Abstract
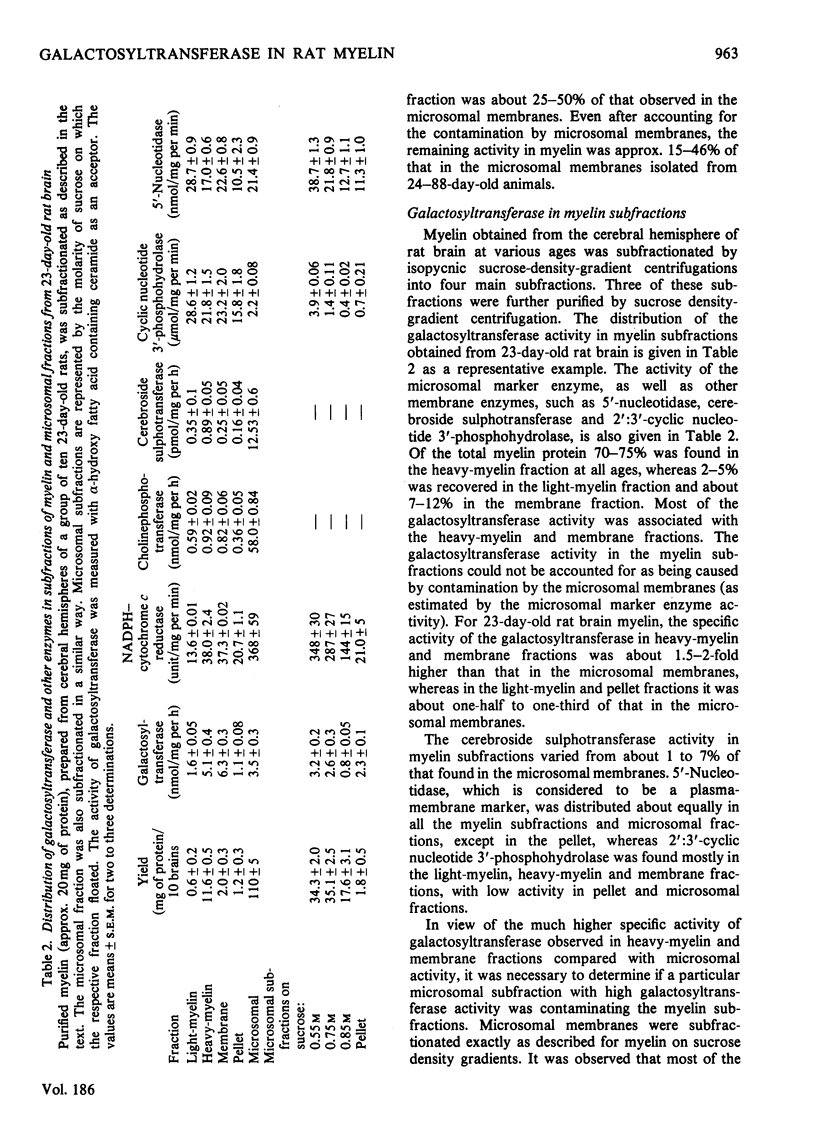
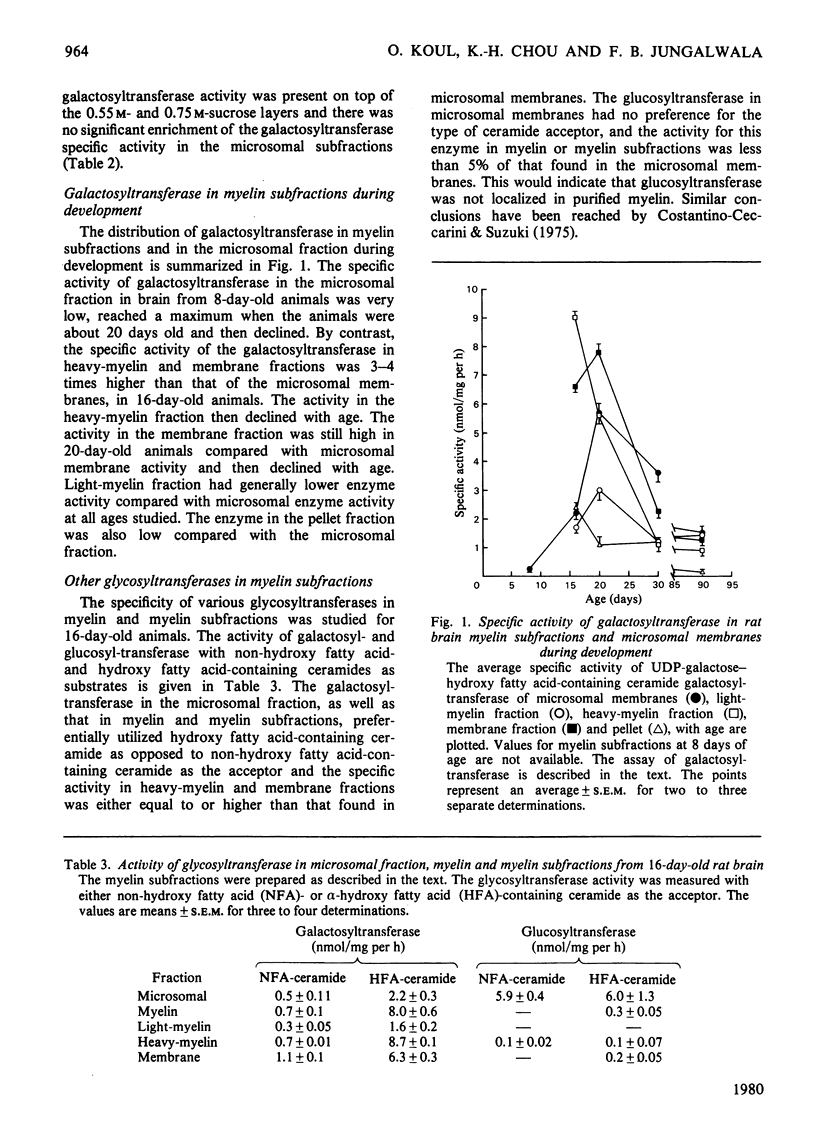
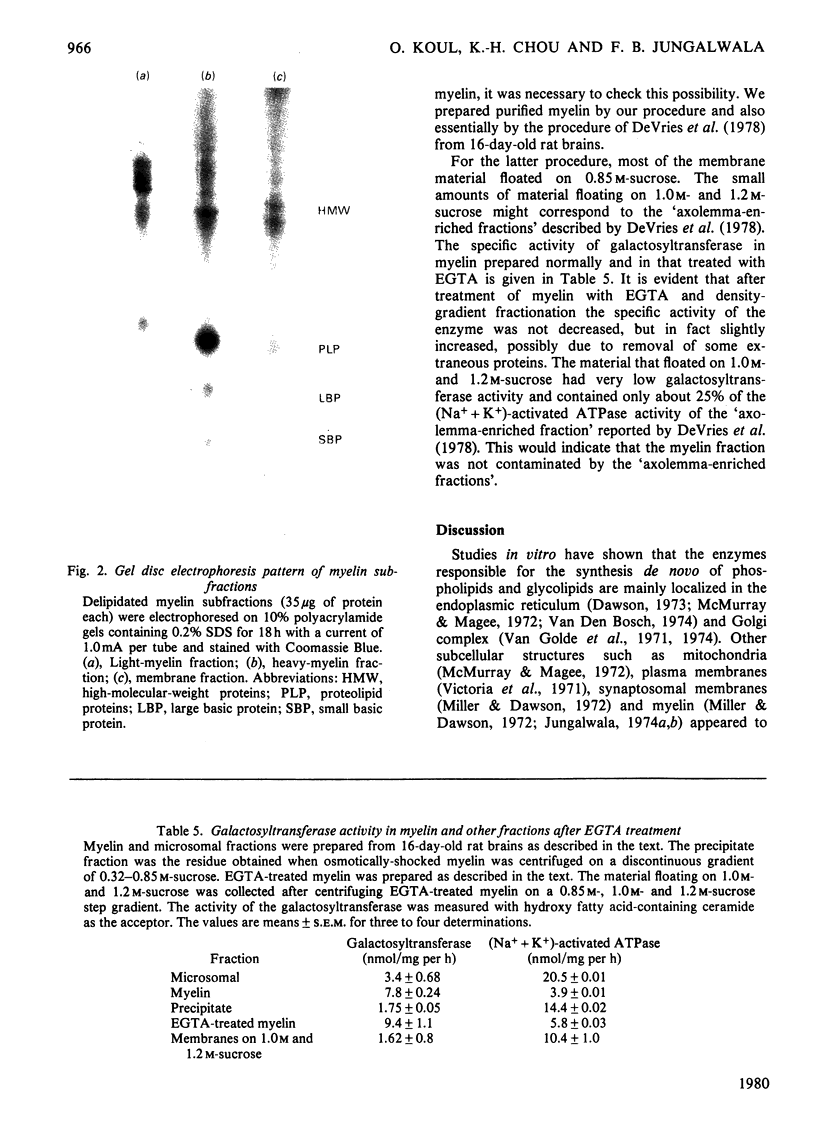
The localization and activity of the enzyme UDP-galactose-hydroxy fatty acid-containing ceramide galactosyltransferase is described in rat brain myelin subfractions during development. Other lipid-synthesizing enzymes, such as cerebroside sulphotransferase, UDP-glucose-ceramide glucosyltransferase and CDP-choline-1,2-diacylglycerol cholinephosphotransferase, were also studied for comparison in myelin subfractions and microsomal membranes. The purified myelin was subfractionated by isopycnic sucrose-density-gradient centrifugation. Four myelin subfractions, three floating respectively on 0.55 M- (light-myelin fraction), 0.75 M- (heavy-myelin fraction) and 0.85 M-sucrose (membrane fraction), and a pellet, were isolated and purified. At all ages, 70--75% of the total myelin proteins was found in the heavy-myelin fraction, whereas 2--5% of the protein was recovered in the light-myelin fraction, and about 7--12% in the membrane fraction. Most of the galactosyltransferase was associated with the heavy-myelin and membrane fractions. Other lipid-synthesizing enzymes studied appeared not to associate with purified myelin or myelin subfractions, but were enriched in the microsomal-membrane fraction. During development, the specific activity of the microsomal galactosyltransferase reached a maximum when the animals were about 20 days old and then declined. By contrast the specific activity of the galactosyltransferase in the heavy-myelin and membrane fractions was 3--4 times higher than that of the microsomal membranes in 16-day-old animals. The specific activity of the enzyme in the heavy-myelin fraction sharply declined with age. Chemical and enzymic analyses of the heavy-myelin and membrane myelin subfractions at various ages showed that the membrane fraction contained more proteins in relation to lipids than the heavy-myelin fraction. The membrane fraction was also enriched in phospholipids compared with cholesterol and contrined equivalent amounts of 2':3'-cyclic nucleotide 3'-phosphohydrolase compared with heavy- and light-myelin fractions. The membrane fraction was deficient in myelin basic protein and proteolipid protein and enriched in high-molecular-weight proteins. The specific localization of galactosyltransferase in heavy-myelin and membrane fractions at an early age when myelination is just beginning suggests that it may have some role in the myelination process.
Full text
PDF







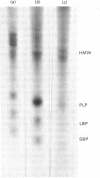
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal H. C., Trotter J. L., Burton R. M., Mitchell R. F. Metabolic studies on myelin. Evidence for a precursor role of a myelin subfraction. Biochem J. 1974 Apr;140(1):99–109. doi: 10.1042/bj1400099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Balázs R., Brooksbank B. W., Patel A. J., Johnson A. L., Wilson D. A. Incorporation of ( 35 S) sulphate into brain constituents during development and the effects of thyroid hormone on myelination. Brain Res. 1971 Jul 23;30(2):273–293. doi: 10.1016/0006-8993(71)90079-5. [DOI] [PubMed] [Google Scholar]
- Barnett R. E. Effect of monovalent cations on the ouabain iniibition of the sodium and potassium ion activated adenosine triphosphatase. Biochemistry. 1970 Nov 24;9(24):4644–4648. doi: 10.1021/bi00826a004. [DOI] [PubMed] [Google Scholar]
- Basu S., Schultz A. M., Basu M., Roseman S. Enzymatic synthesis of galactocerebroside by a galactosyltransferase from embryonic chicken brain. J Biol Chem. 1971 Jul 10;246(13):4272–4279. [PubMed] [Google Scholar]
- Benjamins J. A., Miller K., McKhann G. M. Myelin subfractions in developing rat brain: characterization and sulphatide metabolism. J Neurochem. 1973 Jun;20(6):1589–1603. doi: 10.1111/j.1471-4159.1973.tb00276.x. [DOI] [PubMed] [Google Scholar]
- Brenkert A., Radin N. S. Synthesis of galactosyl ceramide and glucosyl ceramide by rat brain: assay procedures and changes with age. Brain Res. 1972 Jan 14;36(1):183–193. doi: 10.1016/0006-8993(72)90774-3. [DOI] [PubMed] [Google Scholar]
- Costantino-Ceccarini E., Suzuki K. Evidence for presence of UDP-galactose:ceramide galactosyltransferase in rat myelin. Brain Res. 1975 Aug 8;93(2):358–362. doi: 10.1016/0006-8993(75)90359-5. [DOI] [PubMed] [Google Scholar]
- Dawson R. M. The exchange of phospholipids between cell membranes. Subcell Biochem. 1973 Jan;2(1):69–89. [PubMed] [Google Scholar]
- DeVries G. H., Matthieu J. M., Beny M., Chicheportiche R., Lazdunski M., Dolivo M. Isolation and partial characterization of rat CNS axolemma enriched fractions. Brain Res. 1978 May 26;147(2):339–352. doi: 10.1016/0006-8993(78)90844-2. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fry J. M., Weissbarth S., Lehrer G. M., Bornstein M. B. Cerebroside antibody inhibits sulfatide synthesis and myelination and demyelinates in cord tissue cultures. Science. 1974 Feb 8;183(4124):540–542. doi: 10.1126/science.183.4124.540. [DOI] [PubMed] [Google Scholar]
- Fujimoto K., Roots B. I., Burton R. M., Agrawal H. C. Morphological and biochemical characterization of light and heavy myelin isolated from developing rat brain. Biochim Biophys Acta. 1976 Apr 5;426(4):659–668. doi: 10.1016/0005-2736(76)90130-9. [DOI] [PubMed] [Google Scholar]
- Hauser G. Cerebroside and sulphatide levels in developing rat brain. J Neurochem. 1968 Oct;15(10):1237–1238. doi: 10.1111/j.1471-4159.1968.tb06842.x. [DOI] [PubMed] [Google Scholar]
- Hayes L. W., Jungalwala F. B. Synthesis and turnover of cerebrosides and phosphatidylserine of myelin and microsomal fractions of adult and developing rat brain. Biochem J. 1976 Nov 15;160(2):195–204. doi: 10.1042/bj1600195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschkowitz N., McKhann G. M., Saxena S., Shooter E. M. Characterization of sulphatide-containing lipoproteins in rat brain. J Neurochem. 1968 Oct;15(10):1181–1188. doi: 10.1111/j.1471-4159.1968.tb06835.x. [DOI] [PubMed] [Google Scholar]
- Hruby S., Alvord E. C., Jr, Seil F. J. Synthetic galactocerebrosides evoke myelination-inhibiting antibodies. Science. 1977 Jan 14;195(4274):173–175. doi: 10.1126/science.831265. [DOI] [PubMed] [Google Scholar]
- Jungalwala F. B., Dawson R. M. The turnover of myelin phospholipids in the adult and developing rat brain. Biochem J. 1971 Aug;123(5):683–693. doi: 10.1042/bj1230683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungalwala F. B., Hayes L., McCluer R. H. Determination of less than a nanomol of cerebrosides by high performance liquid chromatography with gradient elution analysis. J Lipid Res. 1977 May;18(3):285–292. [PubMed] [Google Scholar]
- Jungalwala F. B. Synthesis and turnover of cerebroside sulfate of myelin in adult and developing rat brain. J Lipid Res. 1974 Mar;15(2):114–123. [PubMed] [Google Scholar]
- Jungalwala F. B. The turnover of myelin phosphatidylcholine and sphingomyelin in the adult rat brain. Brain Res. 1974 Sep 20;78(1):99–108. doi: 10.1016/0006-8993(74)90356-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Latovitzki N., Silberberg D. H. Ceramide glycosyltransferases in cultured rat cerebellum: changes with age, with demyelination, and with inhibition of myelination by 5-bromo-2'-deoxyuridine or experimental allergic encephalomyelitis serum. J Neurochem. 1975 May;24(5):1017–1022. doi: 10.1111/j.1471-4159.1975.tb03671.x. [DOI] [PubMed] [Google Scholar]
- Lees M. B., Paxman S. Modification of the Lowry procedure for the analysis of proteolipid protein. Anal Biochem. 1972 May;47(1):184–192. doi: 10.1016/0003-2697(72)90291-6. [DOI] [PubMed] [Google Scholar]
- Matthieu J. M., Quarles R. H., Brady R. O., Webster H. de F. Variation of proteins, enzyme markers and gangliosides in myelin subfractions. Biochim Biophys Acta. 1973 Dec 5;329(2):305–317. doi: 10.1016/0304-4165(73)90295-x. [DOI] [PubMed] [Google Scholar]
- McMurray W. C., Magee W. L. Phospholipid metabolism. Annu Rev Biochem. 1972;41(10):129–160. doi: 10.1146/annurev.bi.41.070172.001021. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Hawthorne J. N. The site of diphosphoinositide synthesis in rat liver. Biochem Biophys Res Commun. 1965 Nov 22;21(4):333–338. doi: 10.1016/0006-291x(65)90198-1. [DOI] [PubMed] [Google Scholar]
- Miller E. K., Dawson R. M. Exchange of phospholipids between brain membranes in vitro. Biochem J. 1972 Feb;126(4):823–835. doi: 10.1042/bj1260823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell P., Costantino-Ceccarini E., Radin N. S. The biosynthesis by brain microsomes of cerebrosides containing nonhydroxy fatty acids. Arch Biochem Biophys. 1970 Dec;141(2):738–748. doi: 10.1016/0003-9861(70)90192-x. [DOI] [PubMed] [Google Scholar]
- Morell P., Radin N. S. Synthesis of cerebroside by brain from uridine diphosphate galactose and ceramide containing hydroxy fatty acid. Biochemistry. 1969 Feb;8(2):506–512. doi: 10.1021/bi00830a008. [DOI] [PubMed] [Google Scholar]
- Neskovic N. M., Sarlieve L. L., Mandel P. Subcellular and submicrosomal distribution of glycolipid-synthesizing transferases in young rat brain. J Neurochem. 1973 May;20(5):1419–1430. doi: 10.1111/j.1471-4159.1973.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Norton W. T., Poduslo S. E. Myelination in rat brain: changes in myelin composition during brain maturation. J Neurochem. 1973 Oct;21(4):759–773. doi: 10.1111/j.1471-4159.1973.tb07520.x. [DOI] [PubMed] [Google Scholar]
- Pasquini J. M., Gomez C. J., Najle R., Soto E. F. Lack of phospholipid transport mechanisms in cell membranes of the CNS. J Neurochem. 1975 Mar;24(3):439–443. doi: 10.1111/j.1471-4159.1975.tb07659.x. [DOI] [PubMed] [Google Scholar]
- Possmayer F., Kleine L., Duwe G., Stewart-DeHaan P. J., Wong T., MacPherson C. F., Harding P. G. Differences in the subcellular and subsynaptosomal distribution of the putative endoplasmic reticulum markers, NADPH-cytochrome c reductase, estrone sulfate sulfohydrolase and CDP-choline-diacylglycerol cholinephosphotransferase in rat brain. J Neurochem. 1979 Mar;32(3):889–906. doi: 10.1111/j.1471-4159.1979.tb04573.x. [DOI] [PubMed] [Google Scholar]
- Prohaska J. R., Clark D. A., Wells W. W. Improved rapidity and precision in the determination of brain 2',3'-cyclic nucleotide 3'-phosphohydrolase. Anal Biochem. 1973 Nov;56(1):275–282. doi: 10.1016/0003-2697(73)90189-9. [DOI] [PubMed] [Google Scholar]
- Ruenwongsa P., Singh H., Jungalwala F. B. Protein-catalyzed exchange of phosphatidylinositol between rat brain microsomes and myelin. J Biol Chem. 1979 Oct 10;254(19):9385–9393. [PubMed] [Google Scholar]
- Sun G. Y., Horrocks L. A. Metabolism of palmitic acid in the subcellular fractions of mouse brain. J Lipid Res. 1973 Mar;14(2):206–214. [PubMed] [Google Scholar]
- Van Golde L. M., Fleischer B., Fleischer S. Some studies on the metabolism of phospholipids in Golgi complex from bovine and rat liver in comparison to other subcellular fractions. Biochim Biophys Acta. 1971 Oct 12;249(1):318–330. doi: 10.1016/0005-2736(71)90109-x. [DOI] [PubMed] [Google Scholar]
- Vance D. E., Sweeley C. C. Quantitative determination of the neutral glycosyl ceramides in human blood. J Lipid Res. 1967 Nov;8(6):621–630. [PubMed] [Google Scholar]
- Zimmerman A. W., Quarles R. H., de Webster H., Matthieu J. M., Brady R. O. Characterization and protein analysis of myelin subfractions in rat brain: developmental and regional comparisons. J Neurochem. 1975 Dec;25(6):749–757. doi: 10.1111/j.1471-4159.1975.tb04404.x. [DOI] [PubMed] [Google Scholar]
- van Golde L. M., Raben J., Batenburg J. J., Fleischer B., Zambrano F., Fleischer S. Biosynthesis of lipids in Golgi complex and other subcellular fractions from rat liver. Biochim Biophys Acta. 1974 Aug 22;360(2):179–192. doi: 10.1016/0005-2760(74)90168-4. [DOI] [PubMed] [Google Scholar]
- van den Bosch H. Phosphoglyceride metabolism. Annu Rev Biochem. 1974;43(0):243–277. doi: 10.1146/annurev.bi.43.070174.001331. [DOI] [PubMed] [Google Scholar]