Abstract
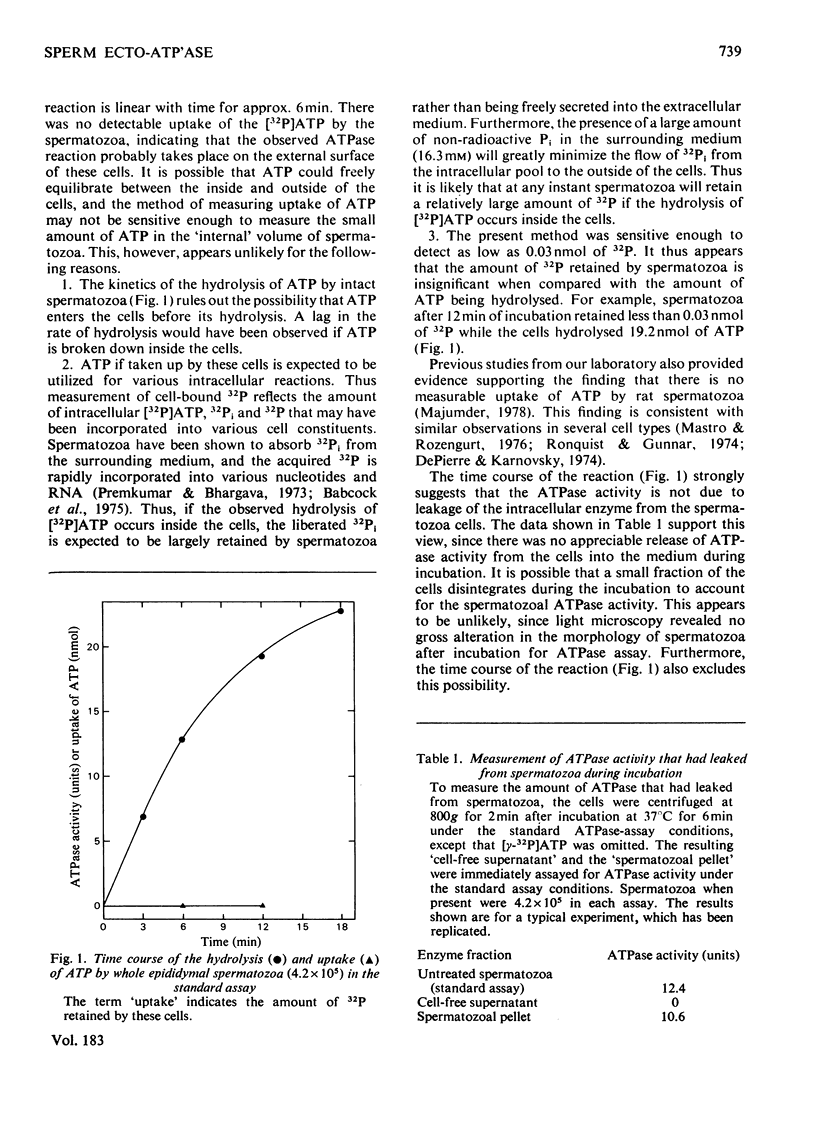
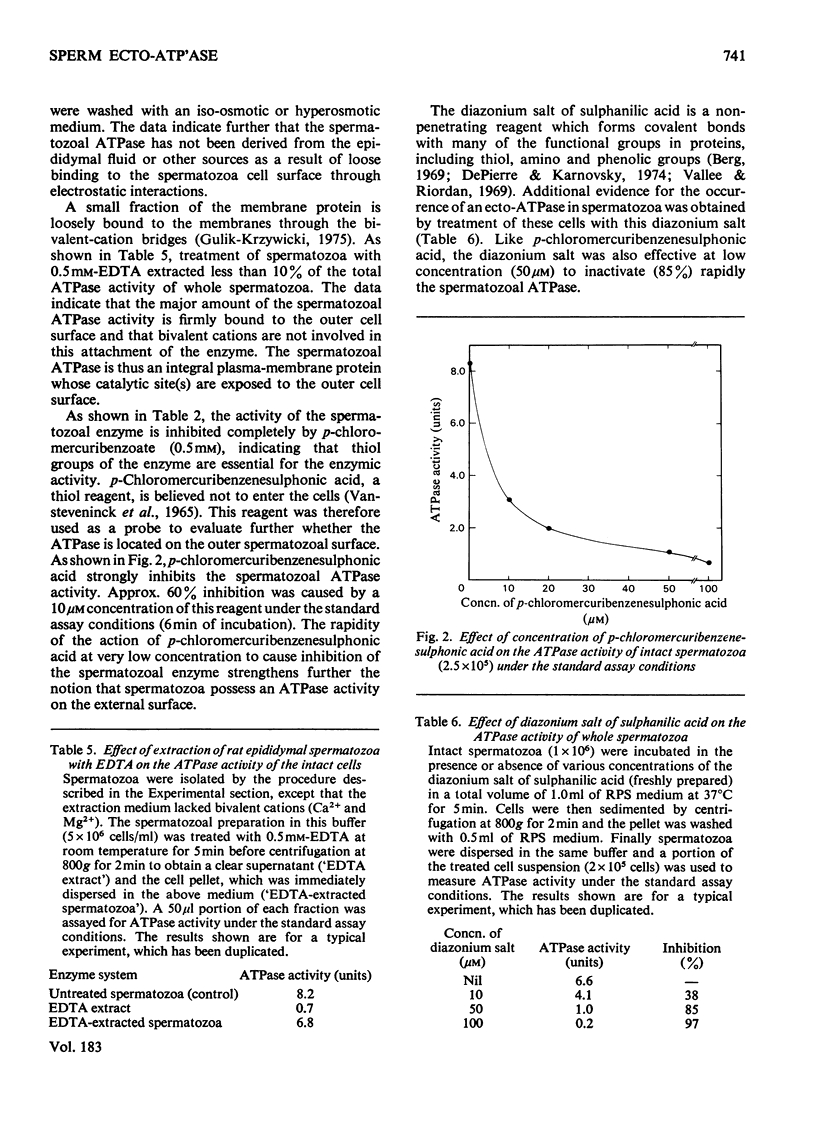
Intact spermatozoa from rat cauda epididymis possess a Mg2+-dependent ATPase activity that hydrolyses externally added [gamma-32P]ATP. The ATPase reaction was linear with time for approx. 6 min and there was no detectable uptake of ATP by these cells. The ATPase activity of the whole spermatozoa was not due to leakage of the intracellular enzymic activity, contamination of the broken cells or any possible cell damage during incubation and isolation of spermatozoa. The activity of the enzyme was strongly inhibited (approx. 85%) by p-chloromercuribenzenesulphonic acid (50 microM) or the diazonium salt of sulphanilic acid (50 microM), which are believed not to enter the cells, whereas ouabain (0.5 mM), NaF (10 mM), NaN3 (2.5 mM) and oligomycin (5 microM) had no appreciable effect on the activity of the spermatozoal APTase. There was little loss of ATPase activity from the cells when washed with 0.5 mM-EDTA and an iso-osmotic or hyperosmotic medium. These data are consistent with the view that the observed ATPase activity is located on the external surface of spermatozoa. The sperm ecto-ATPase activity is resistant to the action of proteinases (50 micrograms/ml), namely trypsin, chymotrypsin and Pronase. Studies with various unlabelled phosphate esters indicate that the sperm ecto-ATPase is not a non-specific phosphatase and it has high degree of substrate specificity for ATP.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babcock D. F., First N. L., Lardy H. A. Transport mechanism for succinate and phosphate localized in the plasma membrane of bovine spermatozoa. J Biol Chem. 1975 Aug 25;250(16):6488–6495. [PubMed] [Google Scholar]
- Berg H. C. Sulfanilic acid diazonium salt: a label for the outside of the human erythrocyte membrane. Biochim Biophys Acta. 1969 Jun 3;183(1):65–78. doi: 10.1016/0005-2736(69)90130-8. [DOI] [PubMed] [Google Scholar]
- CRANE R. K., LIPMANN F. The effect of arsenate on aerobic phosphorylation. J Biol Chem. 1953 Mar;201(1):235–243. [PubMed] [Google Scholar]
- Chulavatnatol M., Yindepit S. Changes in surface ATPase of rat spermatozoa in transit from the caput to the cauda epididymidis. J Reprod Fertil. 1976 Sep;48(1):91–97. doi: 10.1530/jrf.0.0480091. [DOI] [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Ecto-enzymes of the guinea pig polymorphonuclear leukocyte. I. Evidence for an ecto-adenosine monophosphatase, adenosine triphosphatase, and -p-nitrophenyl phosphates. J Biol Chem. 1974 Nov 25;249(22):7111–7120. [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Plasma membranes of mammalian cells: a review of methods for their characterization and isolation. J Cell Biol. 1973 Feb;56(2):275–303. doi: 10.1083/jcb.56.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durr I. F., Abla A., Mroueh A. The hydrolysis of adenosine triphosphate by human spermatozoa. J Reprod Fertil. 1972 Nov;31(2):313–316. doi: 10.1530/jrf.0.0310313. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulik-Krzywicki T. Structural studies of the associations between biological membrane components. Biochim Biophys Acta. 1975 Mar 25;415(1):1–28. doi: 10.1016/0304-4157(75)90015-5. [DOI] [PubMed] [Google Scholar]
- Hudgins P. M., Bond G. H. (Mg2+ + K+)-dependent inhibition of NaK-ATPase due to a contaminant in equine muscle ATP. Biochem Biophys Res Commun. 1977 Aug 8;77(3):1024–1029. doi: 10.1016/s0006-291x(77)80080-6. [DOI] [PubMed] [Google Scholar]
- LARDY H. A., JOHNSON D., McMURRAY W. C. Antibiotics as tools for metabolic studies. I. A survey of toxic antibiotics in respiratory, phosphorylative and glycolytic systems. Arch Biochem Biophys. 1958 Dec;78(2):587–597. doi: 10.1016/0003-9861(58)90383-7. [DOI] [PubMed] [Google Scholar]
- Lindemann C. B., Gibbons I. R. Adenosine triphosphate-induced motility and sliding of filaments in mammalian sperm extracted with Triton X-100. J Cell Biol. 1975 Apr;65(1):147–162. doi: 10.1083/jcb.65.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder G. C. Occurrence of a cyclic AMP-dependent protein kinase on the outer surface of rat epididymal spermatozoa. Biochem Biophys Res Commun. 1978 Aug 14;83(3):829–836. doi: 10.1016/0006-291x(78)91469-9. [DOI] [PubMed] [Google Scholar]
- Majumder G. C., Turkington R. W. Hormone-dependent phosphorylation of ribosomal and plasma membrane proteins in mouse mammary gland in vitro. J Biol Chem. 1972 Nov 25;247(22):7207–7217. [PubMed] [Google Scholar]
- Mastro A. M., Rozengurt E. Endgoenous protein kinase in outer plasma membrane of cultured 3T3 cells. Nature of the membrane-bound substrate and effect of cell density, serum addition, and oncogenic transformation. J Biol Chem. 1976 Dec 25;251(24):7899–7906. [PubMed] [Google Scholar]
- Mustafa M. G., Ibrahim A. B., Le C. T., Cross C. E. Pulmonary alveolar macrophage: demonstration of Na+ - K+, Mg++ ATPase activity. Life Sci. 1969 Dec 15;8(24):1343–1351. doi: 10.1016/0024-3205(69)90191-x. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Gibbons I. R. Dynein 2. A new adenosine triphosphatase from sea urchin sperm flagella. J Biol Chem. 1976 Sep 25;251(18):5793–5801. [PubMed] [Google Scholar]
- Ogawa K., Mori H. Preparation of antiserum against a tryptic fragment (fragment A) of dynein and an immunological approach to the subunit composition of dynein. J Biol Chem. 1975 Aug 25;250(16):6476–6483. [PubMed] [Google Scholar]
- PULLMAN M. E., PENEFSKY H. S., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem. 1960 Nov;235:3322–3329. [PubMed] [Google Scholar]
- Premkumar E., Bhargava P. M. Isolation and characterization of newly synthesized RNA and protein in mature bovine spermatozoa and effect of inhibitors on these syntheses. Indian J Biochem Biophys. 1973 Dec;10(4):239–253. [PubMed] [Google Scholar]
- Quinn P. J., White I. G. Distribution of adenosinetriphosphatase activity in ram and bull spermatozoa. J Reprod Fertil. 1968 Apr;15(3):449–452. doi: 10.1530/jrf.0.0150449. [DOI] [PubMed] [Google Scholar]
- Ronquist G., Agren G. Isolation of 32P-labeled phosphorylserine and phosphorylthreonine from Ehrlich mouse ascites tumor cells suspended in different isotonic media containing 32P-labeled adenosine triphosphate. Acta Chem Scand B. 1974;28(10):1169–1174. doi: 10.3891/acta.chem.scand.28b-1169. [DOI] [PubMed] [Google Scholar]
- Ronquist G. Formation of extracellular adenosine triphosphate by human erythrocytes. Acta Physiol Scand. 1968 Dec;74(4):594–605. doi: 10.1111/j.1748-1716.1968.tb04270.x. [DOI] [PubMed] [Google Scholar]
- Smolen J. E., Weissmann G. Mg2+-ATPase as a membrane ecto-enzyme of human granulocytes. Inhibitors, activators and response to phagocytosis. Biochim Biophys Acta. 1978 Oct 4;512(3):525–538. doi: 10.1016/0005-2736(78)90162-1. [DOI] [PubMed] [Google Scholar]
- Uesugi S., Yamazoe S. Presence of sodium-potassium-stimulated ATPase in boar epididymal spermatozoon. Nature. 1966 Jan 22;209(5021):403–403. doi: 10.1038/209403a0. [DOI] [PubMed] [Google Scholar]
- VANSTEVENINCK J., WEED R. I., ROTHSTEIN A. LOCALIZATION OF ERYTHROCYTE MEMBRANE SULFHYDRYL GROUPS ESSENTIAL FOR GLUCOSE TRANSPORT. J Gen Physiol. 1965 Mar;48:617–632. doi: 10.1085/jgp.48.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Riordan J. F. Chemical approaches to the properties of active sites of enzymes. Annu Rev Biochem. 1969;38:733–794. doi: 10.1146/annurev.bi.38.070169.003505. [DOI] [PubMed] [Google Scholar]
- Voglmayr J. K., Quinn P. J., White I. G. Characteristics of adenosinetriphosphatase of ram ejaculated spermatozoa and isolated sperm tails. Biol Reprod. 1969 Sep;1(3):215–222. doi: 10.1095/biolreprod1.3.215. [DOI] [PubMed] [Google Scholar]
- Wyker R., Howards S. S. Micropuncture studies of the motility of rete testis and epididymal spermatozoa. Fertil Steril. 1977 Jan;28(1):108–112. [PubMed] [Google Scholar]
- Young L. G., Smithwick E. B. Effects of mechanical and chemical disruption on the ATP-phosphohydrolase activity and ultrastructure of sperm flagella. Exp Cell Res. 1976 Oct 1;102(1):179–190. doi: 10.1016/0014-4827(76)90313-x. [DOI] [PubMed] [Google Scholar]


