Abstract
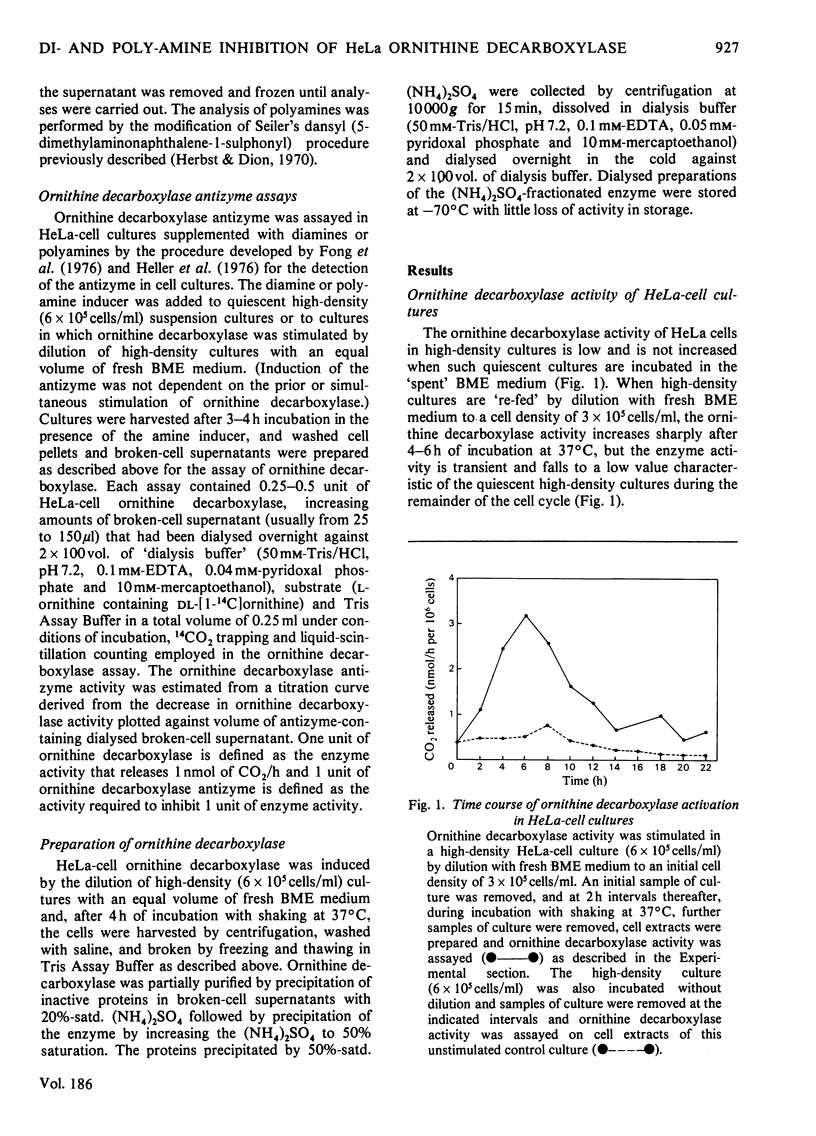
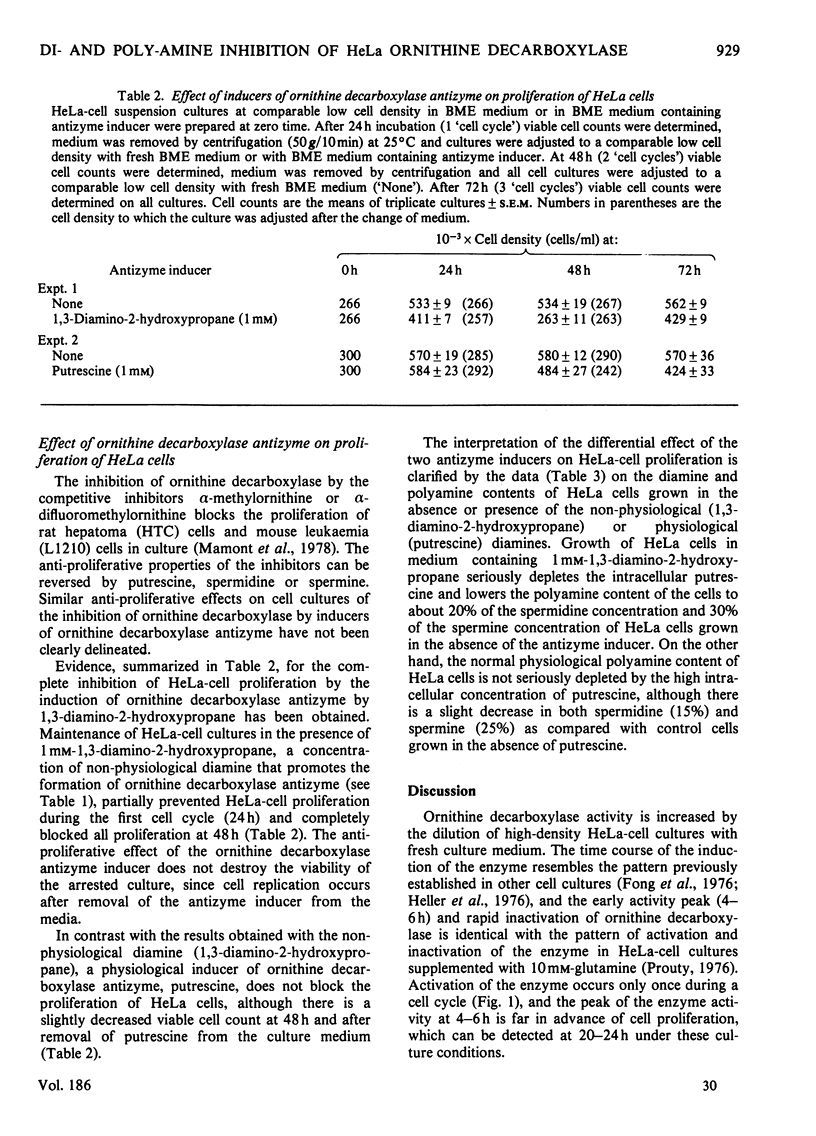
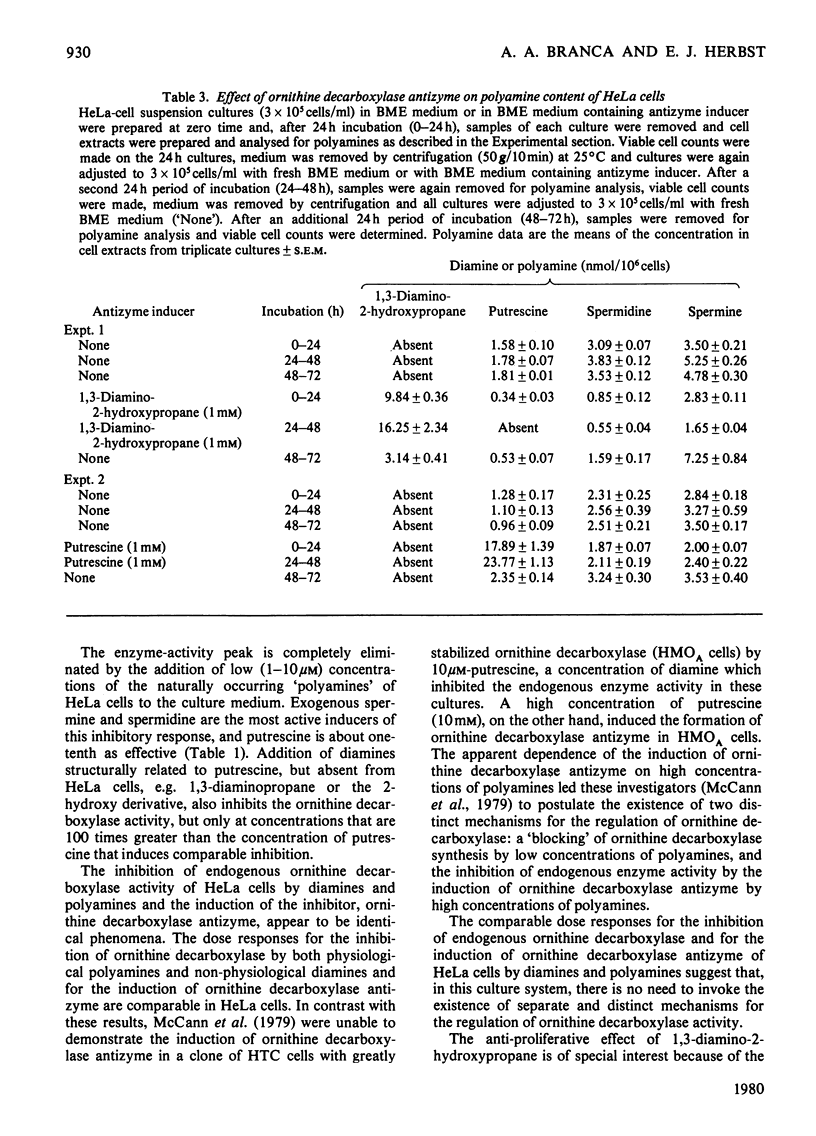
1. Ornithine decarboxylase activity is stimulated in high-density HeLa-cell cultures by dilution of or replacement of spent culture medium with fresh medium containing 10% (v/v) horse serum. 2. After stimulation, ornithine decarboxylase activity reaches a peak at 4–6h, then rapidly declines to the low enzyme activity characteristic of quiescent cultures, where it remains during the remainder of the cell cycle. 3. The stimulation of ornithine decarboxylase is eliminated by the addition of 0.5μm-spermine or -spermidine or 10μm-putrescine to the HeLa-cell cultures at the time of re-feeding with fresh medium. Much higher concentrations (1mm) of the non-physiological diamines, 1,3-diamino-propane or 1,3-diamino-2-hydroxypropane, are required to eliminate the stimulation of ornithine decarboxylase in re-fed HeLa-cell cultures. 4. A heat-labile, non-diffusible inhibitor, comparable with the inhibitory protein ornithine decarboxylase antizyme, is induced in HeLa cells by the addition of exogenous diamines or polyamines. 5. Intracellular putrescine is eliminated, intracellular spermidine and spermine are severely decreased and proliferation of HeLa cells is inhibited when cultures are maintained for 48h in the presence of the non-physiological inducer of ornithine decarboxylase antizyme, 1,3-diamino-2-hydroxypropane. Exogenous putrescine, a physiological inducer of the antizyme, does not decrease intracellular polyamines or interfere with proliferation of HeLa cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fong W. F., Heller J. S., Canellakis E. S. The appearance of an ornithine decarboxylase inhibitory protein upon the addition of putrescine to cell cultures. Biochim Biophys Acta. 1976 Apr 23;428(2):456–465. doi: 10.1016/0304-4165(76)90054-4. [DOI] [PubMed] [Google Scholar]
- Friedman Y., Park S., Levasseur S., Burke G. Regulation of thyroid ornithine decarboxylase by the polyamines. Induction of a protein inhibitor of ornithine decarboxylase by the end-products of the reaction. Biochim Biophys Acta. 1977 Dec 22;500(2):291–303. doi: 10.1016/0304-4165(77)90021-6. [DOI] [PubMed] [Google Scholar]
- Heller J. S., Fong W. F., Canellakis E. S. Induction of a protein inhibitor to ornithine decarboxylase by the end products of its reaction. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1858–1862. doi: 10.1073/pnas.73.6.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller J. S., Kyriakidis D., Fong W. F., Canellakis E. S. Ornithine decarboxylase antizyme is a normal component of uninduced H-35 cells and rat liver. Eur J Biochem. 1977 Dec;81(3):545–550. doi: 10.1111/j.1432-1033.1977.tb11980.x. [DOI] [PubMed] [Google Scholar]
- Herbst E. J., Dion A. S. Polyamine changes during development of Drosophila melanogaster. Fed Proc. 1970 Jul-Aug;29(4):1563–1567. [PubMed] [Google Scholar]
- Kallio A., Löfman M., Pösö H., Jänne J. Diamine-induced inhibition of liver ornithine decarboxylase. Biochem J. 1979 Jan 1;177(1):63–69. doi: 10.1042/bj1770063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio A., Pösö H., Guha S. K., Jänne J. Polyamines and their biosynthetic enzymes in Ehrlich ascites-carcinoma cells. Modification of tumour polyamine pattern by diamines. Biochem J. 1977 Jul 15;166(1):89–94. doi: 10.1042/bj1660089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakidis D. A., Heller J. S., Canellakis E. S. Modulation of ornithine decarboxylase activity in Escherichia coli by positive and negative effectors. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4699–4703. doi: 10.1073/pnas.75.10.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUELLER G. C., KAJIWARA K., STUBBLEFIELD E., RUECKERT R. R. Molecular events in the reproduction of animal cells. I. The effect of puromycin on the duplication of DNA. Cancer Res. 1962 Oct;22:1084–1090. [PubMed] [Google Scholar]
- Mamont P. S., Duchesne M. C., Grove J., Bey P. Anti-proliferative properties of DL-alpha-difluoromethyl ornithine in cultured cells. A consequence of the irreversible inhibition of ornithine decarboxylase. Biochem Biophys Res Commun. 1978 Mar 15;81(1):58–66. doi: 10.1016/0006-291x(78)91630-3. [DOI] [PubMed] [Google Scholar]
- McCann P. P., Tardif C., Hornsperger J. M., Böhlen P. Two distinct mechanisms for ornithine decarboxylase regulation by polyamines in rat hepatoma cells. J Cell Physiol. 1979 May;99(2):183–190. doi: 10.1002/jcp.1040990204. [DOI] [PubMed] [Google Scholar]
- McCann P. P., Tardif C., Mamont P. S. Regulation of ornithine decarboxylase by ODC-antizyme in HTC cells. Biochem Biophys Res Commun. 1977 Apr 25;75(4):948–954. doi: 10.1016/0006-291x(77)91474-7. [DOI] [PubMed] [Google Scholar]
- Munro G. F., Miller R. A., Bell C. A., Verderber E. L. Effects of external osmolality on polyamine metabolism in HeLa cells. Biochim Biophys Acta. 1975 Dec 5;411(2):263–281. doi: 10.1016/0304-4165(75)90306-2. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Conover C., Wrona A. Effects of aliphatic diamines on rat liver ornithine decarboxylase activity. Biochem J. 1978 Mar 15;170(3):651–660. doi: 10.1042/bj1700651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piik K., Pösö H., Jänne J. Reversible inhibition of rat liver regeneration by 1,3-diamino-2-propanol, an inhibitor of ornithine decarboxylase. FEBS Lett. 1978 May 15;89(2):307–312. doi: 10.1016/0014-5793(78)80243-9. [DOI] [PubMed] [Google Scholar]
- Prouty W. F. Ornithine decarboxylase inactivation in HeLa cells. J Cell Physiol. 1976 Sep;89(1):65–76. doi: 10.1002/jcp.1040890107. [DOI] [PubMed] [Google Scholar]
- Sunkara P. S., Rao P. N., Nishioka K. Putrescine biosynthesis in mammalial cells: essential for DNA synthesis but not for mitosis. Biochem Biophys Res Commun. 1977 Feb 7;74(3):1125–1153. doi: 10.1016/0006-291x(77)91635-7. [DOI] [PubMed] [Google Scholar]


