Abstract
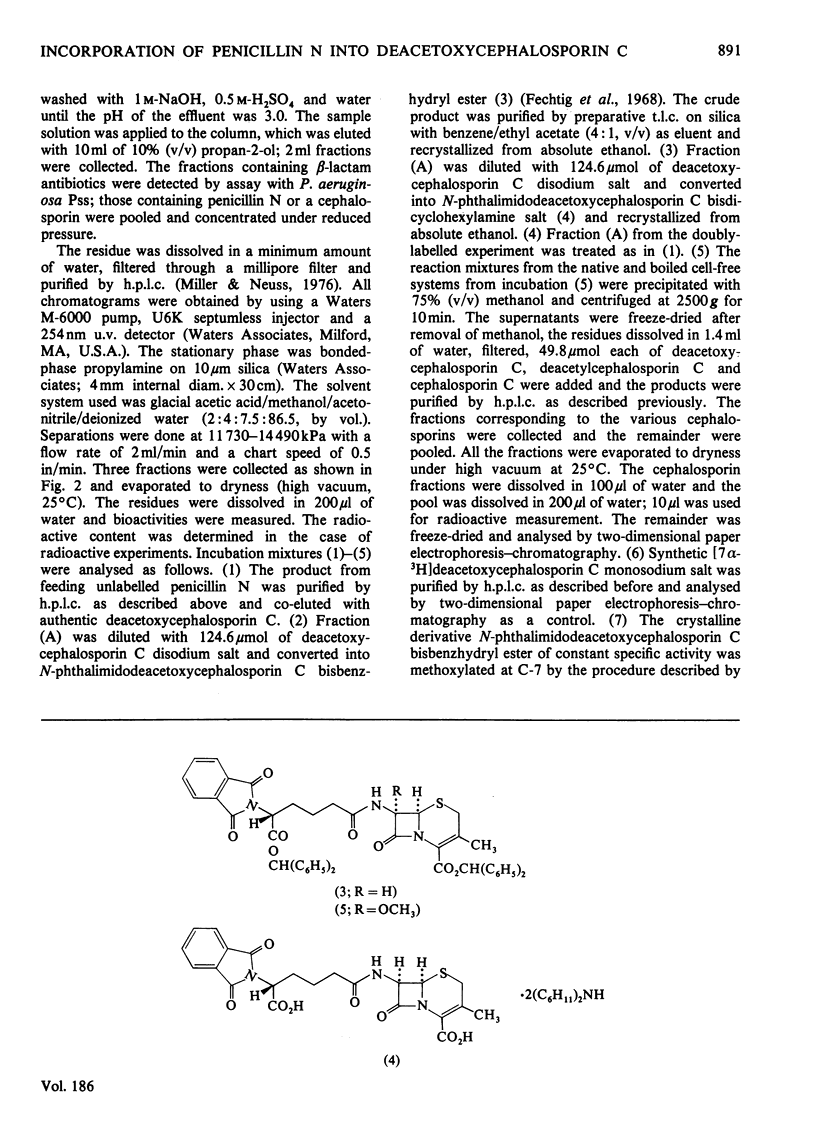
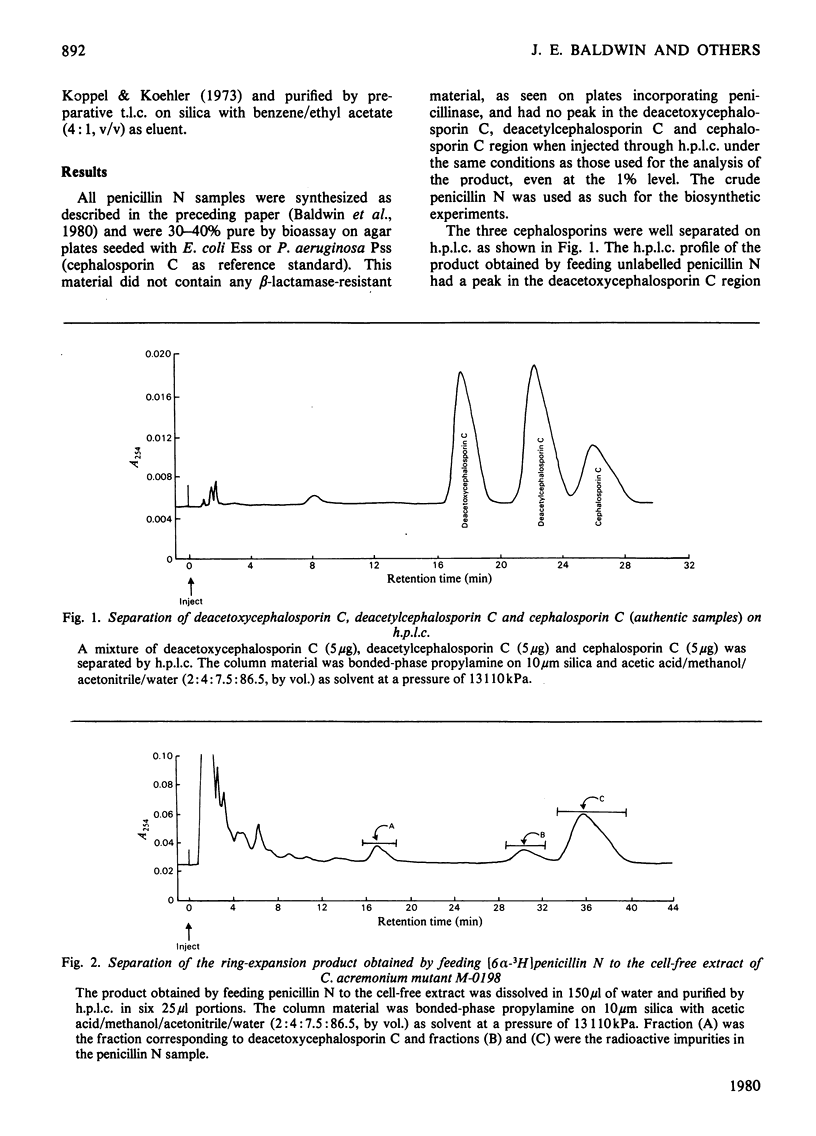
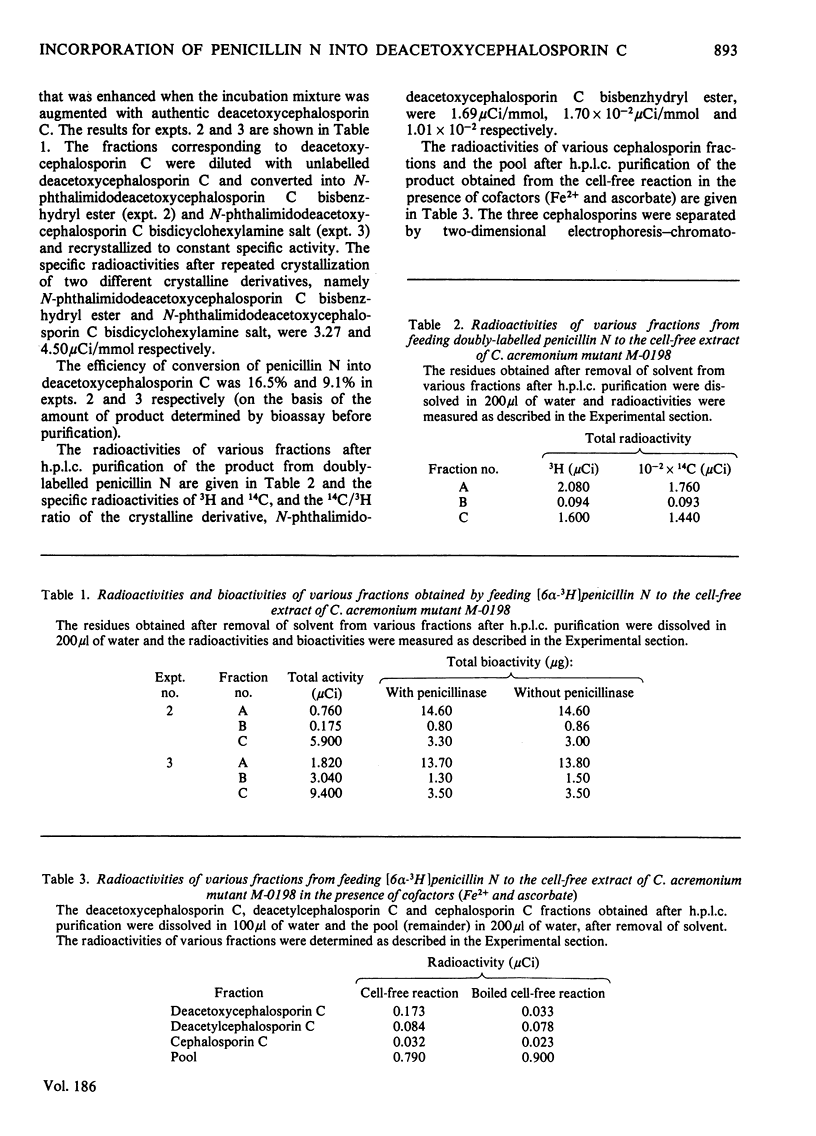
1. In a cell-free system prepared by lysis of protoplasts of Cephalosporium acremonium mutant M-0198, 3H and 14C were incorporated from singly- and doubly-labelled penicillin N into deacetoxycephalosporin C. 2. The deacetoxcephalosporin C obtained from the above feeding experiments was converted into two different crystalline derivatives, namely N-phthalimidodeacetoxycephalosporin C bisbenzhydryl ester and N-phthalimidodeacetoxycephalosporin C bisdicyclohexylamine salt and recrystallized to constant specific activity or constant ratio of specific activity. 3. That 3H is incorporated at C-7 in the biosynthesized deacetoxycephalosporin C was shown by the loss of radioactivity (95.2%) after methoxylating the derived N-phthalimidodeacetoxycephalosporin C bisbenzyhydryl ester. 4. Deacetoxycephalosporin C was also the product of the cell-free reaction conducted in the presence of ferrous ions and ascorbic acid, as shown by two-dimensional paper electrophoresis-chromatography; these additives appreciably improved the efficiency of conversion.
Full text
PDF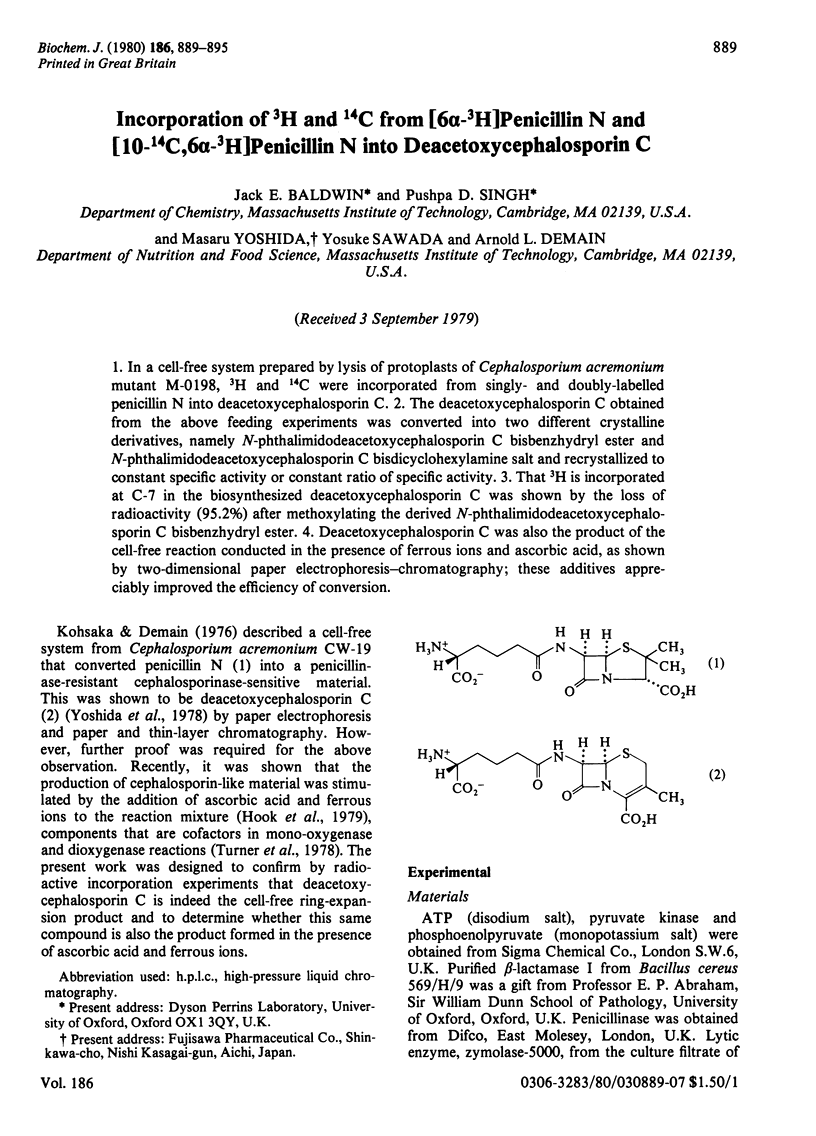



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J. E., Herchen S. R., Singh P. D. Syntheses of penicillin N, [6 alpha-3H]penicillin N and [10-14C,6 alpha-3H]penicillin N. Biochem J. 1980 Mar 15;186(3):881–887. doi: 10.1042/bj1860881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corran P. H., Waley S. G. The tryptic peptides of rabbit muscle triose phosphate isomerase. Biochem J. 1974 Apr;139(1):1–10. doi: 10.1042/bj1390001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fechtig B., Peter H., Bickel H., Vischer E. Uber die Darstellung von 7-Amino-cephalosporansäure. Helv Chim Acta. 1968 Jul 10;51(5):1108–1119. doi: 10.1002/hlca.19680510513. [DOI] [PubMed] [Google Scholar]
- Hook D. J., Chang L. T., Elander R. P., Morin R. B. Stimulation of the conversion of penicillin N to cephalosporin by ascorbic acid, alpha-ketoglutarate, and ferrous ions in cell-free extracts of strains of Cephalosporium acremonium. Biochem Biophys Res Commun. 1979 Mar 15;87(1):258–265. doi: 10.1016/0006-291x(79)91674-7. [DOI] [PubMed] [Google Scholar]
- KATZ A. M., DREYER W. J., ANFINSEN C. B. Peptide separation by two-dimensional chromatography and electrophoresis. J Biol Chem. 1959 Nov;234:2897–2900. [PubMed] [Google Scholar]
- Kohsaka M., Demain A. L. Conversion of penicillin N to cephalosporin(s) by cell-free extracts of Cephalosporium acremonium. Biochem Biophys Res Commun. 1976 May 17;70(2):465–473. doi: 10.1016/0006-291x(76)91069-x. [DOI] [PubMed] [Google Scholar]
- Koppel G. A., Koehler R. E. Functionalization of C 6(7) of penicillins and cephalosporins. A one-step stereoselective synthesis of 7- -methoxycephalosporin C. J Am Chem Soc. 1973 Apr 4;95(7):2403–2404. doi: 10.1021/ja00788a072. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Miller R. D., Neuss N. High performance liquid chromatography of natural products. I. Separation of cephalosporin C derivatives and cephalosporin antibiotics;isolation of cephalosporin C from fermentation broth. J Antibiot (Tokyo) 1976 Sep;29(9):902–906. doi: 10.7164/antibiotics.29.902. [DOI] [PubMed] [Google Scholar]
- O'Sullivan J., Bleaney R. C., Huddleston J. A., Abraham E. P. Incorporation of 3H from delta-(L-alpha-amino (4,5-3H)adipyl)-L-cysteinyl-D-(4,4-3H)valine into isopenicillin N. Biochem J. 1979 Nov 15;184(2):421–426. doi: 10.1042/bj1840421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M. K., Farthing J. E., Brewer S. J. The oxygenation of [3-methyl-3H]desacetoxycephalosporin C [7beta-(5-D-aminadipamido)-3-methylceph-3-em-4-carboxylic acid] to [3-hydroxymethyl-3H]desacetylcephalosporin C by 2-oxoglutarate-linked dioxygenases from Acremonium chrysogenum and Streptomyces clavuligerus. Biochem J. 1978 Sep 1;173(3):839–850. doi: 10.1042/bj1730839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usher J. J., Loder B., Abraham E. P. Synthesis of tritium-labelled isopenicillin N, penicillin N and 6-aminopenicillanic acid. Biochem J. 1975 Dec;151(3):729–739. doi: 10.1042/bj1510729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney J. G., Brannon D. R., Mabe J. A., Wicker K. J. Incorporation of labeled precursors into A16886B, a novel -lactam antibiotic produced by Streptomyces clavuligerus. Antimicrob Agents Chemother. 1972 Mar;1(3):247–251. doi: 10.1128/aac.1.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Konomi T., Kohsaka M., Baldwin J. E., Herchen S., Singh P., Hunt N. A., Demain A. L. Cell-free ring expansion of penicillin N to deacetoxycephalosporin C by Cephalosporium acremonium CW-19 and its mutants. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6253–6257. doi: 10.1073/pnas.75.12.6253. [DOI] [PMC free article] [PubMed] [Google Scholar]


