Abstract
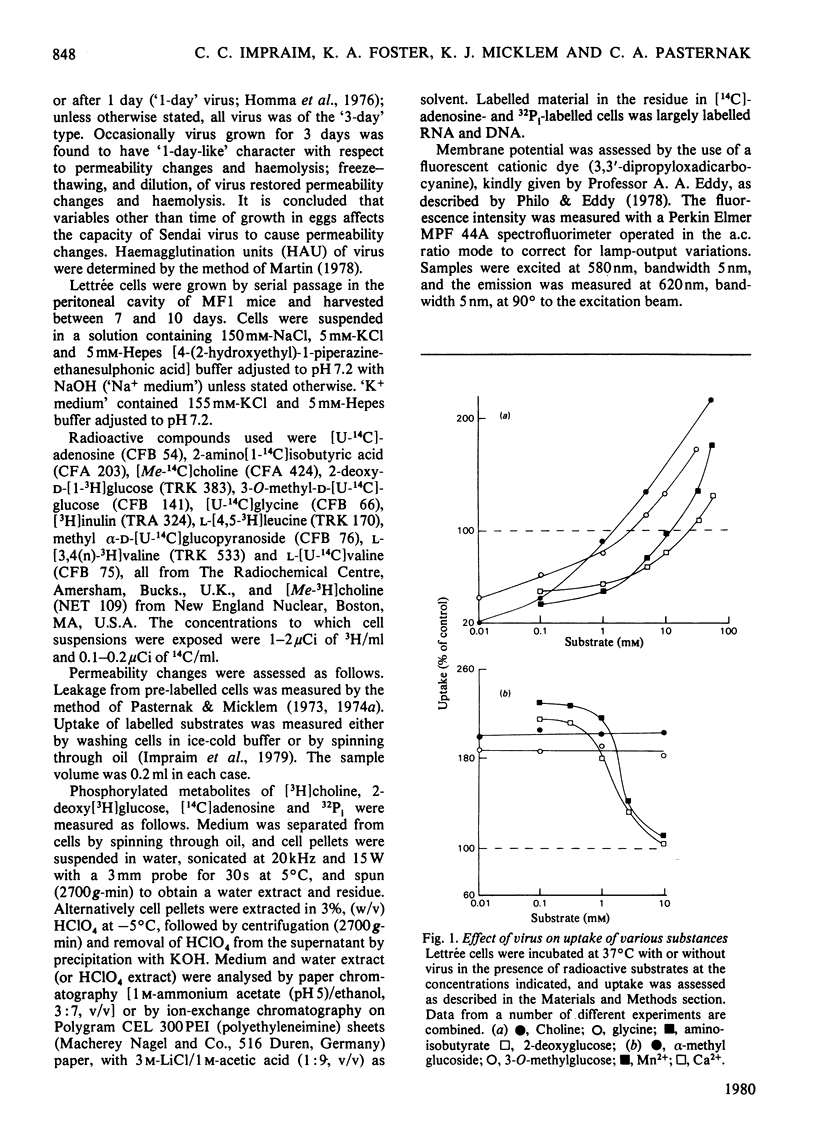
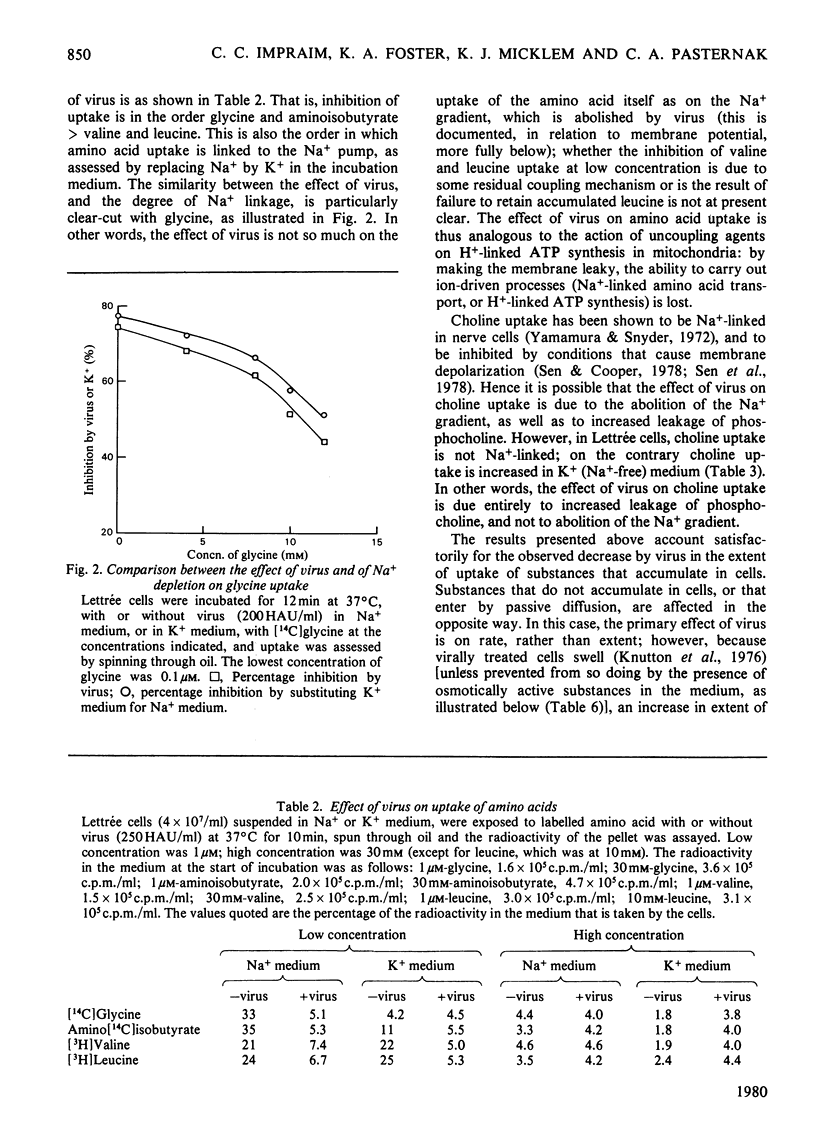
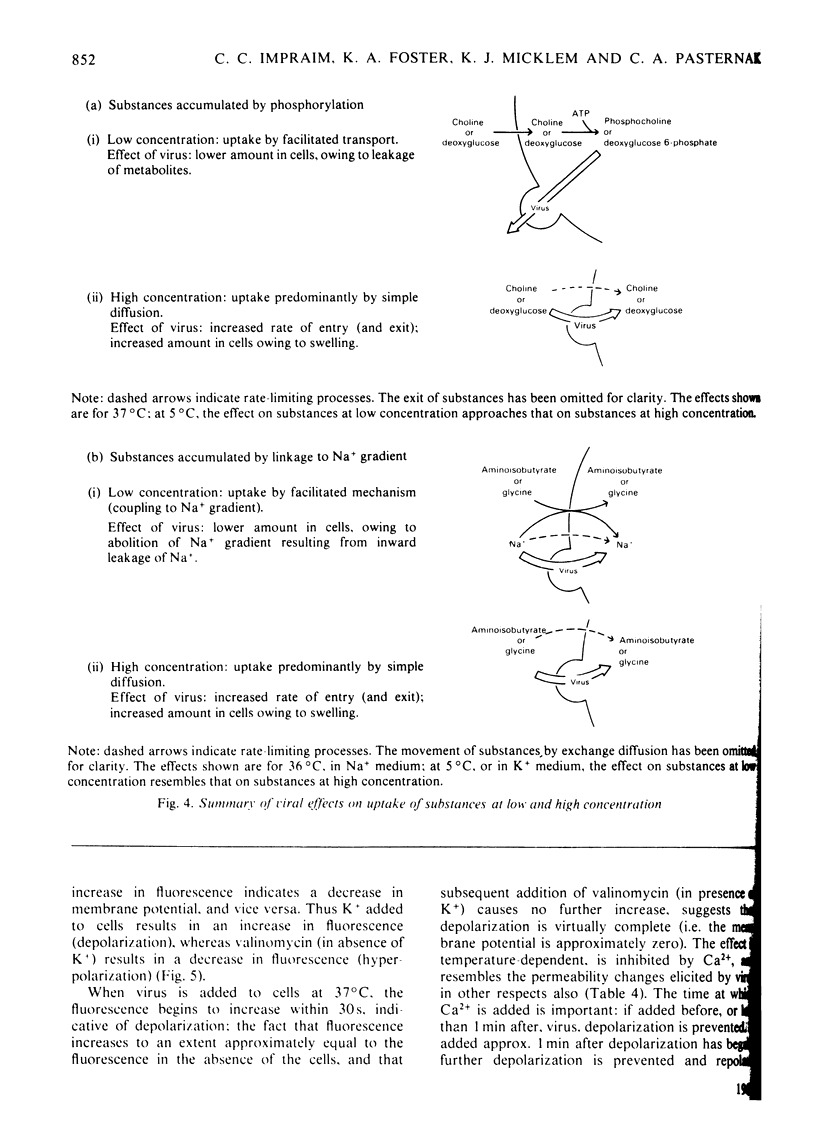
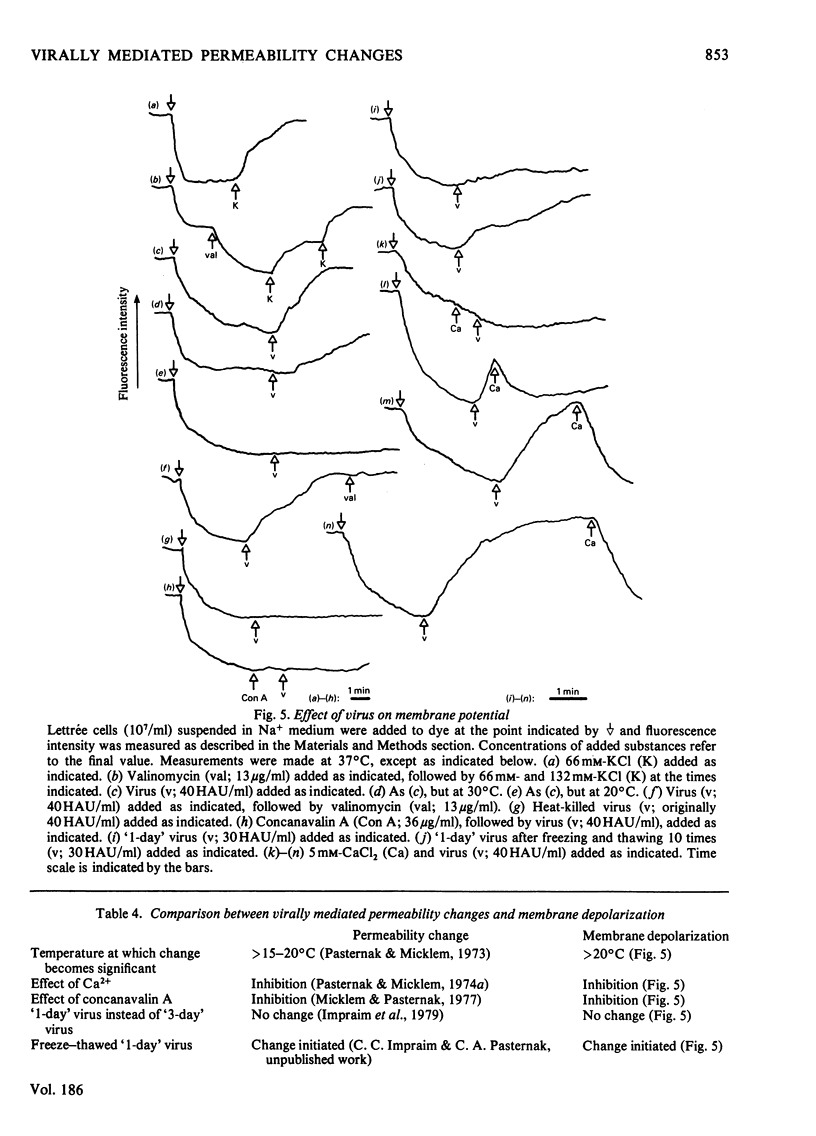
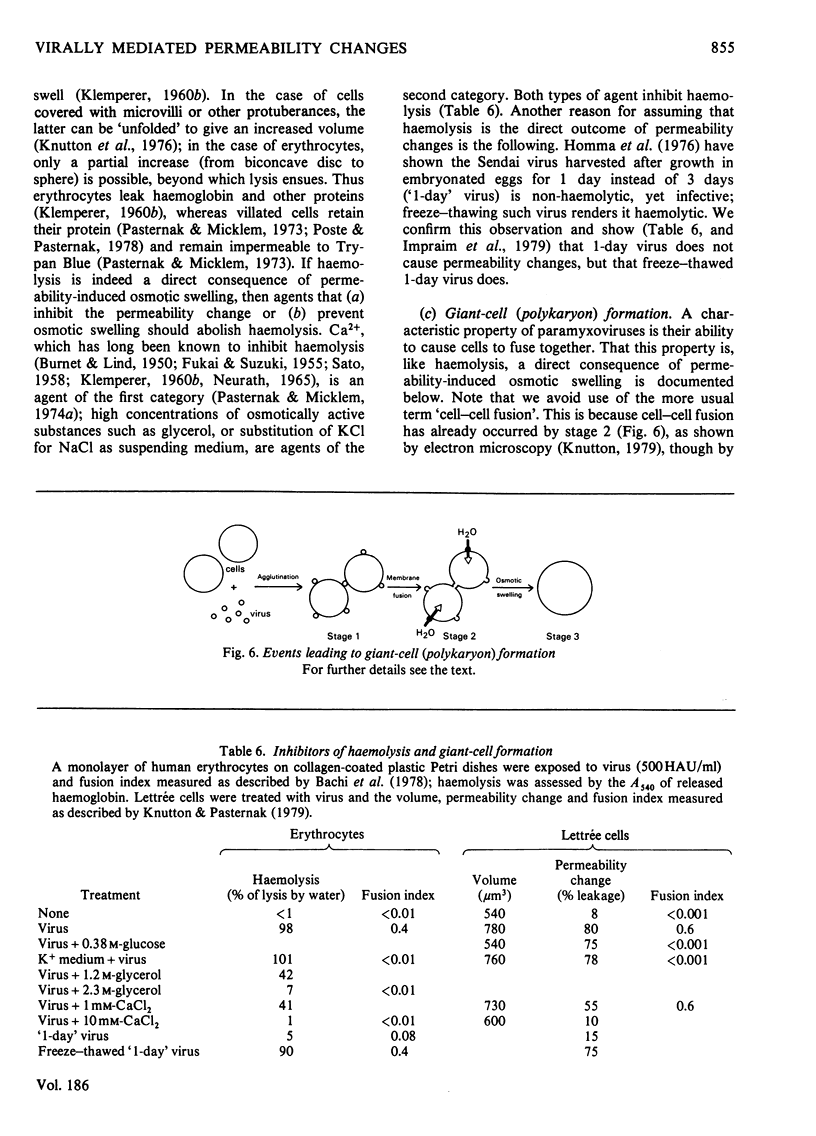
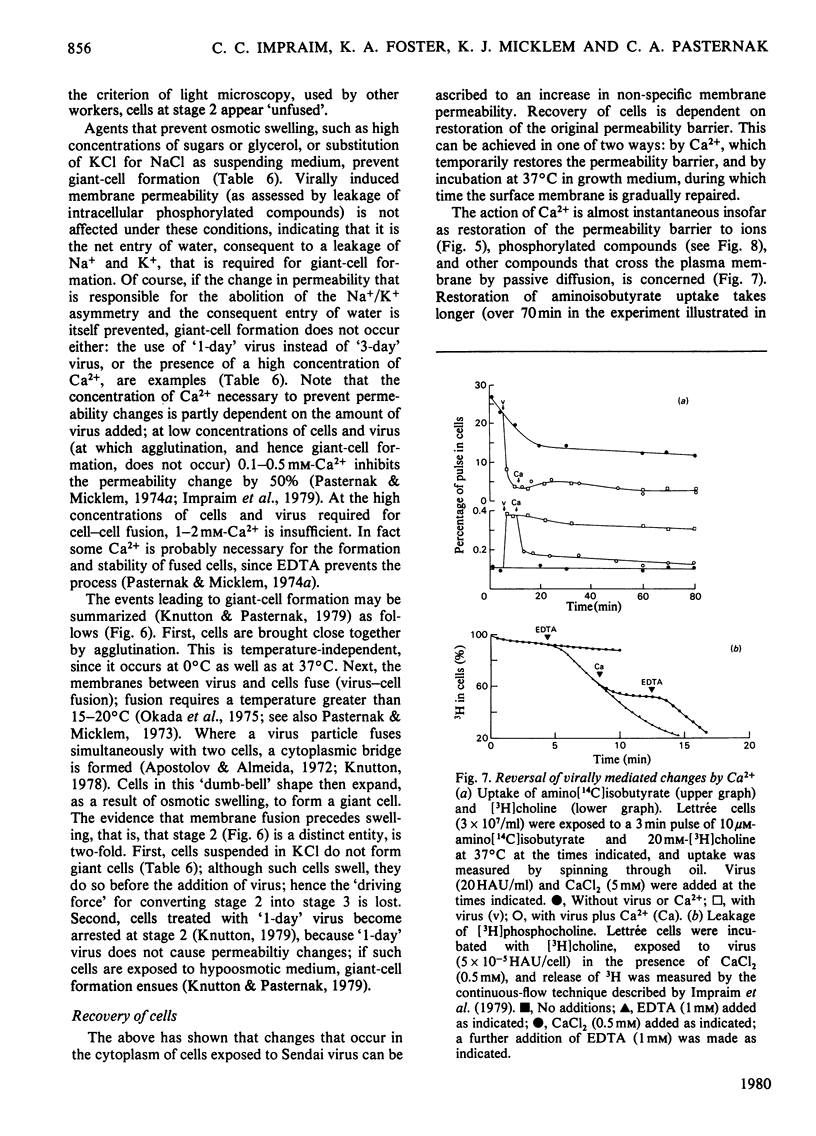
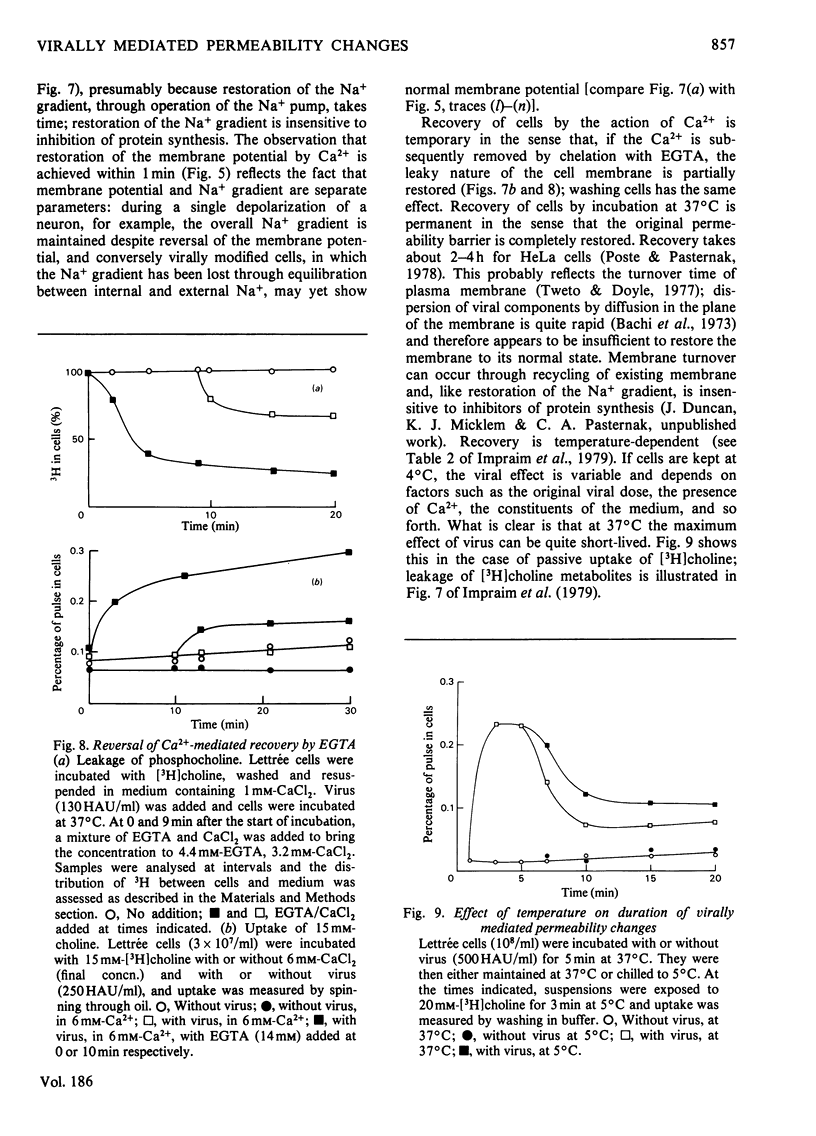
1. The changes in membrane permeability to small molecules caused by Sendai virus [Pasternak & Micklem (1973) J. Membr. Biol. 14, 293-303] have been further characterized. The uptake of substances that are concentrated within cells is inhibited. Choline and 2-deoxyglucose, which become phosphorylated, and aminoisobutyrate and glycine, which are driven by a Na+-linked mechanism, are examples. The uptake of each compound under conditons where its diffusion across the plasma membrane is rate-limiting is stimulated by virus. Choline, 2-deoxyglucose and amino acids at high concentration, amino acids in Na+-free medium, and most substances at low temperature, are examples. It is concluded that virally mediated decrease of uptake is due to one of two causes. Substances that are accumulated by phosphorylation are not retained because of leakage of the phosphorylated metabolites out of cells. Substances that are accumulated by linkage to a Na+ gradient are no longer accumulated because of collapse of the gradient resulting from an increased permeability to Nat 2. Increased permeability to K+ and Na+ results in (a) membrane depolarization and (b) cell swelling. The latter event leads to haemolysis (for erythrocytes) and can lead to giant-cell (polykaryon) formation (for several cell types). 3. Recovery of cells can be temporarily achieved by the addition of Ca2+; permanent recovery requires incubation for some hours at 37 degrees C. 4. The possible significance of virally mediated permeability changes, with regard to clinical situations and to cell biology, is discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apostolov K., Almeida J. D. Interaction of Sendai (HVJ) virus with human erythrocytes: a morphological study of haemolysis cell fusion. J Gen Virol. 1972 Jun;15(3):227–234. doi: 10.1099/0022-1317-15-3-227. [DOI] [PubMed] [Google Scholar]
- BURNET F. M., LIND P. E. Haemolysis by Newcastle disease virus. II. General character of haemolytic action. Aust J Exp Biol Med Sci. 1950 Mar;28(2):129–150. doi: 10.1038/icb.1950.13. [DOI] [PubMed] [Google Scholar]
- Bächi T., Aguet M., Howe C. Fusion of erythrocytes by Sendai virus studied by immuno-freeze-etching. J Virol. 1973 Jun;11(6):1004–1012. doi: 10.1128/jvi.11.6.1004-1012.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bächi T., Eichenberger G., Hauri H. P. Sendai virus hemolysis: influence of lectins and analysis by immune fluorescence. Virology. 1978 Apr;85(2):518–530. doi: 10.1016/0042-6822(78)90458-0. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Miller M. R., Pardee A. B. Animal cells reversibly permeable to small molecules. Proc Natl Acad Sci U S A. 1978 Jan;75(1):351–355. doi: 10.1073/pnas.75.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P., Giberman E. Enhancement of potassium influx, in baby hamster kidney cells and chicken erythrocytes, during adsorption of parainfluenza 1 (Sendai) virus. FEBS Lett. 1973 Apr 1;31(1):127–130. doi: 10.1016/0014-5793(73)80089-4. [DOI] [PubMed] [Google Scholar]
- Homma M., Shimizu K., Shimizu Y. K., Ishida N. On the study of Sendai virus hemolysis. I. Complete Sendai virus lacking in hemolytic activity. Virology. 1976 May;71(1):41–47. doi: 10.1016/0042-6822(76)90092-1. [DOI] [PubMed] [Google Scholar]
- Impraim C. C., Micklem K. J., Pasternak C. A. Calcium, cells and virus--alterations caused by paramyxoviruses. Biochem Pharmacol. 1979 Jun 15;28(12):1963–1969. doi: 10.1016/0006-2952(79)90652-x. [DOI] [PubMed] [Google Scholar]
- KLEMPERER H. G. An effect of phloridzin on influenza virus elution and on neuraminidase activity. Virology. 1960 Nov;12:495–498. doi: 10.1016/0042-6822(60)90172-0. [DOI] [PubMed] [Google Scholar]
- KLEMPERER H. G. Hemolysis and the release of potassium from cells by Newcastle disease virus (NDV). Virology. 1960 Dec;12:540–552. doi: 10.1016/0042-6822(60)90177-x. [DOI] [PubMed] [Google Scholar]
- Kerzner B., Kelly M. H., Gall D. G., Butler D. G., Hamilton J. R. Transmissible gastroenteritis: sodium transport and the intestinal epithelium during the course of viral enteritis. Gastroenterology. 1977 Mar;72(3):457–461. [PubMed] [Google Scholar]
- Knutton S., Jackson D., Graham J. M., Micklem K. J., Pasternak C. A. Microvilli and cell swelling. Nature. 1976 Jul 1;262(5563):52–54. doi: 10.1038/262052a0. [DOI] [PubMed] [Google Scholar]
- Knutton S. Studies of membrane fusion. II. Fusion of human erythrocytes by Sendai virus. J Cell Sci. 1977 Dec;28:189–210. doi: 10.1242/jcs.28.1.189. [DOI] [PubMed] [Google Scholar]
- Knutton S. Studies of membrane fusion. V. Fusion of erythrocytes with non-haemolytic Sendai virus. J Cell Sci. 1979 Apr;36:85–96. doi: 10.1242/jcs.36.1.85. [DOI] [PubMed] [Google Scholar]
- LEFEVRE P. G. Sugar transport in the red blood cell: structure-activity relationships in substrates and antagonists. Pharmacol Rev. 1961 Mar;13:39–70. [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication and the control of growth. Biochim Biophys Acta. 1979 Feb 4;560(1):1–65. doi: 10.1016/0304-419x(79)90002-7. [DOI] [PubMed] [Google Scholar]
- Micklem K. J., Pasternak C. A. Surface components involved in virally mediated membrane changes. Biochem J. 1977 Feb 15;162(2):405–410. doi: 10.1042/bj1620405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. R., Castellot J. J., Jr, Pardee A. B. A general method for permeabilizing monolayer and suspension cultured animal cells. Exp Cell Res. 1979 May;120(2):421–425. doi: 10.1016/0014-4827(79)90404-x. [DOI] [PubMed] [Google Scholar]
- NEURATH A. R. INTERACTION OF SENDAI VIRUS WITH RED BLOOD CELLS. II. COLLOID--OSMOTIC HAEMOLYSIS. Acta Virol. 1965 Mar;9:119–129. [PubMed] [Google Scholar]
- Okada Y., Koseki I., Kim J., Maeda Y., Hashimoto T. Modification of cell membranes with viral envelopes during fusion of cells with HVJ (Sendai virus). Exp Cell Res. 1975 Jul;93(2):368–378. doi: 10.1016/0014-4827(75)90462-0. [DOI] [PubMed] [Google Scholar]
- Pasternak C. A., Micklem K. J. Permeability changes during cell fusion. J Membr Biol. 1973;14(3):293–303. doi: 10.1007/BF01868082. [DOI] [PubMed] [Google Scholar]
- Pasternak C. A., Micklem K. J. The biochemistry of virus-induced cell fusion. Changes in membrane integrity. Biochem J. 1974 Jun;140(3):405–411. doi: 10.1042/bj1400405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak C. A., Micklem K. J. Virally mediated membrane changes: inverse effects on transport and diffusion. Biochem J. 1974 Dec;144(3):593–595. doi: 10.1042/bj1440593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philo R. D., Eddy A. A. The membrane potential of mouse ascites-tumour cells studied with the fluorescent probe 3,3'-dipropyloxadicarbocyanine. Amplitude of the depolarization caused by amino acids. Biochem J. 1978 Sep 15;174(3):801–810. doi: 10.1042/bj1740801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Permeability of cell junction depends on local cytoplasmic calcium activity. Nature. 1975 Mar 20;254(5497):250–252. doi: 10.1038/254250a0. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A., Friedberg I. Effect of exogenous ATP on the permeability properties of transformed cultures of mouse cell lines. J Biol Chem. 1977 Jul 10;252(13):4584–4590. [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A. Reciprocal control of membrane permeability of transformed cultures of mouse cell lines by external and internal ATP. J Biol Chem. 1979 Feb 10;254(3):708–714. [PubMed] [Google Scholar]
- Sen I., Baba A., Schulz R. A., Cooper J. R. Mechanism of action of notexin and notechis II-5 on synaptosomes. J Neurochem. 1978 Oct;31(4):969–976. doi: 10.1111/j.1471-4159.1978.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Sen I., Cooper J. R. Similarities of beta-bungarotoxin and phospholipase A2 and their mechanism of action. J Neurochem. 1978 Jun;30(6):1369–1372. doi: 10.1111/j.1471-4159.1978.tb10468.x. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Sekiguchi M., Okada Y. Restoration of ultraviolet-induced unscheduled DNA synthesis of xeroderma pigmentosum cells by the concomitant treatment with bacteriophage T4 endonuclease V and HVJ (Sendai virus). Proc Natl Acad Sci U S A. 1975 Oct;72(10):4071–4075. doi: 10.1073/pnas.72.10.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner A. Optical probes of membrane potential. J Membr Biol. 1976 Jun 30;27(4):317–334. doi: 10.1007/BF01869143. [DOI] [PubMed] [Google Scholar]
- Yamaizumi M., Uchida T., Okada Y. Macromolecules can penetrate the host cell membrane during the early period of incubation with HVJ (Sendai virus). Virology. 1979 May;95(1):218–221. doi: 10.1016/0042-6822(79)90418-5. [DOI] [PubMed] [Google Scholar]
- Yamamura H. I., Snyder S. H. Choline: high-affinity uptake by rat brain synaptosomes. Science. 1972 Nov 10;178(4061):626–628. doi: 10.1126/science.178.4061.626. [DOI] [PubMed] [Google Scholar]
- van den Berghe G., Bronfman M., Vanneste R., Hers H. G. The mechanism of adenosine triphosphate depletion in the liver after a load of fructose. A kinetic study of liver adenylate deaminase. Biochem J. 1977 Mar 15;162(3):601–609. doi: 10.1042/bj1620601. [DOI] [PMC free article] [PubMed] [Google Scholar]


