Abstract
1. Chloroplasts isolated from spinach leaves by using the low-ionic-strength buffers of Nakatani & Barber [(1977) Biochim. Biophys. Acta. 461, 510–512] had higher rates of HCO3−-dependent oxygen evolution (up to 369μmol/h per mg of chlorophyll) and higher rates of [1-14C]acetate incorporation into long-chain fatty acids (up to 1500nmol/h per mg of chlorophyll) than chloroplasts isolated by using alternative procedures. 2. Acetate appeared to be the preferred substrate for fatty acid synthesis by isolated chloroplasts, although high rates of synthesis were also measured from H14CO3− in assays permitting high rats of photosynthesis. Incorporation of H14CO3− into fatty acids was decreased by relatively low concentrations of unlabelled acetate. Acetyl-CoA synthetase activity was present 3–4 times in excess of that required to account for rates of [1-14C]acetate incorporation into fatty acids, but pyruvate dehydrogenase was either absent or present in very low activity in spinach chloroplasts. 3. Rates of long-chain-fatty acid synthesis from [1-14C]acetate in the highly active chloroplast preparations, compared with those used previously, were less dependent on added cofactors, but showed a greater response to light. The effects of added CoA plus ATP, Triton X-100 and sn-glycerol 3-phosphate on the products of [1-14C]acetate incorporation were similar to those reported for less active chloroplast preparations. 4. Endogenous [14C]acetyl-CoA plus [14C]malonyl-CoA was maintained at a constant low level even when fatty acid synthesis was limited by low HCO3− concentrations. Endogenous [14C]acyl-(acyl-carrier protein) concentrations increased with increasing HCO3− concentration and higher rates of fatty acid synthesis, but were slightly lower in the presence of Triton X-100. It is proposed that rates of long-chain-fatty acid synthesis in isolated chloroplasts at saturating [1-14C]acetate concentrations and optimal HCO3− concentrations may be primarily controlled by rates of removal of the products of the fatty acid synthetase.
Full text
PDF





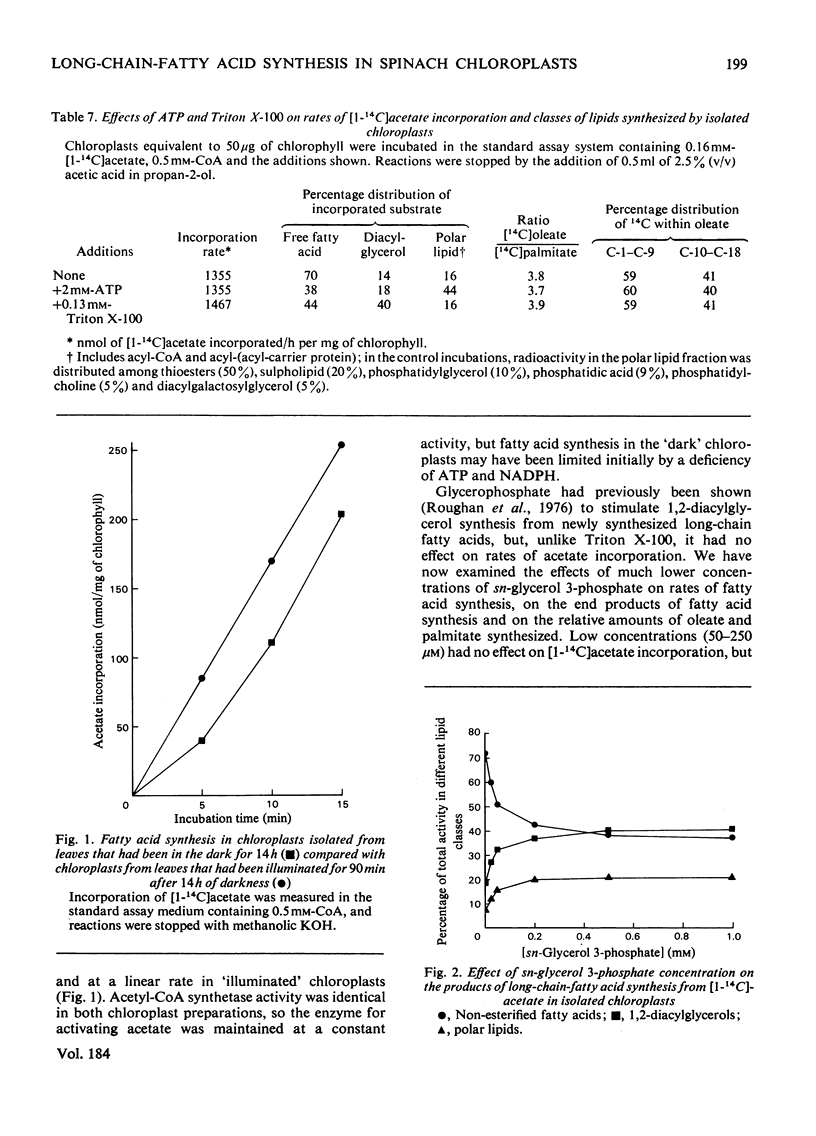
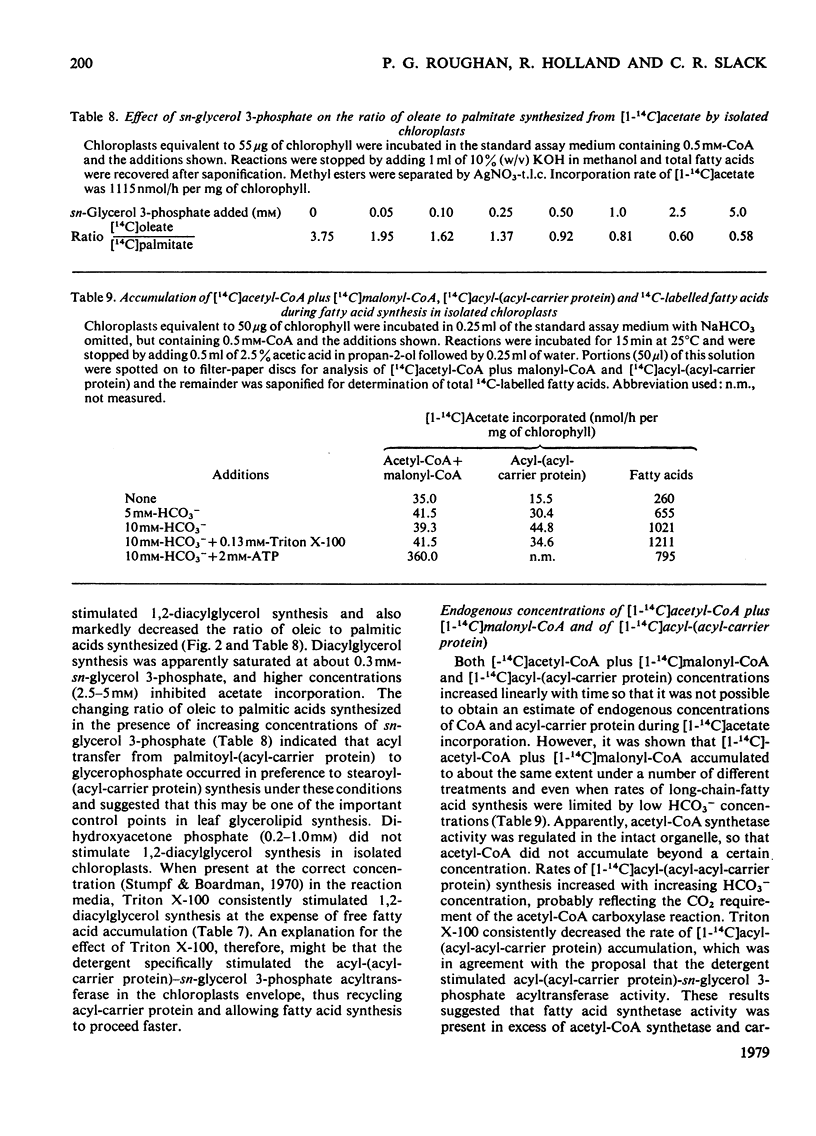


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cockburn W., Walker D. A., Baldry C. W. The isolation of spinach chloroplasts in pyrophosphate media. Plant Physiol. 1968 Sep;43(9):1415–1418. doi: 10.1104/pp.43.9.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R. Site of biosynthesis of galactolipids in spinach chloroplasts. Science. 1974 Mar 1;183(4127):852–853. doi: 10.1126/science.183.4127.852. [DOI] [PubMed] [Google Scholar]
- Downing D. T., Greene R. S. Rapid determination of double-bond positions in monoenoic fatty acids by periodate-permanganate oxidation. Lipids. 1968 Jan;3(1):96–100. doi: 10.1007/BF02530977. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Givan C. V., Stumpf P. K. Fat Metabolism in Higher Plants: XLV. Some Factors Regulating Fatty Acid Synthesis by Isolated Spinach Chloroplasts. Plant Physiol. 1971 Apr;47(4):510–515. doi: 10.1104/pp.47.4.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurr M. I., Brawn P. The biosynthesis of polyunsaturated fatty acids by photosynthetic tissue. The composition of phosphatidyl choline species in Chlorella vulgaris during the formation of linoleic acid. Eur J Biochem. 1970 Nov;17(1):19–22. doi: 10.1111/j.1432-1033.1970.tb01126.x. [DOI] [PubMed] [Google Scholar]
- Jacobson B. S., Jaworski J. G., Stumpf P. K. Fat Metabolism in Higher Plants: LXII. Stearl-acyl Carrier Protein Desaturase from Spinach Chloroplasts. Plant Physiol. 1974 Oct;54(4):484–486. doi: 10.1104/pp.54.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyard J., Douce R. Site of synthesis of phosphatidic acid and diacyglycerol in spinach chloroplasts. Biochim Biophys Acta. 1977 Feb 23;486(2):273–285. doi: 10.1016/0005-2760(77)90023-6. [DOI] [PubMed] [Google Scholar]
- Kaiser W. The effect of hydrogen peroxide on CO2 fixation of isolated intact chloroplasts. Biochim Biophys Acta. 1976 Sep 13;440(3):476–482. doi: 10.1016/0005-2728(76)90035-9. [DOI] [PubMed] [Google Scholar]
- Kannangara C. G., Jacobson B. S., Stumpf P. K. Fat Metabolism in Higher Plants: LVII. A Comparison of Fatty Acid-Synthesizing Enzymes in Chloroplasts Isolated from Mature and Immature Leaves of Spinach. Plant Physiol. 1973 Aug;52(2):156–161. doi: 10.1104/pp.52.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannangara C. G., Stumpf P. K. Fat metabolism in higher plants. I. The biosynthesis of polyunsaturated fatty acids by isolated spinach chloroplasts. Arch Biochem Biophys. 1972 Feb;148(2):414–424. doi: 10.1016/0003-9861(72)90159-2. [DOI] [PubMed] [Google Scholar]
- Kleinig H., Liedvogel B. Fatty acid synthesis by isolated chromoplasts from the daffodil. [14C]Acetate incorporation and distribution of labelled acids. Eur J Biochem. 1978 Feb;83(2):499–505. doi: 10.1111/j.1432-1033.1978.tb12116.x. [DOI] [PubMed] [Google Scholar]
- Mancha M., Stokes G. B., Stumpf P. K. Fat metabolism in higher plants. The determination of acyl-acyl carrier protein and acyl coenzyme A in a complex lipid mixture 1,2. Anal Biochem. 1975 Oct;68(2):600–608. doi: 10.1016/0003-2697(75)90655-7. [DOI] [PubMed] [Google Scholar]
- Nakatani H. Y., Barber J. An improved method for isolating chloroplasts retaining their outer membranes. Biochim Biophys Acta. 1977 Sep 14;461(3):500–512. [PubMed] [Google Scholar]
- Nothelfer H. G., Barckhaus R. H., Spener F. Localisation and characterization of the fatty acid synthesizing system in cells of Glycine max (soubean) suspension cultures. Biochim Biophys Acta. 1977 Dec 21;489(3):370–380. doi: 10.1016/0005-2760(77)90157-6. [DOI] [PubMed] [Google Scholar]
- Reitzel L., Nielsen N. C. Acetyl-CoA carboxylase during development of plastids in wild-type and mutant barley seedlings. Eur J Biochem. 1976 May 17;65(1):131–138. doi: 10.1111/j.1432-1033.1976.tb10397.x. [DOI] [PubMed] [Google Scholar]
- Roughan P. G., Slack C. R., Holland R. High rates of [1-14C]acetate incorporation into the lipid of isolated spinach chloroplasts. Biochem J. 1976 Sep 15;158(3):593–601. doi: 10.1042/bj1580593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Slack C. R. Long-chain acyl-coenzyme A synthetase activity of spinach chloroplasts is concentrated in the envelope. Biochem J. 1977 Feb 15;162(2):457–459. doi: 10.1042/bj1620457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUMPF P. K., JAMES A. T. The biosynthesis of long-chain fatty acids by lettuce chloroplast preparations. Biochim Biophys Acta. 1963 Feb 19;70:20–32. doi: 10.1016/0006-3002(63)90715-7. [DOI] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Schwartz E. R., Old L. O., Reed L. J. Regulatory properties of pyruvate dehydrogenase from Escherichia coli. Biochem Biophys Res Commun. 1968 May 10;31(3):495–500. doi: 10.1016/0006-291x(68)90504-4. [DOI] [PubMed] [Google Scholar]
- Shine W. E., Mancha M., Stumpf P. K. Fat metabolism in higher plants. The function of acyl thioesterases in the metabolism of acyl-coenzymes A and acyl-acyl carrier proteins. Arch Biochem Biophys. 1976 Jan;172(1):110–116. doi: 10.1016/0003-9861(76)90054-0. [DOI] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Balasingham N. Labelling studies in vivo on the metabolism of the acyl and glycerol moieties of the glycerolipids in the developing maize leaf. Biochem J. 1977 Feb 15;162(2):289–296. doi: 10.1042/bj1620289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G. The kinetics of incorporation in vivo of (14C)acetate and (14C)carbon dioxide into the fatty acids of glycerolipids in developing leaves. Biochem J. 1975 Nov;152(2):217–228. doi: 10.1042/bj1520217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf P. K., Boardman N. K. Fat metabolism in higher plants. XXXIX. Effect of adenosine triphosphate and triton X-100 on lipid synthesis by isolated spinach chloroplasts. J Biol Chem. 1970 May 25;245(10):2579–2587. [PubMed] [Google Scholar]
- VONKORFF R. W. PYRUVATE-C14, PURITY AND STABILITY. Anal Biochem. 1964 Jun;8:171–178. doi: 10.1016/0003-2697(64)90043-0. [DOI] [PubMed] [Google Scholar]
- Vick B., Beevers H. Fatty Acid synthesis in endosperm of young castor bean seedlings. Plant Physiol. 1978 Aug;62(2):173–178. doi: 10.1104/pp.62.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B., Beevers H. Phosphatidic Acid synthesis in castor bean endosperm. Plant Physiol. 1977 Mar;59(3):459–463. doi: 10.1104/pp.59.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. A., Baldry C. W., Cockburn W. Photosynthesis by isolated chloroplasts, simultaneous measurement of carbon assimilation and oxygen evolution. Plant Physiol. 1968 Sep;43(9):1419–1422. doi: 10.1104/pp.43.9.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaire P. J., Kekwick R. G. The fractionation of the fatty acid synthetase activities of avocado mesocarp plastids. Biochem J. 1975 Feb;146(2):439–445. doi: 10.1042/bj1460439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaire P. J., Kekwick R. G. The synthesis of fatty acids in avocado mesocarp and cauliflower bud tissue. Biochem J. 1975 Feb;146(2):425–437. doi: 10.1042/bj1460425. [DOI] [PMC free article] [PubMed] [Google Scholar]


