway exposure in the rat. Toxicology. 2009;263(2–3):117–26. [DOI] [PubMed] [Google Scholar]
- 92.Han Y, et al. Correction to: no prominent toxicity of polyethylene microplastics observed in neonatal mice following intratracheal instillation to dams during gestational and neonatal period. Toxicol Res. 2022;38(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fournier SB, et al. Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy. Part Fibre Toxicol. 2020;17(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang S et al. Microplastics in the Lung tissues Associated with Blood Test Index. Toxics, 2023. 11(9). [DOI] [PMC free article] [PubMed]
- 95.Li C, et al. Electrostatic attraction of cationic pollutants by microplastics reduces their joint cytotoxicity. Chemosphere. 2021;282:131121. [DOI] [PubMed] [Google Scholar]
- 96.Banerjee A, Shelver WL. Micro- and nanoplastic induced cellular toxicity in mammals: a review. Sci Total Environ. 2021;755(Pt 2):142518. [DOI] [PubMed] [Google Scholar]
- 97.Qiao R, et al. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere. 2019;236:124334. [DOI] [PubMed] [Google Scholar]
- 98.El Hayek E, et al. Photoaging of polystyrene microspheres causes oxidative alterations to surface physicochemistry and enhances airway epithelial toxicity. Toxicol Sci. 2023;193(1):90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu K, et al. Enhanced cytotoxicity of photoaged phenol-formaldehyde resins microplastics: combined effects of environmentally persistent free radicals, reactive oxygen species, and conjugated carbonyls. Environ Int. 2020;145:106137. [DOI] [PubMed] [Google Scholar]
- 100.Chen C, et al. Microplastics in the Bronchoalveolar Lavage Fluid of Chinese children: associations with Age, City Development, and Disease features. Environ Sci Technol. 2023;57(34):12594–601. [DOI] [PubMed] [Google Scholar]
- 101.Huang S, et al. Detection and analysis of Microplastics in Human Sputum. Environ Sci Technol. 2022;56(4):2476–86. [DOI] [PubMed] [Google Scholar]
- 102.Yang S, et al. Inhalation exposure to polystyrene nanoplastics induces chronic obstructive pulmonary disease-like lung injury in mice through multi-dimensional assessment. Environ Pollut. 2024;347:123633. [DOI] [PubMed] [Google Scholar]
- 103.Dong CD, et al. Polystyrene microplastic particles: in vitro pulmonary toxicity assessment. J Hazard Mater. 2020;385:121575. [DOI] [PubMed] [Google Scholar]
- 104.Jin YJ, et al. Characterisation of changes in global genes expression in the lung of ICR mice in response to the inflammation and fibrosis induced by polystyrene nanoplastics inhalation. Toxicol Res. 2023;39(4):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li Y, et al. Inhaled tire-wear microplastic particles induced pulmonary fibrotic injury via epithelial cytoskeleton rearrangement. Environ Int. 2022;164:107257. [DOI] [PubMed] [Google Scholar]
- 106.Halimu G, et al. Toxic effects of nanoplastics with different sizes and surface charges on epithelial-to-mesenchymal transition in A549 cells and the potential toxicological mechanism. J Hazard Mater. 2022;430:128485. [DOI] [PubMed] [Google Scholar]
- 107.Li X, et al. Intratracheal administration of polystyrene microplastics induces pulmonary fibrosis by activating oxidative stress and Wnt/beta-catenin signaling pathway in mice. Ecotoxicol Environ Saf. 2022;232:113238. [DOI] [PubMed] [Google Scholar]
- 108.Chen QQ et al. An emerging role of microplastics in the etiology of lung ground glass nodules. Environ Sci Europe, 2022. 34(1).
- 109.Pambianchi E, Pecorelli A, Valacchi G. Gastrointestinal tissue as a new target of pollution exposure. IUBMB Life. 2022;74(1):62–73. [DOI] [PubMed] [Google Scholar]
- 110.Kreutz J, et al. Environmental factors and their impact on the COVID-19 pandemic. Herz. 2023;48(3):234–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang C, et al. Polystyrene microplastics significantly facilitate influenza a virus infection of host cells. J Hazard Mater. 2023;446:130617. [DOI] [PubMed] [Google Scholar]
- 112.Zhang F, et al. Theoretical investigation on the interactions of microplastics with a SARS-CoV-2 RNA fragment and their potential impacts on viral transport and exposure. Sci Total Environ. 2022;842:156812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kumar R, et al. Micro(nano)plastics pollution and human health: how plastics can induce carcinogenesis to humans? Chemosphere. 2022;298:134267. [DOI] [PubMed] [Google Scholar]
- 114.Edaes FS, de Souza CB. BPS and BPF are as carcinogenic as BPA and are not viable Alternatives for its replacement. Endocr Metab Immune Disord Drug Targets. 2022;22(9):927–34. [DOI] [PubMed] [Google Scholar]
- 115.Jin W, et al. Microplastics exposure causes the senescence of human lung epithelial cells and mouse lungs by inducing ROS signaling. Environ Int. 2024;185:108489. [DOI] [PubMed] [Google Scholar]
- 116.Zhao Y, et al. Real-time quantification of nanoplastics-Induced oxidative stress in stretching alveolar cells. ACS Nano. 2024;18(8):6176–85. [DOI] [PubMed] [Google Scholar]
- 117.Felix L, Carreira P, Peixoto F. Effects of chronic exposure of naturally weathered microplastics on oxidative stress level, behaviour, and mitochondrial function of adult zebrafish (Danio rerio). Chemosphere. 2023;310:136895. [DOI] [PubMed] [Google Scholar]
- 118.Zhang H, et al. Pulmonary toxicology assessment of polyethylene terephthalate nanoplastic particles in vitro. Environ Int. 2022;162:107177. [DOI] [PubMed] [Google Scholar]
- 119.Lim SL, et al. Targeted metabolomics reveals differential biological effects of nanoplastics and nanoZnO in human lung cells. Nanotoxicology. 2019;13(8):1117–32. [DOI] [PubMed] [Google Scholar]
- 120.Zhang T et al. Polystyrene nanoplastics induce Lung Injury via activating oxidative stress: Molecular insights from Bioinformatics Analysis. Nanomaterials (Basel), 2022. 12(19). [DOI] [PMC free article] [PubMed]
- 121.Yang S, et al. Ferritinophagy mediated by oxidative stress-driven mitochondrial damage is involved in the Polystyrene nanoparticles-Induced ferroptosis of Lung Injury. ACS Nano. 2023;17(24):24988–5004. [DOI] [PubMed] [Google Scholar]
- 122.K CP, et al. Polytetrafluorethylene microplastic particles mediated oxidative stress, inflammation, and intracellular signaling pathway alteration in human derived cell lines. Sci Total Environ. 2023;897:165295. [DOI] [PubMed] [Google Scholar]
- 123.Baysal A, et al. Exposure to phagolysosomal simulated fluid altered the cytotoxicity of PET micro(nano)plastics to human lung epithelial cells. Toxicol Mech Methods. 2024;34(1):72–97. [DOI] [PubMed] [Google Scholar]
- 124.Yang S, et al. In vitro evaluation of nanoplastics using human lung epithelial cells, microarray analysis and co-culture model. Ecotoxicol Environ Saf. 2021;226:112837. [DOI] [PubMed] [Google Scholar]
- 125.Prado Y, et al. Small plastics, big inflammatory problems. Adv Exp Med Biol. 2023;1408:101–27. [DOI] [PubMed] [Google Scholar]
- 126.Bengalli R, et al. Characterization of microparticles derived from waste plastics and their bio-interaction with human lung A549 cells. J Appl Toxicol. 2022;42(12):2030–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee S et al. Toxicity study and Quantitative Evaluation of Polyethylene Microplastics in ICR mice. Polym (Basel), 2022. 14(3). [DOI] [PMC free article] [PubMed]
- 128.Shi Q, et al. Combined cytotoxicity of polystyrene nanoplastics and phthalate esters on human lung epithelial A549 cells and its mechanism. Ecotoxicol Environ Saf. 2021;213:112041. [DOI] [PubMed] [Google Scholar]
- 129.Jeon MS, et al. Polystyrene microplastic particles induce autophagic cell death in BEAS-2B human bronchial epithelial cells. Environ Toxicol. 2023;38(2):359–67. [DOI] [PubMed] [Google Scholar]
- 130.Danso IK, Woo JH, Lee K. Pulmonary toxicity of polystyrene, polypropylene, and polyvinyl chloride microplastics in mice. Molecules, 2022. 27(22). [DOI] [PMC free article] [PubMed]
- 131.Lim D, et al. Inhalation toxicity of polystyrene micro(nano)plastics using modified OECD TG 412. Chemosphere. 2021;262:128330. [DOI] [PubMed] [Google Scholar]
- 132.Xu M, et al. Internalization and toxicity: a preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci Total Environ. 2019;694:133794. [DOI] [PubMed] [Google Scholar]
- 133.Kwabena Danso I, et al. Pulmonary toxicity assessment of polypropylene, polystyrene, and polyethylene microplastic fragments in mice. Toxicol Res. 2024;40(2):313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen X, et al. Endoplasmic reticulum stress: molecular mechanism and therapeutic targets. Signal Transduct Target Ther. 2023;8(1):352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lu H, et al. Endoplasmic reticulum stress-induced NLRP3 inflammasome activation as a novel mechanism of polystyrene microplastics (PS-MPs)-induced pulmonary inflammation in chickens. J Zhejiang Univ Sci B. 2024;25(3):233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alizadeh J, et al. Ceramides and ceramide synthases in cancer: focus on apoptosis and autophagy. Eur J Cell Biol. 2023;102(3):151337. [DOI] [PubMed] [Google Scholar]
- 137.Lu H, et al. Polystyrene microplastics induce autophagy and apoptosis in birds lungs via PTEN/PI3K/AKT/mTOR. Environ Toxicol. 2023;38(1):78–89. [DOI] [PubMed] [Google Scholar]
- 138.Ji Y, et al. Revisiting the cellular toxicity of benzo[a]pyrene from the view of nanoclusters: size- and nanoplastic adsorption-dependent bioavailability. Nanoscale. 2021;13(2):1016–28. [DOI] [PubMed] [Google Scholar]
- 139.Annangi B, et al. Effects of true-to-life PET nanoplastics using primary human nasal epithelial cells. Environ Toxicol Pharmacol. 2023;100:104140. [DOI] [PubMed] [Google Scholar]
- 140.Sipos A et al. Kinetics of autophagic activity in nanoparticle-exposed lung adenocarcinoma (A549) cells. Autophagy Rep, 2023. 2(1). [DOI] [PMC free article] [PubMed]
- 141.Chen GL et al. Mechanosensitive channels TMEM63A and TMEM63B mediate lung inflation-induced surfactant secretion. J Clin Invest, 2024. 134(5). [DOI] [PMC free article] [PubMed]
- 142.Shi W, et al. Potential health risks of the interaction of microplastics and lung surfactant. J Hazard Mater. 2022;429:128109. [DOI] [PubMed] [Google Scholar]
- 143.Cao Y, et al. Interactions between inhalable aged microplastics and lung surfactant: potential pulmonary health risks. Environ Res. 2024;245:117803. [DOI] [PubMed] [Google Scholar]
- 144.Xu X, et al. Microplastics and nanoplastics impair the Biophysical function of pulmonary surfactant by forming heteroaggregates at the alveolar-capillary interface. Environ Sci Technol. 2023;57(50):21050–60. [DOI] [PubMed] [Google Scholar]
- 145.Zha H, et al. Airborne polystyrene microplastics and nanoplastics induce nasal and lung microbial dysbiosis in mice. Chemosphere. 2023;310:136764. [DOI] [PubMed] [Google Scholar]
- 146.Zhang X, et al. Effect of microplastics on nasal and gut microbiota of high-exposure population: protocol for an observational cross-sectional study. Med (Baltim). 2022;101(34):e30215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Goodman KE, et al. Exposure of human lung cells to Polystyrene Microplastics significantly retards cell proliferation and triggers morphological changes. Chem Res Toxicol. 2021;34(4):1069–81. [DOI] [PubMed] [Google Scholar]
- 148.Luo H, et al. The regulation of circRNA_kif26b on alveolar epithelial cell senescence via mir-346-3p is involved in microplastics-induced lung injuries. Sci Total Environ. 2023;882:163512. [DOI] [PubMed] [Google Scholar]
- 149.Xu D, et al. Systematic toxicity evaluation of polystyrene nanoplastics on mice and molecular mechanism investigation about their internalization into Caco-2 cells. J Hazard Mater. 2021;417:126092. [DOI] [PubMed] [Google Scholar]
- 150.Florance I, et al. Exposure to polystyrene nanoplastics impairs lipid metabolism in human and murine macrophages in vitro. Ecotoxicol Environ Saf. 2022;238:113612. [DOI] [PubMed] [Google Scholar]
- 151.Xuan L, et al. Metabolomics reveals that PS-NPs promote lung injury by regulating prostaglandin B1 through the cGAS-STING pathway. Chemosphere. 2023;342:140108. [DOI] [PubMed] [Google Scholar]
- 152.Wu Y, et al. Polystyrenenanoplastics lead to ferroptosis in the lungs. J Adv Res. 2024;56:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Alzaben M, et al. Nanoplastics from ground polyethylene terephthalate food containers: genotoxicity in human lung epithelial A549 cells. Mutat Res Genet Toxicol Environ Mutagen. 2023;892:503705. [DOI] [PubMed] [Google Scholar]
- 154.Sipos A et al. Evidence for Nanoparticle-Induced lysosomal dysfunction in lung adenocarcinoma (A549) cells. Int J Mol Sci, 2019. 20(21). [DOI] [PMC free article] [PubMed]
- 155.Borm PJA. The parallels between particle induced lung overload and particle induced periprosthetic osteolysis (PPOL). Open Res Eur. 2021;1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Boraschi D, et al. Editorial: exploring impacts of combined exposures to particles and chemicals on immune reactions across living organisms. Front Toxicol. 2023;5:1148374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Song S, et al. Inhalable Textile Microplastic fibers impair Airway Epithelial differentiation. Am J Respir Crit Care Med. 2024;209(4):427–43. [DOI] [PubMed] [Google Scholar]
- 158.Liu J, et al. Exposure to microplastics reduces the bioaccumulation of sulfamethoxazole but enhances its effects on gut microbiota and the antibiotic resistome of mice. Chemosphere. 2022;294:133810. [DOI] [PubMed] [Google Scholar]
- 159.Yacobi NR, et al. Nanoparticle effects on rat alveolar epithelial cell monolayer barrier properties. Toxicol Vitro. 2007;21(8):1373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.