Abstract
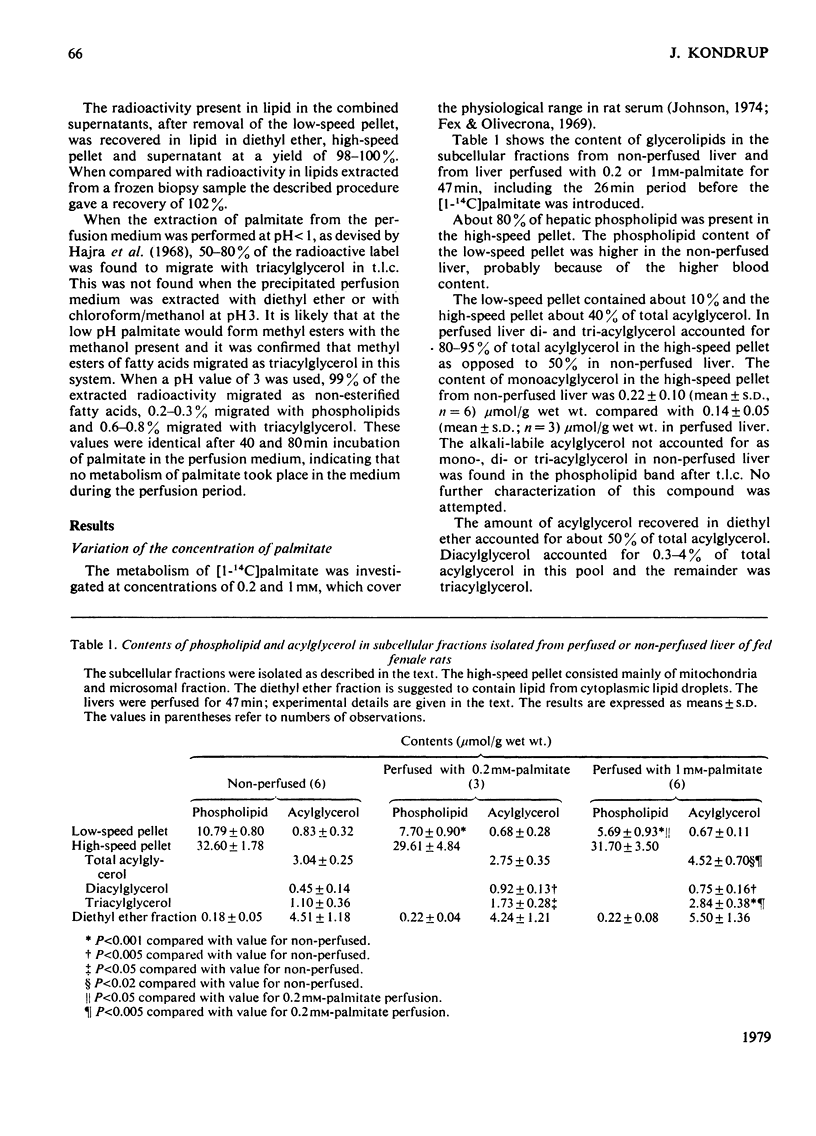
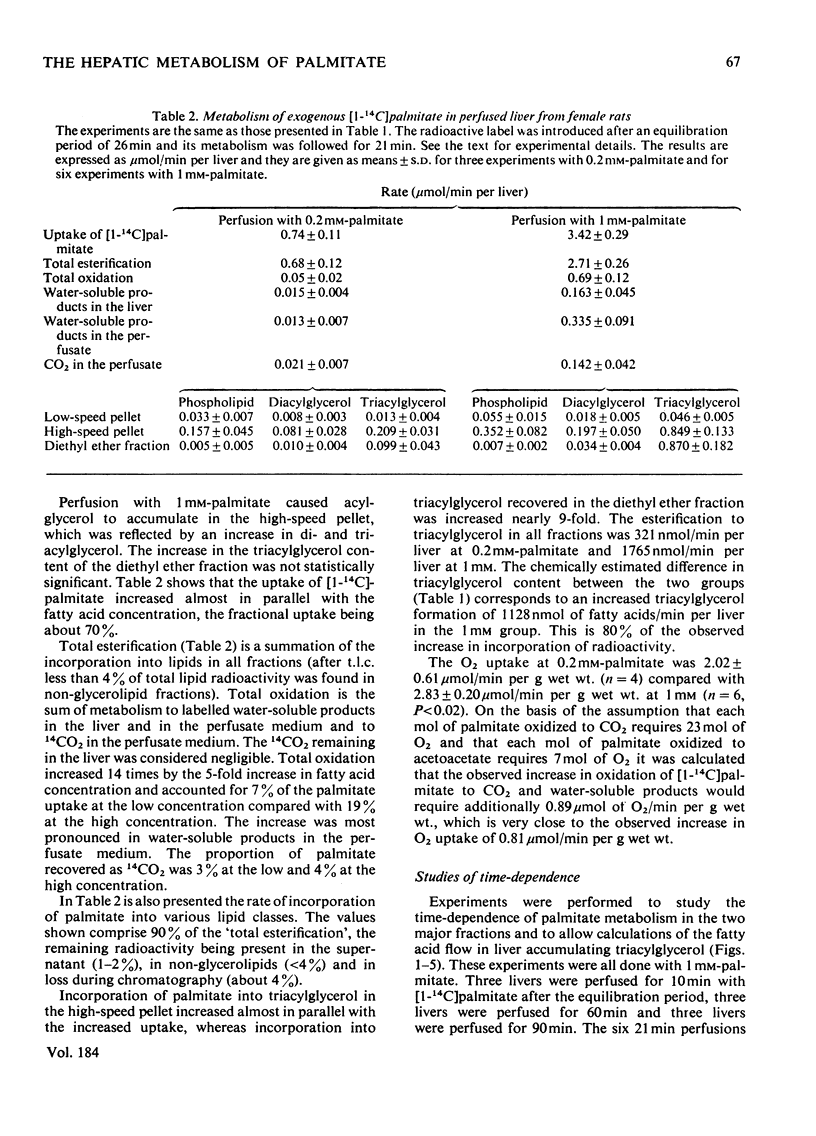
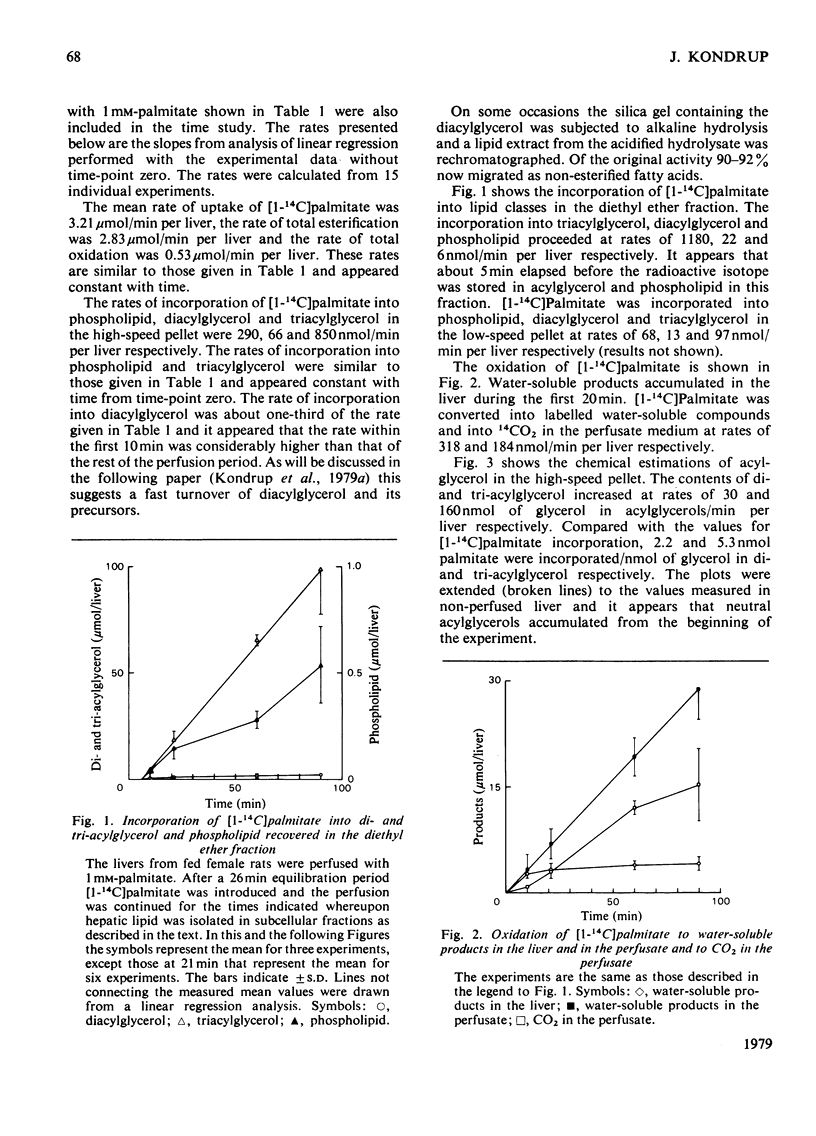
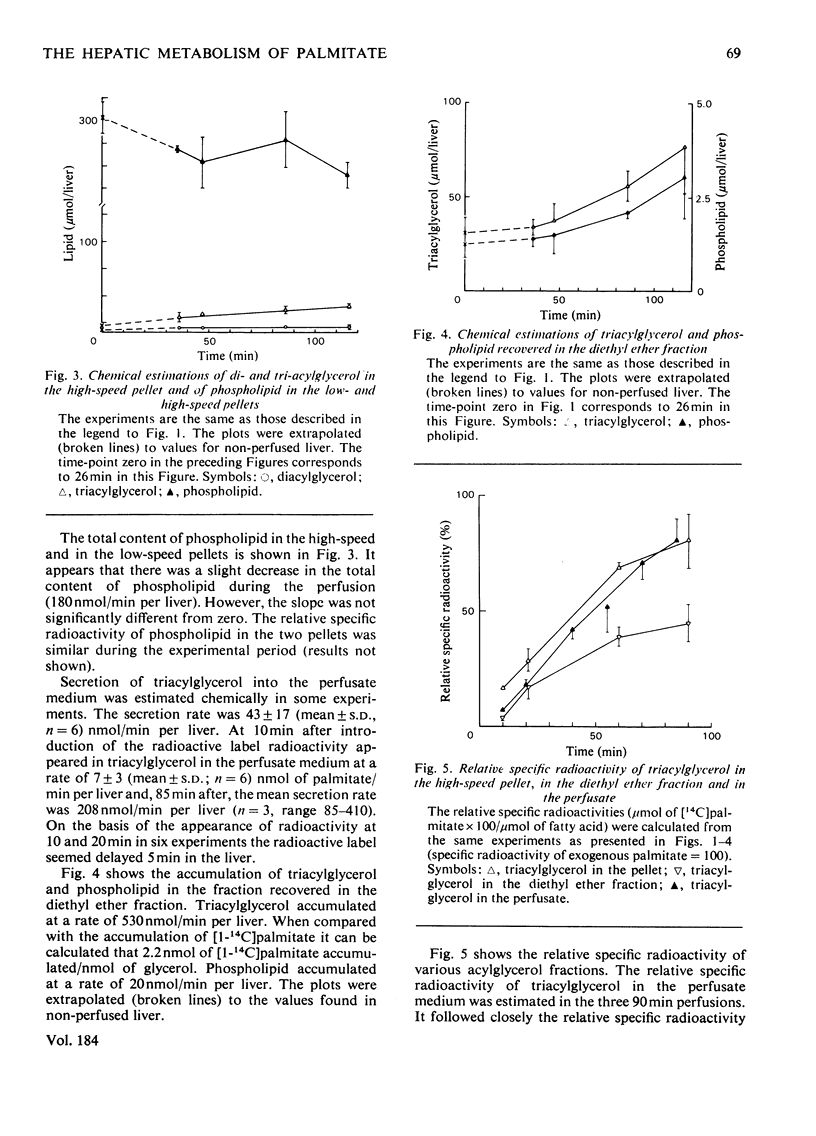
1. The metabolism of [1-14C]palmitate in rat liver was studied in a single-pass perfusion system at concentrations of 0.2 or 1 mM. 2. After the perfusion the liver was homogenized and the floating fat was isolated. The incorporation of [1-14C]palmitate into triacylglycerol in this pool increased 9-fold when the palmitate concentration in the medium was increased from 0.2 to 1 mM. In time studies with 1 mM-[1-14C]palmitate 75% of the total accumulation of triacylglycerol occurred in this pool. Our results support the concept that the floating-fat fraction contains the storage pool of triacylglycerol, i.e. the cytoplasmic lipid droplets. 3. In a particulate preparation consisting mainly of mitochondria and microsomal fraction the incorporation of [1-14C]palmitate into triacylglycerol was proportional to the fatty acid concentration. Triacylglycerol in the perfusate medium and in the particulate fraction was in isotopic equilibrium, which indicates that the particulate fraction contained the precursor pool for secreted triacylglycerol, i.e. the pool in endoplasmic reticulum and Golgi apparatus. 4. The oxidation to labelled water-soluble products and to CO2 was increased 14-fold by the 5-fold increase in palmitate concentration.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., McClure W. O. An improved scintillation cocktail of high-solubilizing power. Anal Biochem. 1973 Jan;51(1):173–179. doi: 10.1016/0003-2697(73)90465-x. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Breckenridge W. C., Kuksis A. Structure of bovine milk fat triglycerides : I. Short and medium chain lengths. Lipids. 1968 Jul;3(4):291–300. doi: 10.1007/BF02530927. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- DiAugustine R. P., Schaefer J. M., Fouts J. R. Hepatic lipid droplets. Isolation, morphology and composition. Biochem J. 1973 Feb;132(2):323–327. doi: 10.1042/bj1320323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fex G., Olivecrona T. Role of uptake and oxidation of plasma free fatty acids by the liver in the development of the ethanol-induced fatty liver. Acta Physiol Scand. 1969 Jan-Feb;75(1):78–81. doi: 10.1111/j.1748-1716.1969.tb04358.x. [DOI] [PubMed] [Google Scholar]
- Glaumann H., Bergstrand A., Ericsson J. L. Studies on the synthesis and intracellular transport of lipoprotein particles in rat liver. J Cell Biol. 1975 Feb;64(2):356–377. doi: 10.1083/jcb.64.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajra A. K., Seguin E. B., Agranoff B. W. Rapid labeling of mitochondrial lipids by labeled orthophosphate and adenosine triphosphate. J Biol Chem. 1968 Apr 10;243(7):1609–1616. [PubMed] [Google Scholar]
- Haude W., Wagner H., Theil S., Haase H., Hünicke G., Goetze E. Bestimmung der Umsätze und Flussraten von Fettsäuren und Cholesterin in Serum und Leber der Ratte mit Hilfe der mathematischen Simulierung von Isotopenverdünnungskurven am Analogrechner. Acta Biol Med Ger. 1972;28(6):963–975. [PubMed] [Google Scholar]
- Heimberg M., Weinstein I., Kohout M. The effects of glucagon, dibutyryl cyclic adenosine 3',5'-monophosphate, and concentration of free fatty acid on hepatic lipid metabolism. J Biol Chem. 1969 Oct 10;244(19):5131–5139. [PubMed] [Google Scholar]
- Johnson O. Effect of ethanol on liver triglyceride concentration in fed and fasted rats. Lipids. 1974 Jan;9(1):57–60. doi: 10.1007/BF02533215. [DOI] [PubMed] [Google Scholar]
- Kondrup J., Damgaard S. E., Fleron P. Metabolism of palmitate in perfused rat liver. Computer models of subcellular triacylglycerol metabolism. Biochem J. 1979 Oct 15;184(1):73–81. doi: 10.1042/bj1840073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondrup J., Lundquist F., Damgaard S. E. Metabolism of palmitate in perfused rat liver. Effect of ethanol in livers from rats fed on a high-fat diet with or without ethanol. Biochem J. 1979 Oct 15;184(1):89–95. doi: 10.1042/bj1840089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkilä E. A., Kekki M., Ojala K. Kinetic analysis of triglyceride metabolism in single rats. Ann Med Exp Biol Fenn. 1966;44(2):348–355. [PubMed] [Google Scholar]
- STEIN Y., SHAPIRO B. Assimilation and dissimilation of fatty acids by the rat liver. Am J Physiol. 1959 Jun;196(6):1238–1241. doi: 10.1152/ajplegacy.1959.196.6.1238. [DOI] [PubMed] [Google Scholar]
- Schlunk F. F., Lombardi B. Liver liposomes. I. Isolation and chemical characterization. Lab Invest. 1967 Jul;17(1):30–38. [PubMed] [Google Scholar]
- Sestoft L. Regulation of fructose metabolism in the perfused rat liver. Interrelation with inorganic phosphate, glucose, ketone body and ethanol metabolism. Biochim Biophys Acta. 1974 Mar 20;343(1):1–16. doi: 10.1016/0304-4165(74)90235-9. [DOI] [PubMed] [Google Scholar]


