Abstract
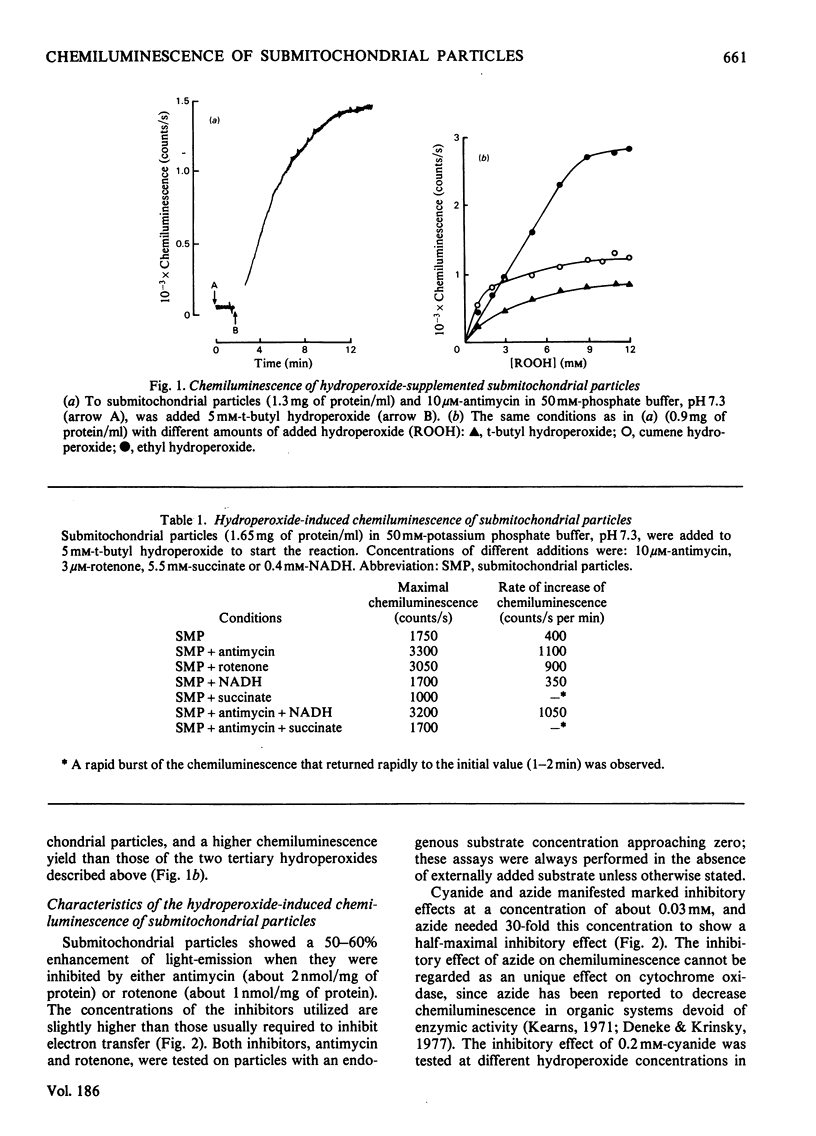
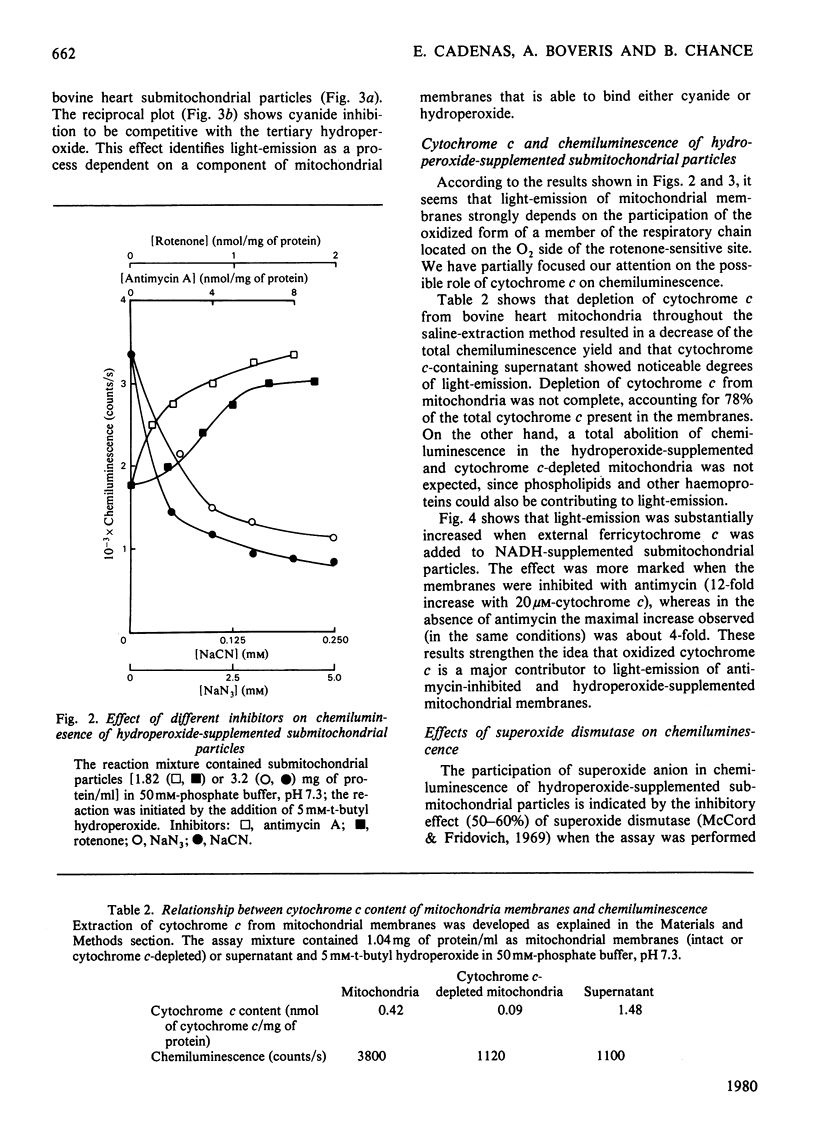
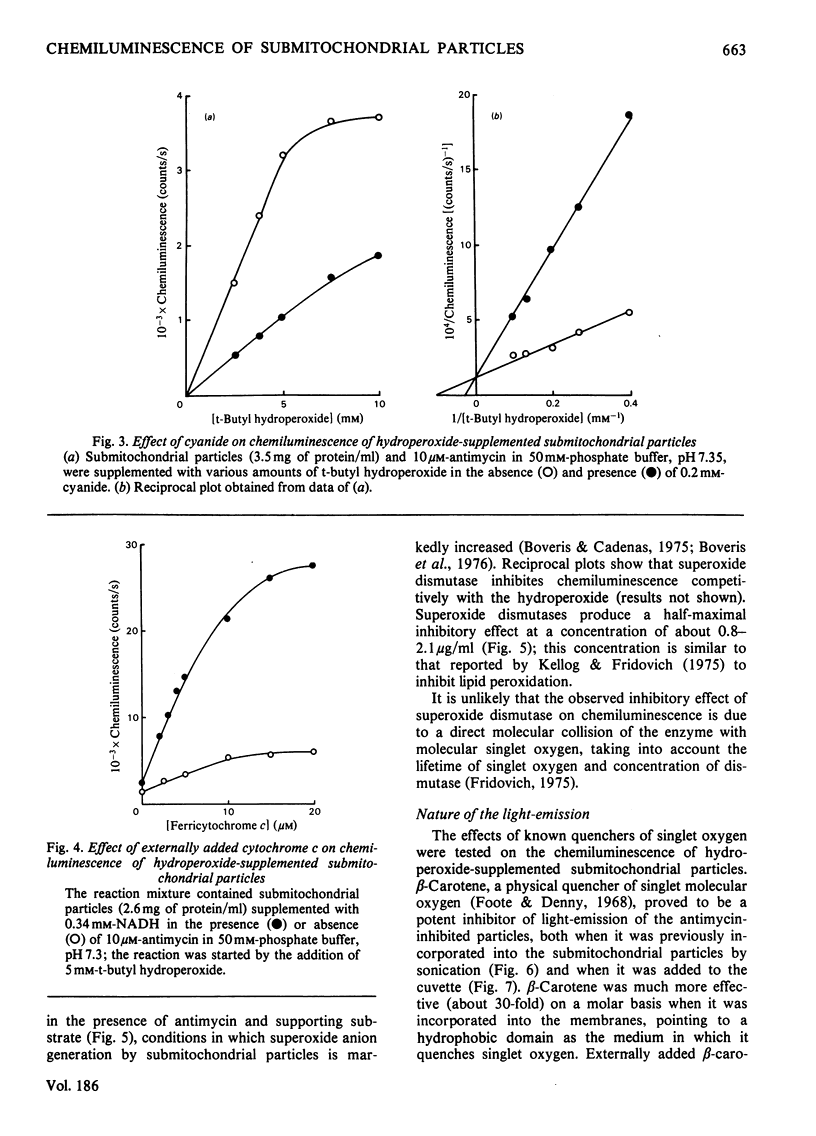
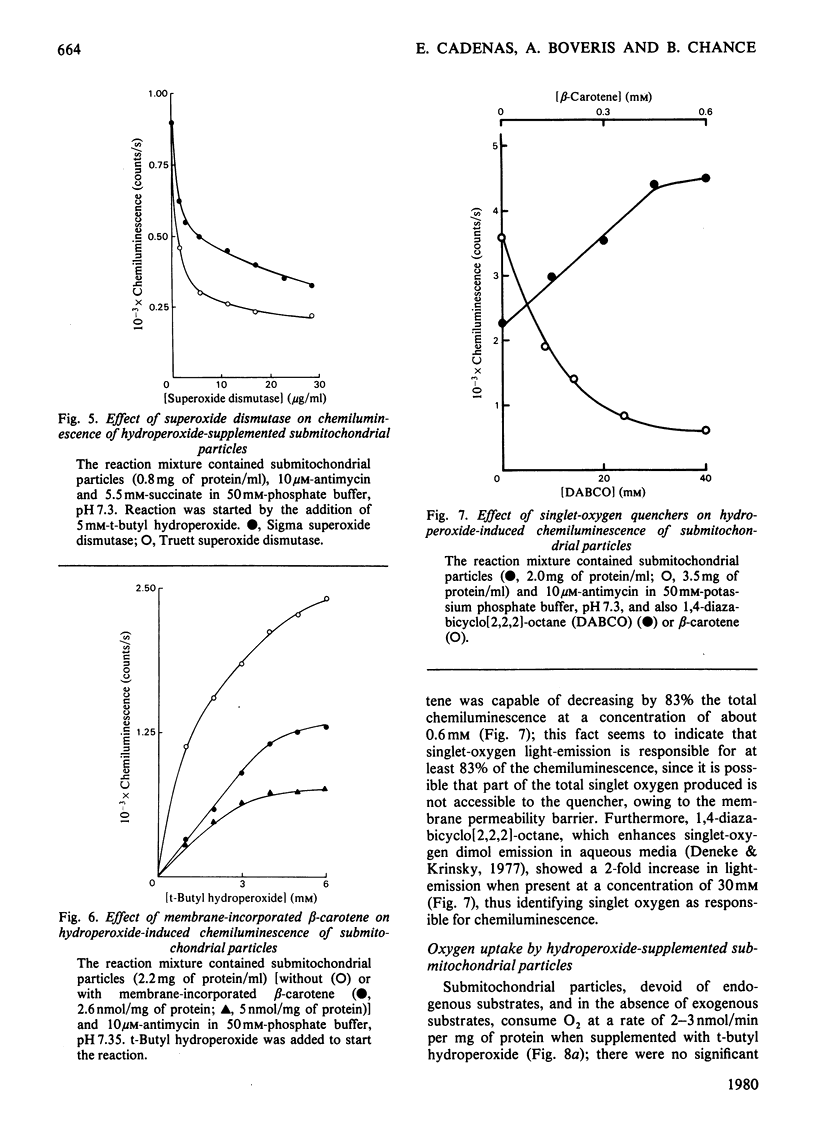
Submitochondrial particles from bovine heart mitochondria showed low-level chemiluminescence when supplemented with organic hydroperoxides. Chemiluminescence seems to measure integratively radical reactions involved in lipid peroxidation and related processes. Maximal light-emission was about 1500 counts/s and was reached 2–10min after addition of hydroperoxides. Ethyl hydroperoxide, cumene hydroperoxide and t-butyl hydroperoxide were effective in that order. Antimycin and rotenone increased chemiluminescence by 50–60%; addition of substrates, NADH and succinate did not produce marked changes in the observed chemiluminescence. Cyanide inhibited chemiluminescence; half-maximal inhibitory effect was obtained with 0.03mm-cyanide and the inhibition was competitive with respect to t-butyl hydroperoxide. Externally added cytochrome c (10–20μm) had a marked stimulatory effect on chemiluminescence, namely a 12-fold increase in light-emission of antimycin-inhibited submitochondrial particles. Stimulation of hydroperoxide-induced chemiluminescence of submitochondrial particles by cytochrome c was matched by a burst of O2 consumption. O2 is believed to participate in the chain radical reactions that lead to lipid peroxidation. Superoxide anion seems to be involved in the chemiluminescence reactions as long as light-emission was 50–60% inhibitible by superoxide dismutase. Singlet-oxygen quenchers, e.g. β-carotene and 1,4-diazabicyclo[2,2,2]-octane, affected light-emission. β-Carotene was effective either when incorporated into the membranes or added to the cuvette. The present paper suggests that singlet molecular oxygen is mainly responsible for the light-emission in the hydroperoxide-supplemented submitochondrial particles.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANKS A., EDDIE E., SMITH J. G. Reactions of cytochrome-c with methyl linoleate hydroperoxide. Nature. 1961 Jun 3;190:908–909. doi: 10.1038/190908a0. [DOI] [PubMed] [Google Scholar]
- Boveris A., Cadenas E. Mitochondrial production of superoxide anions and its relationship to the antimycin insensitive respiration. FEBS Lett. 1975 Jul 1;54(3):311–314. doi: 10.1016/0014-5793(75)80928-8. [DOI] [PubMed] [Google Scholar]
- Boveris A., Cadenas E., Stoppani A. O. Role of ubiquinone in the mitochondrial generation of hydrogen peroxide. Biochem J. 1976 May 15;156(2):435–444. doi: 10.1042/bj1560435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demopoulos H. B. The basis of free radical pathology. Fed Proc. 1973 Aug;32(8):1859–1861. [PubMed] [Google Scholar]
- Diaz P., Jones D. G., Kay A. B. Histamine-coated particles generate superoxide (O-2) and chemiluminescence in alveolar macrophages. Nature. 1979 Mar 29;278(5703):454–456. doi: 10.1038/278454a0. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron chelates: is it a mechanism for hydroxyl radical production in biochemical systems? FEBS Lett. 1978 Aug 15;92(2):321–326. doi: 10.1016/0014-5793(78)80779-0. [DOI] [PubMed] [Google Scholar]
- JACOBS E. E., SANADI D. R. The reversible removal of cytochrome c from mitochondria. J Biol Chem. 1960 Feb;235:531–534. [PubMed] [Google Scholar]
- Kakinuma K., Cadenas E., Boveris A., Chance B. Low level chemiluminescence of intact polymorphonuclear leukocytes. FEBS Lett. 1979 Jun 1;102(1):38–42. doi: 10.1016/0014-5793(79)80923-0. [DOI] [PubMed] [Google Scholar]
- Kaschnitz R. M., Hatefi Y. Lipid oxidation in biological membranes. Electron transfer proteins as initiators of lipid autoxidation. Arch Biochem Biophys. 1975 Nov;171(1):292–304. doi: 10.1016/0003-9861(75)90036-3. [DOI] [PubMed] [Google Scholar]
- Kellogg E. W., 3rd, Fridovich I. Liposome oxidation and erythrocyte lysis by enzymically generated superoxide and hydrogen peroxide. J Biol Chem. 1977 Oct 10;252(19):6721–6728. [PubMed] [Google Scholar]
- Kellogg E. W., 3rd, Fridovich I. Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J Biol Chem. 1975 Nov 25;250(22):8812–8817. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lloyd D., Boveris A., Reiter R., Filipkowski M., Chance B. Chemiluminescence of Acanthamoeba castellanii. Biochem J. 1979 Oct 15;184(1):149–156. doi: 10.1042/bj1840149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch R. E., Fridovich I. Effects of superoxide on the erythrocyte membrane. J Biol Chem. 1978 Mar 25;253(6):1838–1845. [PubMed] [Google Scholar]
- MARGOLIASH E. The use of ion exchangers in the preparation and purification of cytochrome c. Biochem J. 1954 Apr;56(4):529–535. doi: 10.1042/bj0560529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. A peroxide-dependent reduction of cytochrome c by NADH. Biochim Biophys Acta. 1973 Apr 5;292(3):815–824. doi: 10.1016/0005-2728(73)90028-5. [DOI] [PubMed] [Google Scholar]
- Nakano M., Noguchi T., Sugioka K., Fukuyama H., Sato M. Spectroscopic evidence for the generation of singlet oxygen in the reduced nicotinamide adenine dinucleotide phosphate-dependent microsomal lipid peroxidation system. J Biol Chem. 1975 Mar 25;250(6):2404–2406. [PubMed] [Google Scholar]
- Seliger H. H. The origin of bioluminescence. Photochem Photobiol. 1975 May;21(5):355–361. doi: 10.1111/j.1751-1097.1975.tb06684.x. [DOI] [PubMed] [Google Scholar]
- Sugioka K., Nakano M. A possible mechanism of the generation of singlet molecular oxygen in nadph-dependent microsomal lipid peroxidation. Biochim Biophys Acta. 1976 Feb 16;423(2):203–216. doi: 10.1016/0005-2728(76)90179-1. [DOI] [PubMed] [Google Scholar]
- Tappel A. L. Lipid peroxidation damage to cell components. Fed Proc. 1973 Aug;32(8):1870–1874. [PubMed] [Google Scholar]


