Abstract
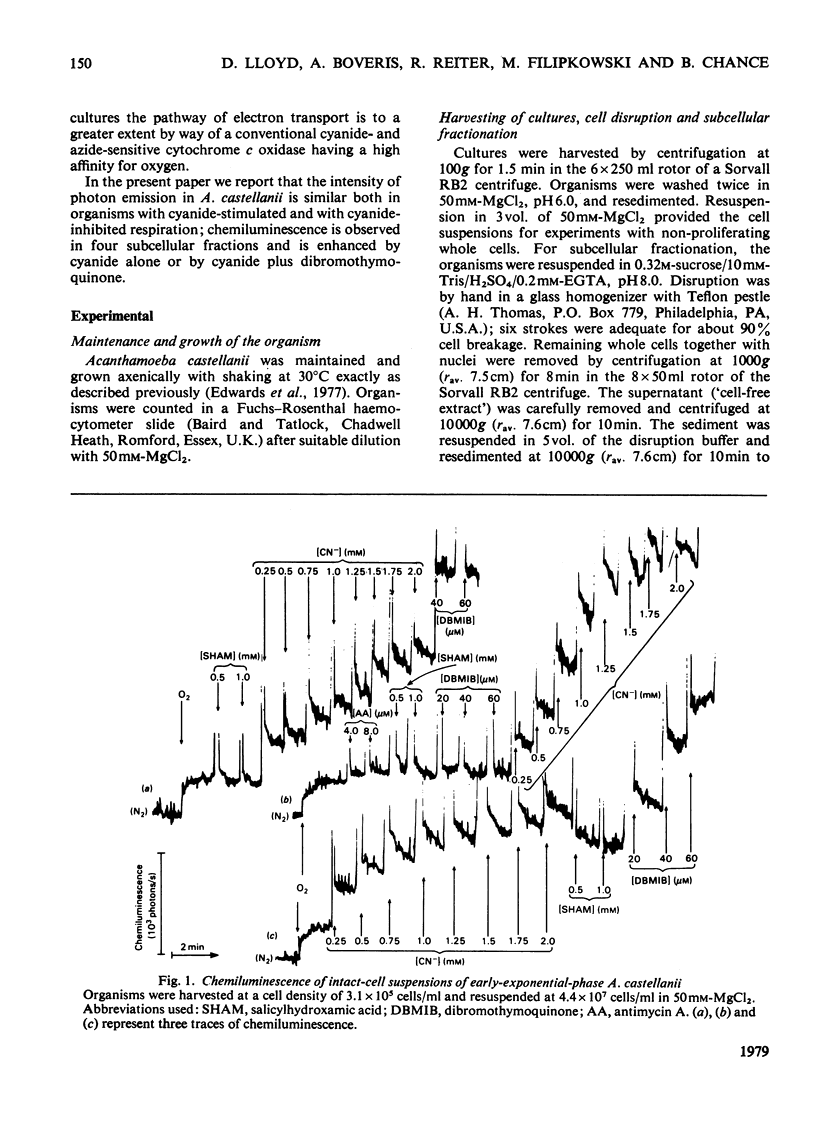
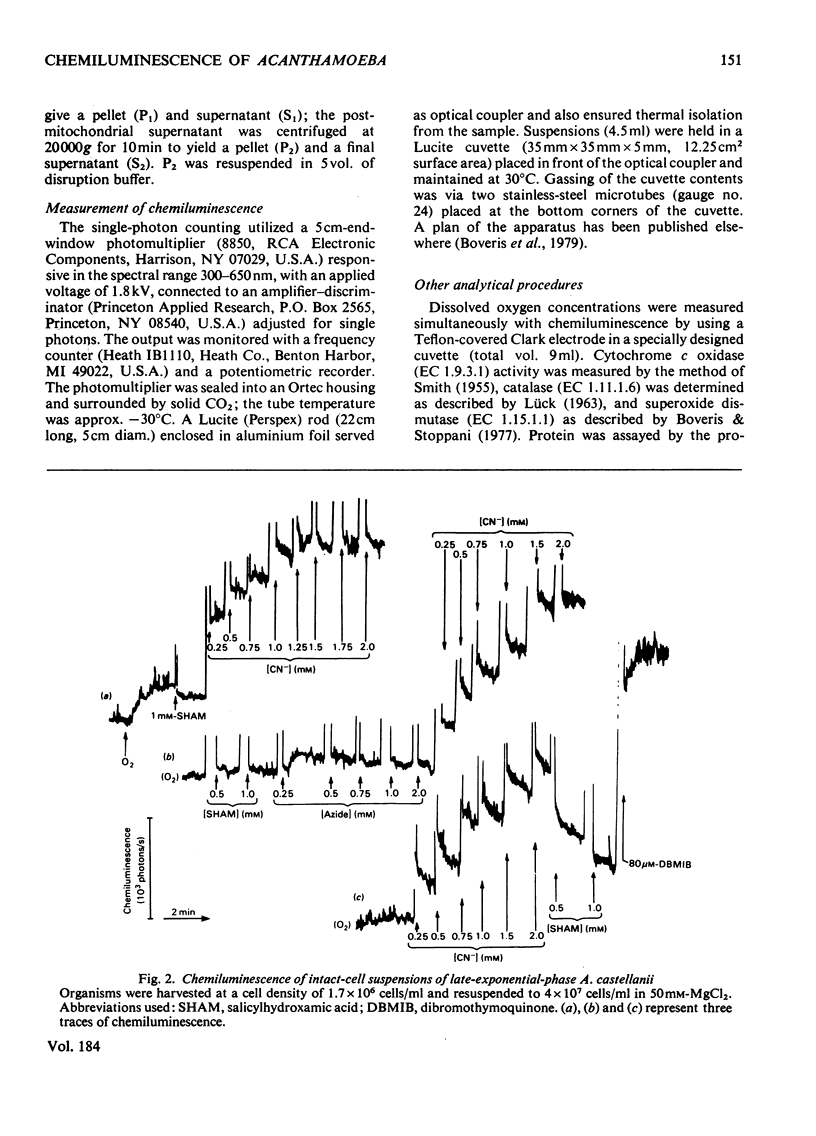
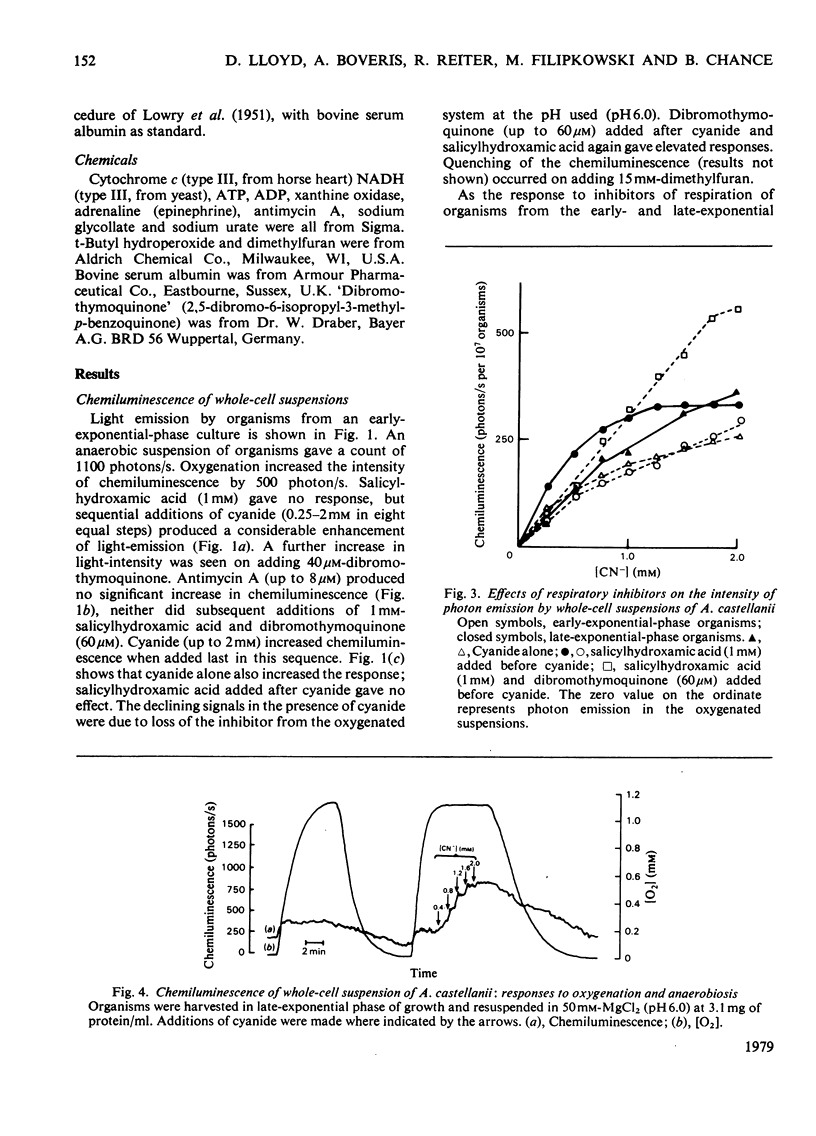
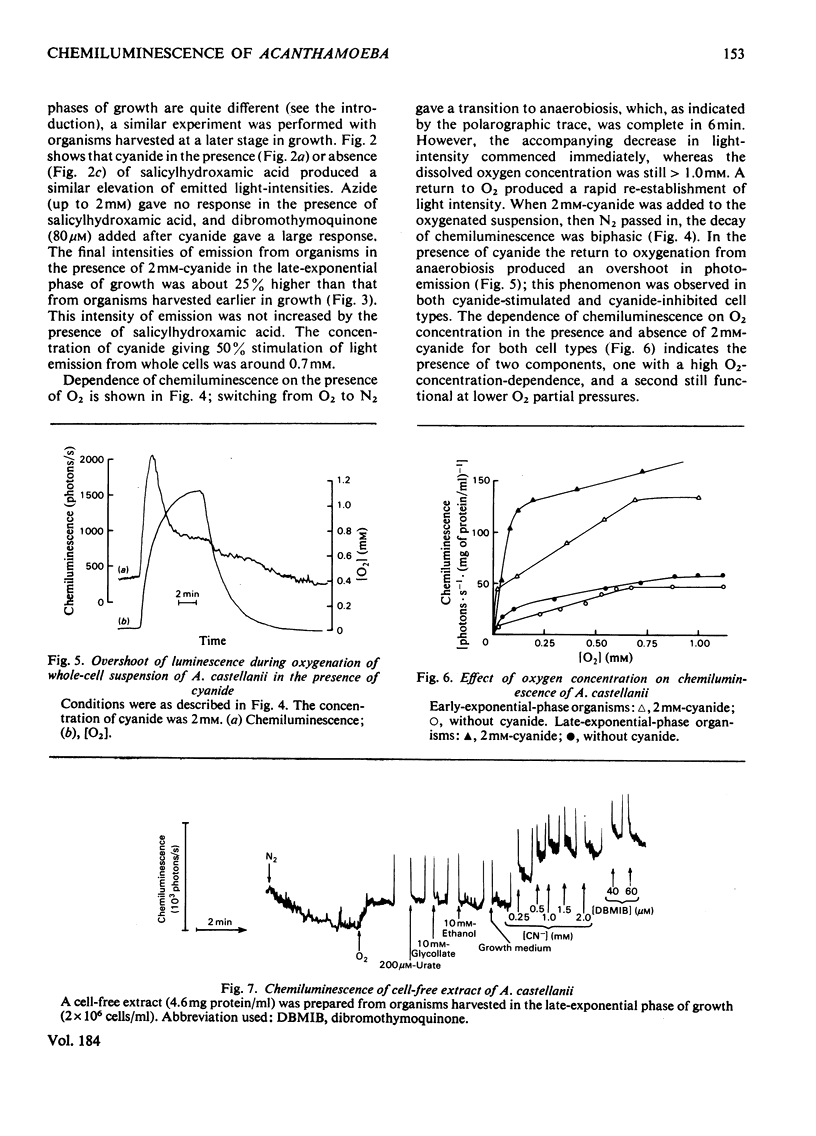
1. Chemiluminescence of Acanthomoeba castellanii in the presence of O2 was of similar intensity in organisms harvested early or late during exponential growth [when cyanide (1 mM) stimulates or inhibits respiration respectively]. 2. Cyanide (up to 1.5 mM) stimulated photoemission in both types of organism by 250--300 photons/s per 10(7) cells above the value observed under aerobic conditions. 3. 'Dibromothymoquinone' (2,5-dibromo-6-isopropyl-3-methyl-p-benzoquinone) (up to 80 microM) further increased chemiluminescence. 4. Similar responses were also demonstrated in whole homogenates and in subcellular fractions; 36% of the chemiluminescence was provided by a fraction sedimenting at 100000g-min, and 20% in that fraction that was non-sedimentable at 200000g-min. 5. Mitochondrial substrates (succinate, 2-oxoglutarate, NADH) in the presence or absence of ADP and Pi or peroxisomal substrates (glycollate, urate or ethanol) gave no increases in light emission by whole homogenates or in any of the fractions. 6. It is suggested that reactions responsible for production of chemiluminescence are those primarily producing superoxide anions and leading to lipid peroxidation and singlet-oxygen formation. Photoemission enhancement and superoxide dismutase inhibition showed similar cyanide concentration-dependencies.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boveris A., Cadenas E. Mitochondrial production of superoxide anions and its relationship to the antimycin insensitive respiration. FEBS Lett. 1975 Jul 1;54(3):311–314. doi: 10.1016/0014-5793(75)80928-8. [DOI] [PubMed] [Google Scholar]
- Boveris A., Cadenas E., Stoppani A. O. Role of ubiquinone in the mitochondrial generation of hydrogen peroxide. Biochem J. 1976 May 15;156(2):435–444. doi: 10.1042/bj1560435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A., Docampo R., Turrens J. F., Stoppani A. O. Effect of beta-lapachone on superoxide anion and hydrogen peroxide production in Trypanosoma cruzi. Biochem J. 1978 Nov 1;175(2):431–439. doi: 10.1042/bj1750431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A., Oshino N., Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972 Jul;128(3):617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A., Stoppani A. O. Hydrogen peroxide generation in Trypanosoma cruzi. Experientia. 1977 Oct 15;33(10):1306–1308. doi: 10.1007/BF01920148. [DOI] [PubMed] [Google Scholar]
- Cadenas E., Boveris A., Ragan C. I., Stoppani A. O. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch Biochem Biophys. 1977 Apr 30;180(2):248–257. doi: 10.1016/0003-9861(77)90035-2. [DOI] [PubMed] [Google Scholar]
- Draber W., Trebst A., Harth E. On a new inhibitor of photosynthetic electron-transport in isolated chloroplasts. Z Naturforsch B. 1970 Oct;25(10):1157–1159. doi: 10.1515/znb-1970-1018. [DOI] [PubMed] [Google Scholar]
- Edwards S. W., Chagla A. H., Griffiths A. J., Lloyd D. The cytochromes of Acanthamoeba castellanii. Biochem J. 1977 Oct 15;168(1):113–121. doi: 10.1042/bj1680113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. W., Lloyd D. Properties of mitochondria isolated from cyanide-sensitive and cyanide-stimulated cultures of Acanthamoeba castellanii. Biochem J. 1978 Jul 15;174(1):203–211. doi: 10.1042/bj1740203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser A., Stauff J. Lumineszenz von Hefe. Z Naturforsch B. 1968 Nov;23(11):1554–1555. [PubMed] [Google Scholar]
- Howes R. M., Steele R. H. Microsomal ( S) chemiluminescence (CL) induced by NADPH and its relation to lipid peroxidation. Res Commun Chem Pathol Pharmacol. 1971 Jul-Sep;2(4):619–626. [PubMed] [Google Scholar]
- Howes R. M., Steele R. H. Microsomal (muS) chemiluminescence (CL) induced by NADPH and its relation to aryl-hydroxylations. Res Commun Chem Pathol Pharmacol. 1972 Mar;3(2):349–357. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lloyd D., Edwards S., Kristensen B., Degn H. The effect of inhibitors on the oxygen kinetics of terminal oxidases of Acanthamoeba castellanii. Biochem J. 1979 Jul 15;182(1):11–15. doi: 10.1042/bj1820011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loschen G., Azzi A., Flohé L. Mitochondrial H2O2 formation: relationship with energy conservation. FEBS Lett. 1973 Jun 15;33(1):84–87. doi: 10.1016/0014-5793(73)80165-6. [DOI] [PubMed] [Google Scholar]
- Loschen G., Azzi A., Richter C., Flohé L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974 May 15;42(1):68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- Loschen G., Flohé L., Chance B. Respiratory chain linked H(2)O(2) production in pigeon heart mitochondria. FEBS Lett. 1971 Nov 1;18(2):261–264. doi: 10.1016/0014-5793(71)80459-3. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- Nakano M., Noguchi T., Sugioka K., Fukuyama H., Sato M. Spectroscopic evidence for the generation of singlet oxygen in the reduced nicotinamide adenine dinucleotide phosphate-dependent microsomal lipid peroxidation system. J Biol Chem. 1975 Mar 25;250(6):2404–2406. [PubMed] [Google Scholar]
- PIATENKO V. S., TARUSOV B. N. KATODNOE SVECHENIE NORMAL'NYKH I RAKOVYKH KLETOK. Biofizika. 1964;9:134–135. [PubMed] [Google Scholar]
- Quickenden T. I., Que Hee S. S. Weak luminescence from the yeast Saccharomyces cerevisiae and the existence of mitogenetic radiation. Biochem Biophys Res Commun. 1974 Sep 23;60(2):764–770. doi: 10.1016/0006-291x(74)90306-4. [DOI] [PubMed] [Google Scholar]
- Rich P. R., Bonner W. D. The sites of superoxide anion generation in higher plant mitochondria. Arch Biochem Biophys. 1978 May;188(1):206–213. doi: 10.1016/0003-9861(78)90373-9. [DOI] [PubMed] [Google Scholar]
- Sugioka K., Nakano M. A possible mechanism of the generation of singlet molecular oxygen in nadph-dependent microsomal lipid peroxidation. Biochim Biophys Acta. 1976 Feb 16;423(2):203–216. doi: 10.1016/0005-2728(76)90179-1. [DOI] [PubMed] [Google Scholar]
- TARUSOV B. N., POLIVODA A. I., ZHURAVLEV A. I., SEKAMOVA E. N. [Ultraweak spontaneous luminescence in animal tissue]. Tsitologiia. 1962 Nov-Dec;4:696–699. [PubMed] [Google Scholar]


