Abstract
The division of a cellular compartment culminates with the scission of a highly constricted membrane neck. Scission requires lipid rearrangements, topology changes, and transient formation of nonbilayer intermediate structures driven by curvature stress. Often, a side effect of this stress is pore-formation, which may lead to content leakage and thus breaching of the membrane barrier function. In single-membrane systems, leakage is avoided through the formation of a hemifusion (HF) intermediate, whose structure is still a subject of debate. The consequences of curvature stress have not been explored in double-membrane systems, such as the mitochondrion. Here, we combine experimental and theoretical approaches to study neck constriction and scission driven by tension in biomimetic lipid systems, namely single- and double-membrane nanotubes (sNTs and dNTs), respectively. In sNTs, constriction by high tension gives rise to a metastable HF intermediate (seen as stalk or worm-like micelle), whereas poration is universally slower in a simple neck. In dNTs, high membrane tension causes sequential rupture of each membrane. In contrast, low tension leads to the HF of both membranes, which may lead to a leaky fusion pathway, or may progress to further fusion of the two membranes along a number of transformation pathways. These findings provide a new mechanistic basis for fundamental cellular processes.
Significance
Biological membranes serve as barriers, delimiting cellular compartments. A key feature of cellular membranes is their ability to change topology through transient remodeling events, fusion or fission, without mixing the compartments’ contents. This work investigates the mechanisms and transformation pathways of membrane fission, particularly double-membrane fission as it occurs in mitochondrial division, focusing on the structure and thermodynamics of intermediate states. We uncover a complex landscape of possible membrane rearrangements during fission, some of which may be deleterious to mitochondria. These developments shed light on fission mechanisms but also open questions about how organelles select from a multitude of possible rearrangement pathways.
Introduction
Membrane fission, a process key to cellular membrane dynamics (1), occurs at the later stages of organelle division when one membrane compartment becomes two. From the physiological point of view, this process should proceed without the loss of contents enclosed by the membrane, preserving the membrane’s barrier function. Therefore, membrane fission is assumed to be a highly localized and controlled cellular process, with phospholipids having the leading role in the leakless remodeling process due to their intrinsic polymorphism (2). Indeed, the essence of membrane fission can be distilled to a tiny lipid neck linking the dividing compartments. In steady state and assuming cylindrical geometry, the neck’s radius is related to the membrane tension, σ, by:
| (1) |
where κ is the bending rigidity set by the membrane composition, and the membrane tension, σ, is dictated by the available membrane reservoir (3). To undergo fission, the neck should first be constricted to a critical radius in the order of magnitude of the thickness of a lipid monolayer (4,5). At this point, a spontaneous lipid rearrangement occurs, leading to the neck’s scission due to the accumulated curvature stress (2). However, such membrane constriction is hardly achievable in free-standing lipid systems, where κ is in the range of a few tens of , whereas . Therefore, the process does not occur spontaneously, but cells use proteins to drive fission in a controlled way.
Experimental and early theoretical approaches showed that the stress-driven lipid rearrangements can follow a leakless fission pathway characterized by sequential disconnection of the inner and outer monolayers of the neck’s membrane (4,6,7,8). The major free-energy barrier here is associated with the formation of a hemifusion (HF) intermediate, closely resembling a fusion stalk, i.e., an hourglass-shaped monolayer structure, which can expand, resulting in a structure identical to a worm-like micelle (WLM). The free-energy barriers for the stalk or WLM formation are lowered by membrane constriction so that these structures readily form in silico models of membrane fission (8,9,10). But, so far, these nonbilayer intermediates have eluded direct experimental observation.
Alternatively, membrane stress has been linked to membrane poration as observed experimentally and in coarse-grained (CG) simulations (11,12,13,14,15). Then the question remains, what are the parameters that push the reaction toward leakless fission or another remodeling pathway. In this sense, membrane tension is a major, yet surprisingly overlooked, parameter to explore. It is a crucial constriction cofactor in protein-driven fission (16,17,18,19,20). It is also a major cause of poration, with high tension known to quickly produce pores and rupture of the lipid bilayer (11,14,15,21,22). How these constructive and destructive faces of tension operate in fission remains largely unexplored. In single-membrane systems, lateral tension or similar bulk forces (such as, e.g., friction-induced tension) can produce substantial membrane constriction, critical for protein-driven fission (20). However, it remains unexplored whether tension alone can drive fission via the HF intermediate all the way in a single-membrane system.
Curiously, despite the general consensus on the fission pathway for single-membrane nanotubes (sNTs), there are virtually no such predictions for remodeling of double-membrane systems, e.g., division of mitochondria or formation of annular gap junctions. The coexistence of two concentric membranes during fission-neck constriction complicates the topological outcome of constriction stress, as direct contact between the inner and the outer membranes cannot be ruled out. Indeed, experimental data suggest that close contact between both bilayers during mitochondrial division is possible as 1) close proximity of the inner and outer membranes of mitochondrial fission neck has been documented in ultrastructural studies (23,24,25,26) and 2) mitochondrial fission machinery and mitochondrial outer-membrane poration are linked through the programmed cell death process, where both have been implicated (27,28). Interestingly, membrane tension has been proposed to play a key role in mitochondrial fission (19). However, the exact remodeling pathway promoted by high membrane tension during mitochondrial division remains unknown.
One of the reasons behind the lack of theoretical and experimental descriptions of double-membrane neck remodeling is the inherent topological complexity of the process. So far, there have been virtually no double-membrane systems in place to systematically study this phenomenon. The methods available to theory vary significantly in the level of detail used to represent membranes. At one extreme, particle-based simulations represent the system as a set of particles (often atoms), which spontaneously assemble into a membrane (9,29,30,31,32,33,34,35,36,37,38). These simulations capture all behaviors exhibited by the membrane, but at an extreme computational cost. Modeling large-scale rearrangements requires huge systems to be simulated for long times and is currently out of reach for these fine-grained simulations.
At the other extreme of membrane modeling, one can ignore the molecular degrees of freedom and consider a membrane itself as an elastic sheet. This gives rise to equation-based free-energy descriptions, such as the Helfrich Hamiltonian, which can be adapted to high curvatures and the effects of lipid tilt and splay in the membrane (39,40,41,42,43,44,45,46). These methods coarse grain the behavior of lipids and proteins into just a few parameters and can easily capture the large-scale behavior of membranes. The ignored molecular details, however, contribute significantly to the free-energy and may become important when modeling topology-altering processes that involve complicated lipid rearrangements. If the membrane itself is the basic structure being considered, as opposed to being emergent, it is challenging to describe nonmembrane intermediate structures, such as WLMs.
There are multiple alternative ways to study the free-energy during membrane rearrangements (47,48,49). One highly successful method for studying membranes and amphiphilic molecules is self-consistent field theory (SCFT). This method considers the statistics of each molecular species and represents molecular interactions using fields, modeling the potential felt by each molecule at a given point in space. The statistics of molecules in these fields are calculated, giving the average local densities, and the field is adjusted until it accurately represents the particle interaction potentials expected from these densities. In addition to being very successful for traditional polymer systems, SCFT has already proven useful for studying lipid membranes (22,26,50,51,52,53,54,55,56,57). We will employ SCFT to study membrane fission.
Although SCFT has been highly successful, being a mean-field theory, it lacks some details available to particle-based simulations. 1) Particle-based simulations include fluctuations, which may affect the equilibrium behavior, 2) these fluctuations can allow the system to explore configuration space and discover alternative pathways, and 3) the transition pathway from SCFT contains no timescale, whereas particle-based simulations give access to actual dynamics. For this reason, we use particle-based simulations to validate our SCFT results.
Regardless of the calculation or simulation method, changes in membrane topology during double-membrane fission can involve complicated rearrangements. Tracking the progress of these changes can be challenging, as they are often not amenable to a simple reaction coordinate. The string method offers an elegant solution (34,35) employing a reaction coordinate in terms of changes of the local density as the transformation progresses. A path in density configuration space is then locally optimized, converging to the most likely transition mechanism, the minimum free-energy path (MFEP). The string method has proven useful for obtaining the MFEP in various polymer and membrane systems (22,26,36,37,38,45,58,59,60).
In a recent publication, we combined new experimental and theoretical approaches for the ultrastructural characterization of sNTs and double-membrane nanotubes (dNTs) and discovered new pathways for the fission of membrane tubes, catalyzed by transient fusion events (26). This follow-up study expands upon this work, presenting a detailed description of single-membrane fission and expanding the characterization of double-membrane fission to explore a variety of possible fission pathways available to double-membrane tubes. We use SCFT of amphiphilic molecules to model the fission of single and double NTs under tension. Combining SCFT with the string method, we find the MFEPs connecting stable and metastable membrane configurations. Our results are then validated using a specifically developed cryoelectron microscopy (cryo-EM) methodology alongside particle-based simulations. Taken together, our findings provide new mechanistic insights into the process of membrane fission, particularly for double-membrane systems.
Materials and methods
Our experimental and theoretical methods closely follow those described in (26). The methods are described below and further details can be found in the supplemental information of (26).
Experimental methods
Formation of NTs for cryo-EM observations
Upon mixing 99:1 mol % of POPC and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B silfonyl) (ammonium salt) stocks in chloroform to a final 1 g/L concentration, the chloroform was evaporated under vacuum. The lipid lamellae were then hydrated in 1 mM HEPES (pH 7.0), resulting in the formation of multilamellar vesicles. A 0.4-μL drop of the multilamellar vesicle solution was placed on the edge of the carbon-coated side of a glow-discharged Quantifoil R 2/2300 mesh copper grid, and dried again under vacuum for 30 min. The grid, held with forceps near the lipid reservoir, was placed in a Leica EM GP2 cryoplunger, maintained at 10°C and 90% relative humidity. Next, 3–4 μL of 1 mM HEPES (pH 7.0) was added to the grid from the carbon-coated side. The lipids were allowed to rehydrate for 5 min. After incubation, the liquid was removed by blotting with an absorbent filter paper (Ø55 mm, Grade 595, Hahnemühle) on the grid edge opposite to the lipid reservoir for 3 s. Subsequently, the grid covered with the “ultrathin liquid film” (100–300 nm thickness) was abruptly plunged into liquid ethane.
Cryo-EM observation of the NT specimens
Once the specimen was frozen, the vitrified grids were maintained in liquid nitrogen and visualized on a JEOL JEM-2200FS/CR, equipped with a field emission gun operated at 200 kV and an in-column energy filter. During imaging, no-tilted zero-loss two-dimensional (2D) images were recorded under low-dose conditions, utilizing the “minimum dose system (MDS)” of Jeol software, with a total dose on the order of 30–40 electrons per exposure, at defocus values ranging from 1.5 to 4.0 μm. The in-column Omega energy filter of the microscope helped us to record images with improved signal/noise ratio by zero-loss filtering, using an energy selecting slit width of 20 eV centered at the zero-loss peak of the energy spectra. Digital images were recorded in linear mode on a 3840 × 3712 (5 μm pixels) Gatan K2 Summit direct detection camera (Gatan, Pleasanton, CA, USA) using DigitalMicrograph (Gatan, Pleasanton, CA, USA) software, at nominal magnifications of 2000× and 25,000× with pixel sizes of 1.6 and 0.154 nm, respectively. Images were subsequently treated and analyzed using ImageJ software (61).
Theory
In aqueous environments, amphiphilic lipid molecules spontaneously organize into double-layered membranes. We model each lipid molecule as an AB diblock copolymer with segments of type A (tail) and segments of type B (head). The surrounding medium, generally water, is represented by a simple homopolymer consisting of segments of type B. We employ a flexible Gaussian chain model for the molecules, assigning each segment a statistical length of b and a volume of . The natural end-to-end length of a lipid molecule is represented by , which serves as the unit of length in our analysis.
A system of volume contains lipids and solvent molecules, each consisting of N and segments, respectively. We set and . Interactions between A and B segments are described by a fixed Flory-Huggins parameter, . Our SCFT calculations are performed in the semigrand canonical ensemble, where we fix the exchange chemical potential, allowing and to vary.
SCFT
The structure and thermodynamics of our lipid solution is calculated using SCFT (62,63), the implementation of which follows (26,64). Molecules are modeled as Gaussian chains with the position along the molecule parameterized by t, where for the lipids and for the solvent molecules. Molecular statistics are calculated using partial partition functions, or propagators, represented by , where or for the lipid or solvent, respectively. The propagator satisfies
| (2) |
where is a potential field felt by a γ-type segment. For a system with two types of segments, A and B (i.e., or B) it is convenient to write the fields in terms of a segregation field, and a pressure field, . Whereas the fields and are conjugate to the (dimensionless) segment concentrations and , the new fields are conjugate to the local segregation, , and total concentration , respectively.
The lipid tail groups () are labeled A type, and the headgroups () and water are both B type. is calculated by integrating Eq. 2 with uniform initial conditions, as the ends of the molecules are unconstrained. We also require a “back” propagator, , which is similar, but is solved with one side of Eq. 2 multiplied by −1, as we are integrating from the other end of the molecule. The total partition functions for the lipids and solvents are
| (3) |
| (4) |
Given a particular set of field configurations, , the product of the forward and back propagators, , gives the Boltzmann-weighted number of ways to place segment t at spatial position and is therefore proportional to the average concentration of segment t. The average dimensionless concentrations of each type of segment are found by scaling this product by the fugacity, , and integrating over the relevant parts of the molecule
| (5) |
| (6) |
| (7) |
The total B concentration is thus , and the total lipid concentration is . In the usual manner of SCFT, we calculate the following local exchange chemical potentials, which act on the segregation and total concentration, respectively,
| (8) |
These local exchange chemical potentials drive the system to change configuration, thus stable and metastable configurations occur when they vanish. SCFT is done by initializing the system with some initial fields, and , and relaxing the system toward by updating the fields by an amount proportional to the exchange chemical potentials. We are typically satisfied with a root mean-squared error of .
The grand canonical free-energy, F, is then calculated using
| (9) |
where is the invariant polymerization index. Once we have obtained stable or metastable structures, we compare the free energies to determine the relative stability of different phases. The quantity depends on specific system parameters, such as the molecular weight of the lipids and the density of statistical segments. In practice, we treat the energy scale, , as a fitting parameter and match to experiments by comparing known quantities. A convenient choice is the membrane bending modulus, κ.
We typically solve Eq. 2 using a pseudospectral method (65) with 60 steps along the lipids and 6 along the homopolymers (solvent). We typically use a 3D grid of dimensions and discretization of to represent the concentrations and fields. For the cylindrically symmetric phases, however, we can greatly decrease computation time by using a 2D grid and a cylindrical coordinate system.
String method
To study transitions between stable and metastable structures, we use the string method (34,35,66,67,68,69,70). First, we must describe the system in configuration space: each dimension of this space corresponds to the state of the system (given by ) at the point . The state of the system is given by a point (or vector) in this high-dimensional space, and its evolution is described by motion through the space. The set of local exchange chemical potentials at all points, i.e., the function , corresponds to a vector in this space and drives this transformation along its evolution path. The string method seeks a path that is tangent to , as this corresponds to a (thermodynamically reversible estimate for) the most probable pathway between metastable states given by the MFEP connecting configurations (34,35).
A path between two states is discretized along the reaction coordinate into m configurations indexed by i in the range . Each point in configuration space is described by its segregation field , with incompressibility enforced ( equilibrated) independently. This allows us to define a reaction coordinate, α, determined by calculating the Euclidean distance between adjacent points on the string and normalizing the total string length to 1.
The ends of the string and are updated as described above; however, we only update the intermediate positions perpendicularly to the path of the string to find a path with tangent vector . This involves defining a vector parallel to the string, . We find by fitting pointwise along the α direction using a cubic spline. We then calculate the portion of parallel to ,
| (10) |
where with . Notice that the spatial integral is a scalar product in configuration space. We then subtract from , resulting in the perpendicular component,
| (11) |
In addition to the perpendicular updates, we restrain the points to be equidistant in configuration space by shifting them along the path using the aforementioned fit. The update procedure can in fact be done using the entire local exchange chemical potential (not simply the perpendicular component) followed by redistributing the points; however, the perpendicular update provides a more direct update and a far better estimate of the degree of convergence. The completely converged string satisfies ; however, due to the computationally intensive nature of these calculations, we are typically satisfied with a root mean-squared error of .
The MFEP connecting one (meta)stable state (local free-energy minimum) to another passes through a saddle-point in the free-energy, where . The MFEP from the saddle-point to a metastable state corresponds to a simple “downward” motion, i.e., . The first proportionality is true when is parallel to the path, i.e., when . The MFEP connecting two metastable states may be direct or include other intermediate metastable states, and thus multiple saddle-points (barriers).
Particle simulations
In addition to the mean-field calculations conducted with SCFT and the string method, we also employ particle-based simulations using the versatile and well-tested soft coarse-grained Monte-Carlo acceleration (SOMA) simulation package (71). This allows us to compare our SCFT predictions with particle-based simulation results, which include fluctuations and do not assume that molecular configurations relax quickly as compared with densities. Simulations also allow us to extract a timescale, which is absent in SCFT calculations. The particle-based simulations are conducted using . This is in the experimentally relevant regime, as judged by comparing our bending energy, , with the experimental value of (72). We use a Helfand compressibility energy
| (12) |
where we choose . This large value of is used to approximate the incompressible system considered in SCFT.
Results
We start with the simple case of sNT fission, comparing theoretical and experimental results. Then we investigate how a second NT complicates the topological transition in dNT fission, as dNTs offer many more degrees of freedom. As a result, we explore many possible rearrangements as steps in an overall pathway and then compare the free-energy barriers of each step to conclude the most likely pathway to fission. The preferred pathway is finally presented as a function of membrane tension.
We use the membrane tension as a control parameter in the theoretical calculations, as it is a simple way to implement constriction in tubular membranes. The tension and free-energy are given in units of the bending energy of a bilayer, , and bilayer thickness, , where measures the density of lipid molecules, and is the average end-to-end length of the lipid molecule. The bending energy and bilayer thickness depend on the lipid but, for ease of comparison, a purely POPC membrane used in the experiments throughout this work is characterized by (at ) (4,54) and has a thickness of nm (72). The unit conversion factor is thus simply (see supporting material).
Detailed results are shown for tensions of and . These are on the high end of physiological tensions, (73,74); however so is, presumably, the membrane tension at the constriction site during a fission event. All pathways that we have found available at low tension are also available at higher tension, but the reverse is not true. We therefore use key images during paths at as exemplars, highlighting where reducing the tension leads to differences. Summary data show a full range of tensions, down to almost zero.
Single-membrane tube fission
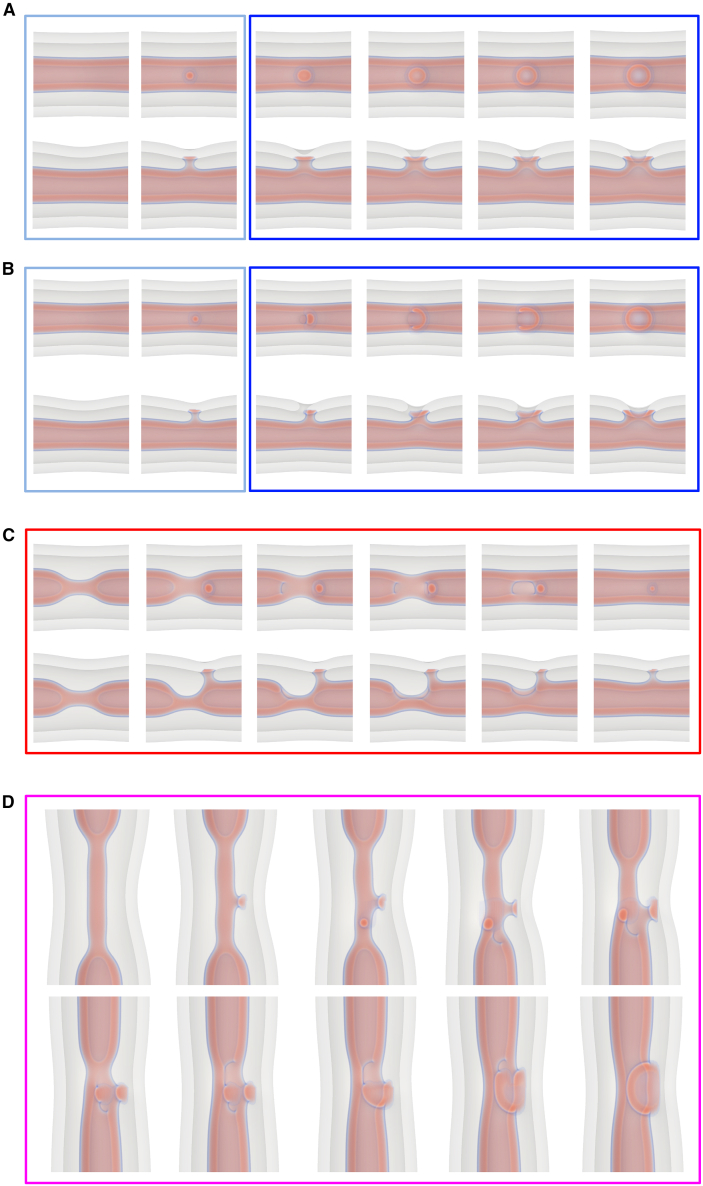
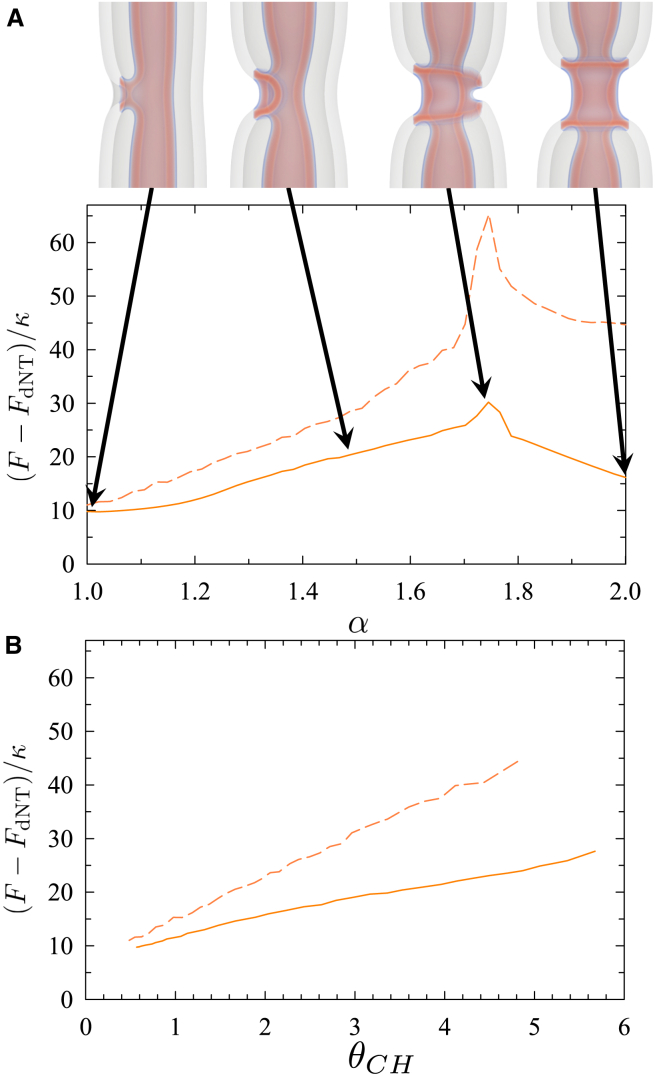
Our first step is to reconstruct the remodeling pathway of NTs under tension with experimental data and theoretical predictions. The free-energy along the MFEP, , of sNT fission is presented in Fig. 1 A. Our calculations indicate that, before fission occurs, the sNT constricts locally, forming an HF intermediate, where the free-energy peaks. The HF intermediate then expands into a metastable WLM, connecting two capped NTs. The WLM subsequently constricts and disconnects close to one of the caps, where the free-energy peaks again. Finally, the WLM retracts into one of the capped NTs, which recedes due to membrane tension.
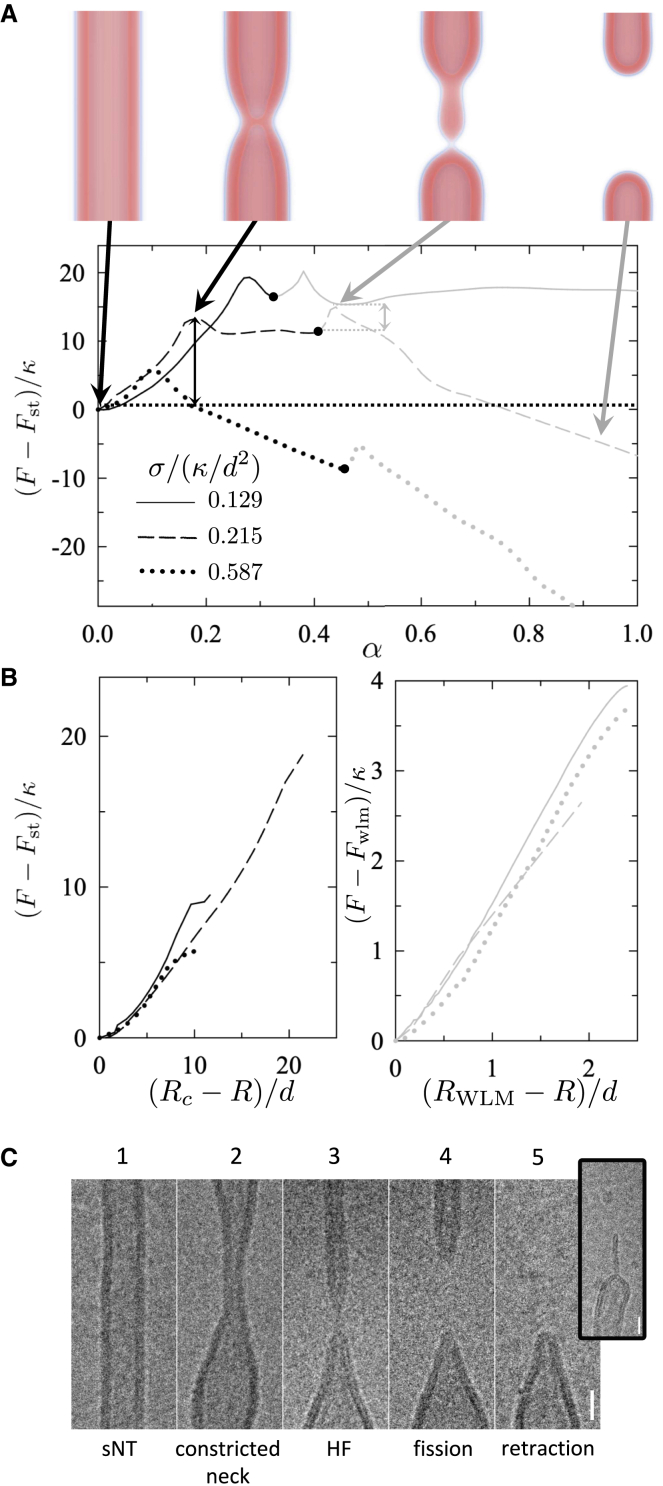
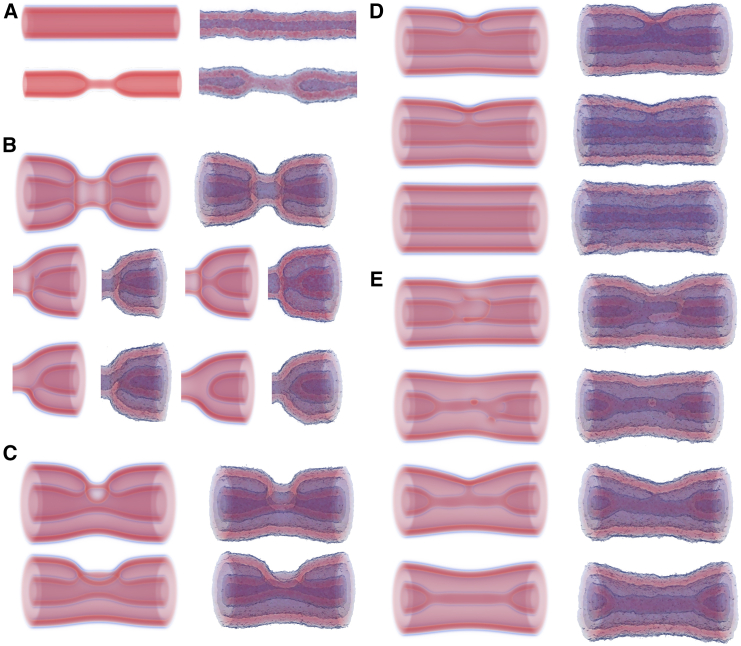
Figure 1.
Pathway of sNT remodeling during scission. (A) Free-energy (relative to that of a sNT, ) along the MFEP during sNT fission. Graphs are shown for (solid) (1.3 mN/m for a POPC membrane), (dashed) (2.1 mN/m), and (dotted) (5.8 mN/m). Images depict lipid densities at characteristic points along the pathway. A sNT divides into two capped tubes, moving apart and exiting the system. The final state still contains two capped tubes. Transition barriers are indicated by vertical arrows for the middle (solid) curve. (B) Fission is divided into two steps (left) constriction and (right) WLM fission, and free energies are shown as functions of calculated order parameters, discussed in the text. The order parameters are only calculated (left) when the tube is constricting and (right) when the WLM is thinning, i.e., in the increases leading to the maxima. (C) Cryo-EM snapshots of main sNTs remodeling steps during fission upon increase of membrane tension ( mN/m). Scale bars, 20 nm. Step 1 corresponds to the sNTs. Constriction of the NT is seen in step 2. At step 3, the NT partially collapses forming an HF/WLM intermediate. The inset shows an elongated WLM sometimes forming at this step. Membranes disconnect at step 4 and retraction to the reservoir is observed in step 5.
Using α as the reaction coordinate captures the cumulative change in the spatial distribution of lipid tails throughout the transformation, but it is not easy to interpret as its correspondence to intuitive descriptions of the system changes along the transformation process. During constriction, α reflects changes in tube radius, then the length of the WLM as it grows. During WLM rupture, α reflects its thickness at the rupture site. Finally, α reflects the distance between capped tubes as they retract. The ability of the MFEP to identify a suitable reaction coordinate, α, makes it ideal to simulate complicated transformations but, when it comes to interpretation, it is useful to calculate coordinates reflecting changes that occur at specific parts of the transformations.
One useful reaction coordinate is the local tube radius, , where r is the distance from the tube axis. R is calculated by evaluating the integral over a plane normal to the tube axis and passing through the constriction site. For an equilibrium tube, this gives the tube radius, , and for a WLM it is . During fission, we find the minimum value of R at the constriction site. In the case of tube constriction this is the radius of the constricted tube, whereas for fissioning WLM, it measures the thickness of the WLM at the fission site. The free-energy of the unperturbed tube is, of course, a local minimum; thus, as seen in Fig. 1 B, the free-energy at small depends quadratically thereon. The quadratic behavior quickly gives way to an approximately linear dependence, with a slope (constriction force) that does not significantly depend on tension. This suggests that the main source of the barrier comes from the degree to which the tube must be constricted, i.e., the equilibrium tube radius, and also that a constant force is required to constrict the tube, independent of radius. It is important to note that this is a local constriction and is markedly different from a global constriction brought about by, for example, tension in the membrane.
To confirm our theoretical predictions experimentally, we developed a method that allows the formation and ultrastructural observation of lipid NT geometry by cryo-EM. Moreover, as the transitional states are highly unstable, and thus difficult to detect in stationary experimental conditions, we cryopreserved NTs right upon inducing NT fission by hydrodynamic flow. Such quasidynamic conditions rendered snapshots of the main stages of the NT fission process (Fig. 1 C) that further confirm our predictions. We find that extreme constriction of the membrane neck (Fig. 2 C, step 2) (4) is followed by its collapse into a stalk or WLM structure (Fig. 1 C, step 3). We also detected the predicted membrane retraction upon rupture of the HF intermediate. (Fig. 1 C, steps 4 and 5).
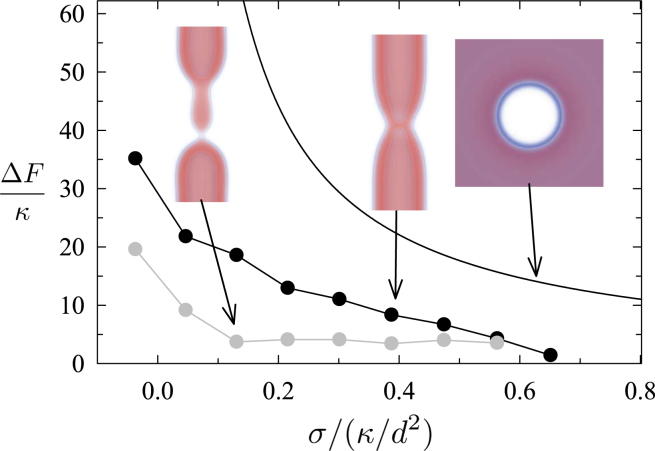
Figure 2.
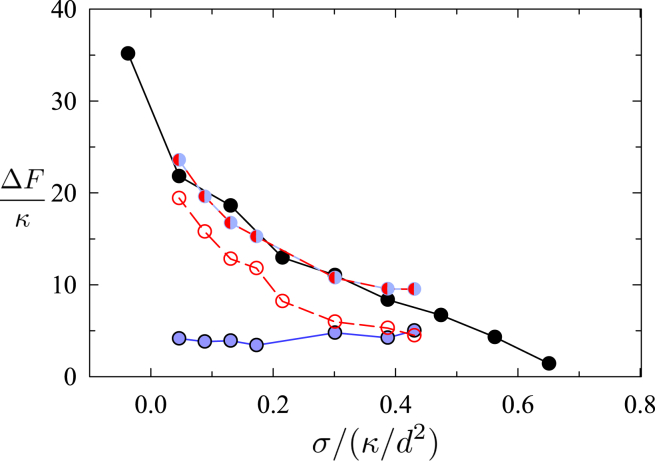
Free-energy barriers are shown for the (black points) first and (gray points) second barriers to sNT scission (see Fig. 1). Also shown is (curve) the barrier to pore formation (, where γ is the line tension of a bilayer edge) calculated from classical nucleation theory.
Interestingly, the probability of the formation of the WLM structure depends on the initial membrane tension. At high membrane tension (dotted curve in Fig. 1 A), the extension of the WLM becomes favorable, as its free-energy, , is smaller than that of the sNT, (see the supporting material for further discussion). Indeed, in our dynamic experimental conditions, where membranes are subjected to high tension, we could observe extended WLMs even after fission occurred (see Fig. 1 C, supporting material, and (26)). For intermediate tension, close to that of the constricted neck (dashed curve in Fig. 1 A), . At low tension (solid curve in Fig. 1 A), the WLM becomes unfavorable as . In this case, the WLM breaks without extending significantly.
In our calculations done at high tension, where , the system can lower its free-energy by elongating the collapsed region. This is apparent from the negative slope seen in the dotted curve of Fig. 1 A as the WLM grows, and continues to be true for arbitrarily extended WLMs. It therefore does not reach a true minimum before fission, but rather WLM fission constitutes a jump over a barrier in a direction nonparallel (in configuration space) to WLM elongation. In practice, this suggests that, at high tension, the gradual conversion of tube into WLM will be randomly interrupted by the fission of the WLM at one of its connection sites.
Of note, high tension in membranes is usually associated with pore formation. Therefore, we address this possibility. Fig. 2 shows that the barrier to pore formation in single membranes is much larger than the free-energy of the leakless fission pathway presented above. However, the poration barrier in Fig. 2 may be overestimated, as we approximated it using the classical nucleation theory in a planar membrane. We decided to corroborate this result by 1) manually inserting pores into the sNT fission sequences near sites of high membrane curvature (e.g., the WLM-membrane fusion site) and 2) creating a fission pathway that starts from a nucleating pore, which “unzips” around the membrane, leading to fission. In both cases, as the string relaxed (i.e., the pathway evolved from our artificial initial state to an optimal mechanism), the pores closed and the sequence converged to the fission mechanism described above. Hence, our results suggest that, in sNTs, fission would preferentially proceed without pores even under relatively high membrane tension. See supporting material for further details.
While formation of a pore is a costly process and not an optimal or expected pathway, in real biological systems, one cannot completely exclude membrane poration as part of the fission mechanism. If an external agent, e.g., a protein, stabilizes a pore at the initial configuration, fission may still progress through the leaky unzipping pathway (see supporting material for further details). This does not preclude the possibility of fission via poration from occurring, but it is not an optimal path.
Double-membrane tube fission
While single-membrane systems undergo fission via the HF intermediate, the first systematic studies about the remodeling paths of double-membrane systems occurred only recently (26). Given the evidence provided therein for the role of intermembrane fusion in membrane fission, and the above success in describing the fission of sNTs, we turned to in silico calculations to find the possible pathways for dNTs remodeling in different membrane-tension regimes.
Sequential fission pathway
The first case we consider is the one of sequential fission of first the inner and then the outer bilayer of a double-membrane system (Fig. 3). The inner NT proceeds to HF, which extends into a WLM and then ruptures and retracts. Both retracting capped NTs remain inside the outer NT until it undergoes fission in a similar way. Free-energy calculations, similar to those presented above, show that the barrier to inner NT rupture is not significantly affected by the presence of the outer NT (the difference is small on the scale of free energies presented) (26). Importantly, this pathway is leakless, as in the case of sNTs discussed above.
Figure 3.
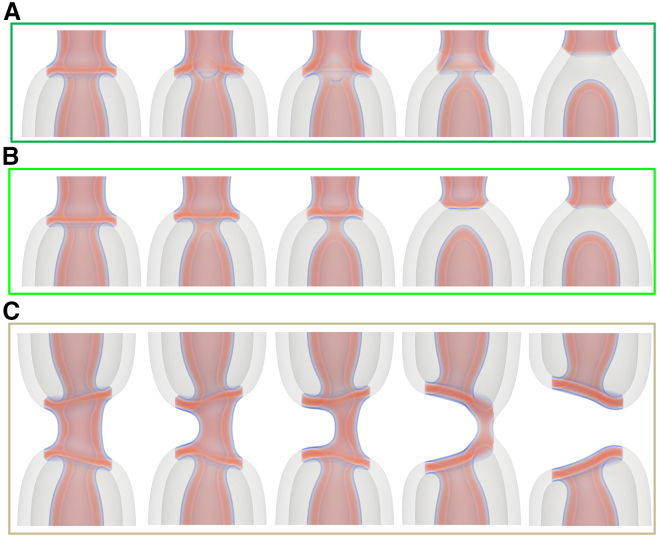
Rupture of the inner tube directly, by way of a WLM intermediate at (top: A and B) and (bottom: C and D) . This pathway is functionally the same as the sNT pathway presented earlier: (left: A and C) the inner NT locally constricts, forms a WLM that elongates only at high tension, i.e., (A); then (right: B and D) the WLM thins and ruptures, resulting in two capped tubes. For the elongated WLM in (A), WLM rupture occurs at one of the connection sites (B), followed by WLM retraction, whereas the short stalk-like WLM in (C) simply ruptures in the middle (D). See also Video S1. The outer tube has been made transparent.
Pathways to intermembrane fusion
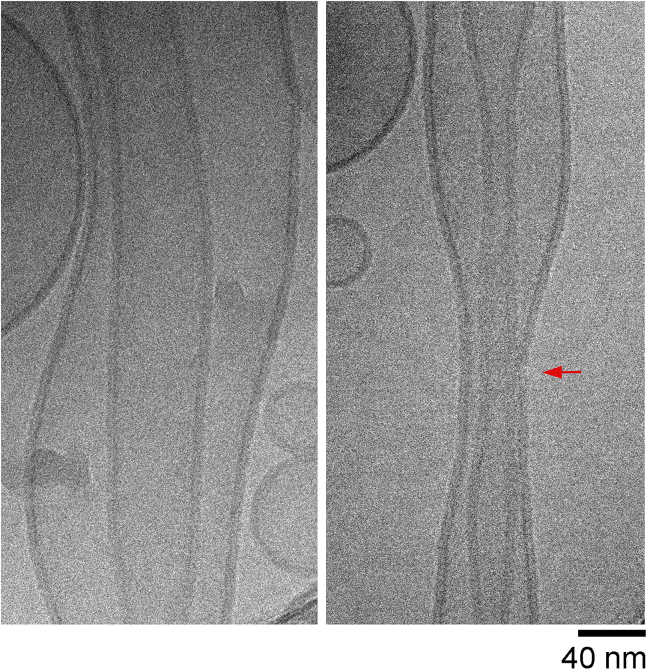
Next we explore the landscape of possibilities that include intermembrane contact. Evidence of intermembrane contact has been presented in (26) and is recapitulated in Fig. 4.
Figure 4.
Cryo-EM snapshots of dNT constriction at low (left) and (right) higher membrane tension. The red arrow points to close proximity (<2 nm) of the inner and outer membrane of the dNT upon tension-induced constriction. Reproduced from (26) under the terms of the Creative Commons Attribution License.
In the simplest case, the inner and outer NTs would fuse—direct-HF pathway. This is illustrated in Fig. 5, A and B (see also Videos S2 and S3). The first step here is the formation of a metastable stalk—i.e., a hydrophobic bridge—between both membranes. At this point we find the first bifurcation in the pathway. The stalk can simply widen (i.e., radially expand) and flatten (Fig. 5 A), forming a metastable hemifusion diaphragm (HD), as has been seen previously (50). Alternatively, the presence of the stalk can lead to a thinning of the surrounding membrane, which then heterogeneously nucleates a pore in the outer (Fig. 5 B) or inner membrane (see supporting material). The connection initiated by the stalk then expands around the pore, as in fusion studies (75,76). In either case, the final topology is the same: an HD connecting the inner and outer membranes on one side of the dNT.
Figure 5.
Intermediate configurations for the fusion of an inner and outer membrane at a tension of . Four mechanisms are shown: (A) direct hemifusion via a stalk connecting the membranes, (B) fusion via a pore in the outer membrane, (C) hemifusion via a WLM intermediate, and (D) a more complicated pathway to the hemifused state from the WLM intermediate as first described in (26). (A–C) Show the same configurations from two perpendicular views. The WLM at the start of (C) and (D) forms according the same mechanism shown previously for a sNT and in Fig. 3. In each case, the outer tube has been made transparent, to emphasize rearrangements of the inner tube.
As illustrated in Fig. 5, A and B, showing the direct-HF pathway, the steps presented in this section can often occur with small variations, such as the transient pores mentioned above. Rather than trying to discuss each possible variation, we combine sets of potential steps, which are very similar in terms of the actual rearrangement as well as their free-energy barriers (differences typically ). We consider, for example, the above to be a direct HF, which may or may not include transient pores. Potential variations will be mentioned where relevant and are discussed further in the supporting material. For stalk formation, α reflects the number of lipids in the stalk, as it tracks changes in local lipid number.
Intriguingly, there is an alternative pathway to HF, via the collapsed state—the WLM pathway. We first presented this result in (26) but expand upon it here. Variations of this pathway are illustrated in Fig. 5, C and D, respectively, and in Videos S3 and S4. The simpler of these, shown in Fig. 5 C, is the reverse of the fission-by-fusion pathway presented in (26), calculated here at higher tension. It proceeds as follows: 1) the WLM bends toward the outer membrane, 2) a stalk forms, connecting the inner and outer membranes, 3) the stalk destabilizes the inner membrane and a pore forms, 4) the other capped NT forms a pore resulting in a dNT with a pore on the inner membrane and a stalk connecting the membranes, and 5) the pore then closes, resulting in a dNT connected by a stalk. This final structure is topologically equivalent to the hemifused states at the end of the other processes. The stalk may then widen to form an HD, similarly to the process in Fig. 5 A. Although framed as fusion pathways, the paths produced by the string method are reversible, and they may equally be seen as pathways from the hemifused to the collapsed state.
At high tension, the stable WLM intermediate allows for a more complicated path (illustrated in Fig. 5 D): 1) the inner NT first collapses into a WLM as in the sNT system, and the outer membrane curves inward, closer to the equilibrium radius of a sNT, 2) this inward bend facilitates the formation of a stalk, connecting the outer membrane and the WLM, 3) a second stalk forms, closer to one of the capped NTs, 4) the stalks move along the WLM toward this capped NT, and a pore forms in the cap, 5) the WLM flattens, and the stalks broaden into diaphragms, 6) subsequently, these diaphragms expand around the pore and form an enclosed connection between one of the inner capped NTs and the outer membrane, and 7) finally, the connected portion expands toward the other capped NT, which fuses with the connected region, reconnecting the two inner NTs. The HD adjusts slightly, forming the same final metastable state as seen in Fig. 5, A and B. This complicated, multistep pathway is not amenable to a simple-order parameter.
The formation of a second stalk in this second pathway may seem extraneous, as a single stalk could expand around the pore, leading to a more direct path. We manually modified the transformation pathways to check whether the second stalk was an artifact of our initialization. If one of the stalks is removed from the string, it simply reforms when the string is allowed to relax. We, therefore, conclude that the presence of a second stalk is an intrinsic part of this transformation path. This is unlike, for example, the pore in the previous direct-HF pathway, which may or may not form. Once the inner and outer membranes have locally fused by any of the pathways illustrated in Fig. 5, the next step is to expand the HD.
Pathway selection
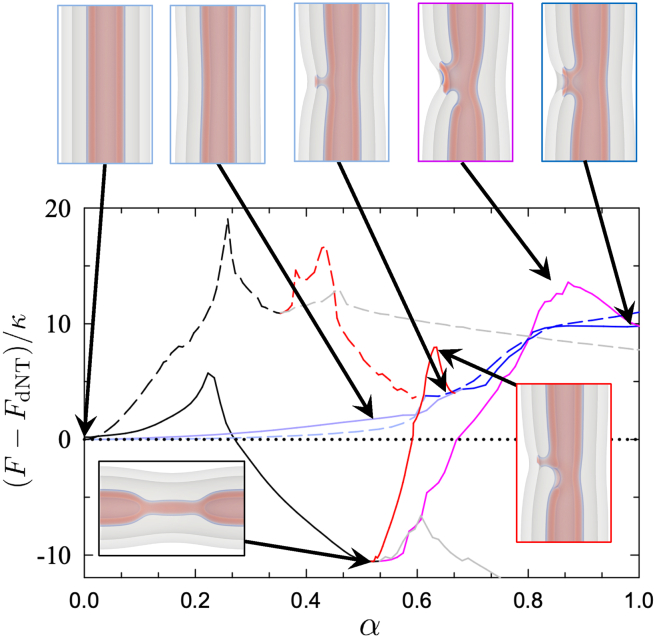
Fig. 5 presents a number of paths by which a dNT system may form or break connections between the inner and outer membranes. To understand how the system will actually proceed, we need to understand the barriers to each of these processes. The free energies along the path are presented in Fig. 6. Each step in the transition can be thought of as a jump between metastable states (local minima on the free-energy landscape) with a barrier given by the difference between the free-energy at the starting metastable state and the highest free-energy before the next metastable state.
Figure 6.
Free-energy along the MFEPs for the pathways linking the fused and unfused dNT states shown in Fig. 5. Data are shown for (dashed) and (solid) , and free energies are given relative to that of a dNT, . Colors correspond to different steps, and are chosen to match those in Figs. 3 and 5. Membrane configurations are shown at key steps for . Barriers are calculated similarly to Fig. 1.
It is important to note that the pathways are reversible, and can proceed in the direction of increasing or decreasing α. In fact, the system can proceed along any of the given paths in either direction, with metastable states serving as junction points connecting between paths. As before, local minima correspond to metastable states and the barrier can be inferred by the free-energy peak between these minima. We present data for a tension on the high end of physiological relevance, , and a higher tension, , to illustrate a change in behavior occurring between these values.
Starting from the unperturbed dNT state, there are two possibilities: collapsing the inner tube, as in the sNT case (black) or forming a stalk between the membranes (light blue). At both tensions, the barrier to stalk formation is . At the higher tension, , the barrier to collapse (black) is also . As the stalk is only weakly metastable (the barrier to its dissolution is small on the scale of the free energies presented) and thus likely to disconnect before proceeding further, we expect inner tube collapse to be the dominant pathway. At the lower tension, however, the barrier to direct tube collapse is far greater () and less likely to occur.
Once the stalk forms, the system can proceed in a number of ways: the stalk could expand (dark blue) into an HD; it could catalyze further fusion of the membranes (red, in the decreasing α direction, i.e., Fig. 5 C); or the stalk could simply dissolve. If the stalk dissolves, the system simply returns to where it started until it “tries” again, thus we ignore this possibility. The barrier to forming an HD is smaller than the alternatives; however, the HD is only weakly metastable. The free energies presented imply that the HD is unstable, as it corresponds to a plateau in the free-energy () or, worse yet, a slope leading back to the stalk state (). Similar simulations conducted in the canonical ensemble (fixing lipid number, as opposed to tension) produce a free-energy minimum, i.e., stable HD. Based on these calculations, as well as particle simulations showing long-lived stalks and HDs (discussed later), we expect that the slow lipid diffusion will lead to a metastable HD. Due to the weak metastability (or instability) of the HD, and (as we will see) the prohibitive barrier to proceeding further along the HD pathway, we expect it to simply shrink back into the stalk state.
The free-energy barriers to these competing processes are shown in Fig. 7. Starting from the stalk, the barrier to collapse into the WLM state is consistently below the barrier to the canonical pathway, showing that the stalk aids the collapse. The total free-energy cost of both jumps is, however, similar between the canonical pathway and stalk intermediate. Nonetheless, the existence of a stalk intermediate serves as a more easily formed jumping off point, to catalyze the collapse. Once in the collapsed state (second image of Fig. 6, top), the WLM easily ruptures (see sNT data) leading to the fission of the inner tube. This is the case for mN/m, i.e., for essentially the entire range of physiologically relevant tensions.
Figure 7.
Free-energy barriers are shown for the (black points) canonical pathway, (blue) stalk formation, (red) transition from the stalk to the collapsed state, and (blue and red) sum of the stalk and collapse barriers, i.e., total barrier to collapse via the stalk pathway.
Expanding the HD
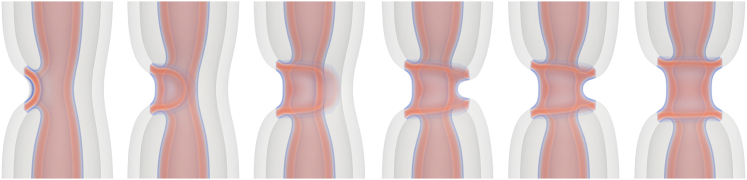
Although inner-tube fission is the most likely possibility, other possibilities remain, which may be of physiological relevance. As illustrated in Fig. 8, the HD may expand (“zip”) around the NT, resulting in a sNT connecting two dNTs—the cylindrically hemifused (CH) state. During this process, a pore opens in the outer membrane. The pore forms during the final stages of the “zipping” process, when and where the membrane is strongly curved and would need to curve even further to continuously deform into the final state. Consequently, the bending energy of the highly curved membrane outweighs the line tension of the pore. A transient pore can also form at the leading edge of the zip due to the high curvature. Each of the mechanisms presented here are further discussed in the supporting material, including images that are enlarged and annotated for clarity and a discussion of possible variations of transition pathways.
Figure 8.
Configurations during the “zipping” of the HD toward the CH state at a tension of . The initial connection between the inner and outer membranes zips along the membranes until the remaining portion is small. A pore then opens (fourth image) and the remaining outer membrane breaks and then fuses with the inner membrane. The outer tube in the double-tube region has been made transparent.
The free-energy curves during zipping are illustrated in Fig. 9, and the barriers are quite large, for both of the tensions. Without intervention, the barriers to forming the CH state appear to be prohibitively large, and the previously presented pathways are likely to dominate. Potential interventions will be discussed later.
Figure 9.
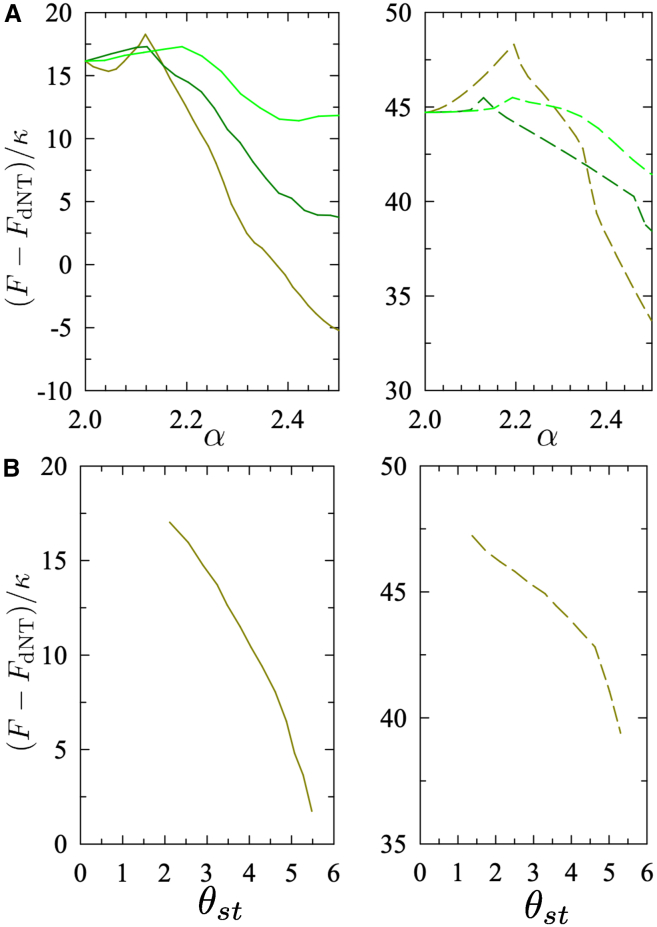
Free-energy along the MFEPs for the HD zipping shown in Fig. 8. Free energies are shown (A) as a function of, α, the order parameter derived from the string method and (B) , the angle through which the HD has “zipped.” is only calculated while the HD is zipping, i.e., the data used in (B) is cut off at the more complicated final rupture of the outer membrane. Data are shown for (dashed) and (solid) , free energies are given relative to that of a dNT, and key steps are illustrated for .
Although a simple-order parameter for the entire process is not forthcoming, we examine the main part of the pathway in terms of the angle around which the HD has zipped, (Fig. 9 B). The free-energy increases roughly linearly with . The process can be seen as the free-energy change associated with replacing the double membrane with a single membrane plus two threefold membrane junctions. The areas of the membrane replaced and the length of the junctions both vary linearly with .
Collapse of the CH state
The sNT is metastable and, once this CH state has formed, the evolution may proceed in a number of ways. The most likely pathways involve either disconnecting the inner and outer membranes or simply rupturing the sNT. This section discusses these mechanisms, which are illustrated in Fig. 10. Fig. 10 A shows a mechanism where a pore forms in the inner membrane, close to its connection to the diaphragm. The pore then expands, “unzipping” the inner NT from the outer one, but leaving a stalk connecting the inner and outer membranes. This is followed by the closure of the inner NT, creating a capped NT connected to the outer membrane by a stalk. The inner region is now disconnected from the region between the membranes. The stalk then disconnects, leaving a capped NT inside of the outer membrane. As the membranes are under tension, the capped NT then retracts. A possible variation on this pathway involves the formation of two pores, leading to two stalks. Another variation involves the stalks remaining intact, as the inner tube retracts, but remains connected to the outer tube.
Figure 10.
Intermediate configurations for the evolution of the CH state at a tension of . (A) and (B) show two mechanisms, by which the inner membrane detaches from the outer membrane, resulting in a capped inner NT. (C) The rupture and unzipping of the connecting sNT. The outer tube in the double-tube region has been made transparent.
Alternatively, as presented in Fig. 10 B, the inner NT may first collapse, creating a WLM, connecting it to a diaphragm that spans the outer NT. The WLM then breaks, followed by the diaphragm. The result is, once again, a capped NT inside the outer NT. As the sNT has two sides, the disconnection process must occur twice to disconnect both inner membranes from the outer membrane and produce a pair of capped NTs. The sides may disconnect separately or concurrently. When calculating the free energies, we consider the case of only one side disconnecting. The barrier to the second disconnection is smaller (not shown).
The third option involves the formation of a pore in the sNT connection (Fig. 10 C). The pore then grows, unzipping the sNT portion, until the two structures are only connected by a WLM, which subsequently breaks. This resembles fission by poration of a sNT, which was discussed previously and found to be unfavorable. In this case, however, the newly created interface is not simply a membrane edge, and the pore resembles an HD rim pore, which can be more stable than simple membrane pores (64). Unlike cases in Fig. 10 A and B, the final result is leaky: the region inside the inner membrane is open to the outer fluid. As the membrane is under tension, the two NTs are then pulled away from one another. In our case, disappearing through the reflecting boundary, but in real system they would simply retract.
Once again, we can compare the likelihood of these pathways by examining the free-energy change throughout the process, shown in Fig. 11. At both tensions, the barriers to disconnecting the inner tube (Fig. 10, A and B) are similar via either process, and smaller than the barrier to rupturing the sNT (Fig. 10 C).
Figure 11.
Free-energy along the MFEPs for the fission of the CH state. Plot colors are chosen to match those of Fig. 10, data are shown for (left, solid) and (right, dashed) . Free energies are given relative to that of a dNT, . Free energies are shown as functions of (A) α and (B) the inferred order parameter, , the angle through which the sNT has unzipped for the pathway shown in Fig. 10C. is only calculated during the growth of the pore, i.e., after pore formation and before the final disconnection of the tubes.
The inner tube disconnection processes are not easily amenable to a simple order-parameter. Rupturing the sNT can be measured in terms of the angle through which the pore expands, . The free-energy is roughly linear in terms of . The reasoning is similar to the zipping process: disappearance of the membrane under tension and conversion of the threefold junction into a curved membrane. The threefold junction has a higher line tension than the curved membrane, and deletion of the membrane under tension is favorable, thus the free-energy decreases. The barrier to this process comes from pore formation.
Another possibility (not shown) is that the single-tube simply collapses and ruptures as in the simple mechanism presented earlier. The barrier to this is significantly larger than for any of the disconnection mechanisms presented. After the tube retracts, the structure continuously transforms into a pair of capped NTs (inner and outer) connected by a stalk. This transformation is shown in the supporting material.
Overview of possible pathways
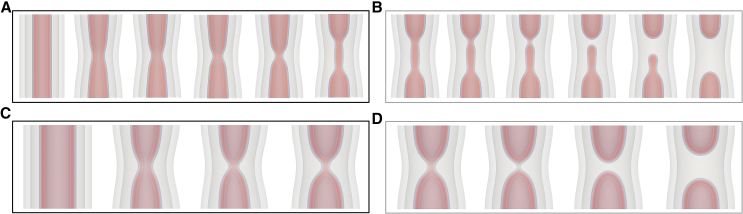
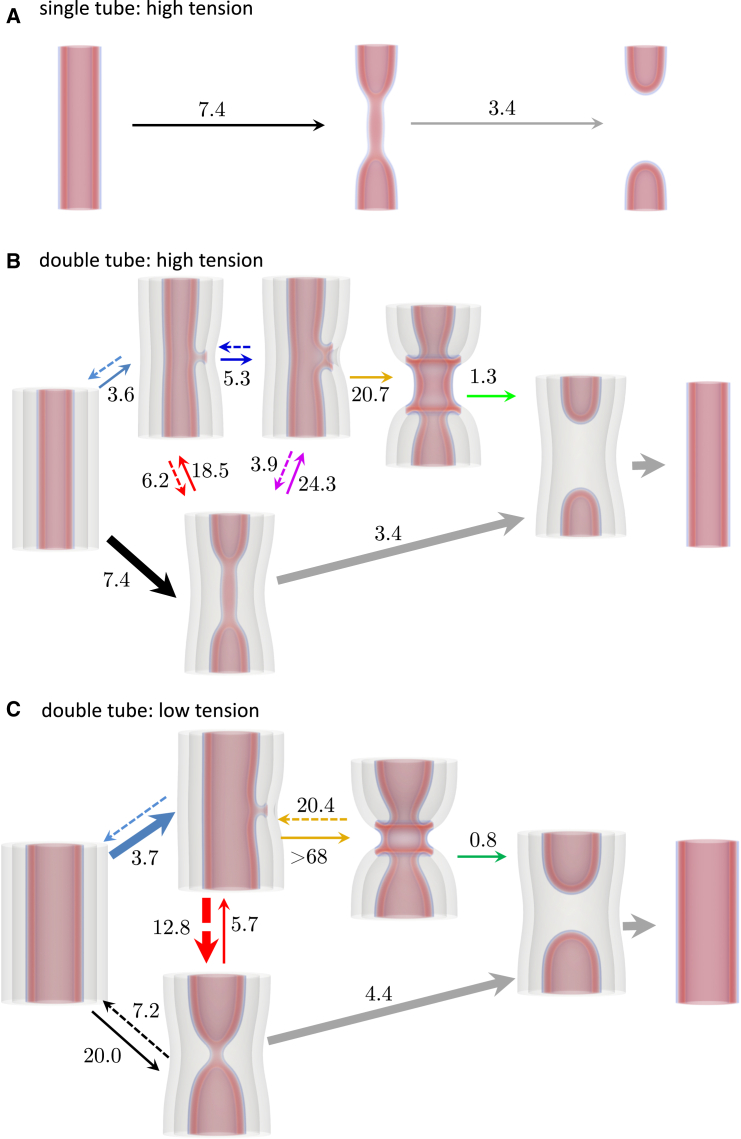
We have presented a number of ways that a dNT can evolve under tension, broken into individual steps or stages. We now wish to combine the steps into an overall picture of membrane tube evolution. To clarify the possible processes, Fig. 12 summarizes potential transformation pathways of sNTs at high tension, and dNTs at high and low tensions, and , respectively. sNT fission (Fig. 12 A) proceeds by partially collapsing the membrane (WLM) and subsequently rupturing the WLM.
Figure 12.
For a Figure360 author presentation of Fig. 12, see https://doi.org/10.1016/j.bpj.2024.10.009.
Illustration of the transition mechanisms with associated free-energy barriers, in units of κ. Colors correspond to the barriers used thus far in the paper. Arrows are shown for increasing α (solid) and decreasing α (dashed) directions, respectively. The most likely pathways to fission is highlighted with thick arrows. Pathways are shown for (A) sNTs at , (B) dNTs at , where sequential fission is more likely, and (C) dNTs at , where hemifusion is more likely. The missing barriers on blue paths likely are nonzero but difficult to assess (see main text). Arrows going only one direction are transitions that appear irreversible, because the transition may proceed arbitrarily far in one direction, e.g., the growth of a WLM intermediate.
The dNT system has many more degrees of freedom than the sNT system and a variety of possible pathways. At high tension, the system may collapse directly (canonical pathway) or using a stalk. The barrier to initially forming a stalk is lower than the canonical pathway. The stalk may catalyze inner-tube fission or may expand to form an HD. From there, it can either collapse (pink pathway) or zip (orange pathway). The barrier to zipping is significantly higher than the other options, thus the next state is likely the collapsed state.
To judge the likelihood of the multistep pathways, we combine the probabilities of each event along the pathway. The probability of a fluctuation with free-energy occurring is proportional to . The probability of a series of such events is proportional to . We therefore choose the sum of the individual barriers to describe the probability of a given process. At high tension, the sum of the individual barriers is lowest for the direct (black) pathway, making it the most likely.
At low tension (Fig. 12 C), the barrier to directly collapsing a part of the inner membrane into a WLM becomes prohibitive, and stalk formation between the inner and outer membranes becomes more favorable by comparison. Once again, from here, the stalk may either follow the upper branch and form an HD and then zip around to form the CH state (orange) or the inner membrane may disconnect via a WLM intermediate (red), i.e., transition to the lower branch in the diagram. The barrier to disconnecting and forming a partially collapsed (WLM) inner NT is substantially lower than zipping the HD. The system is therefore more likely to first connect the inner and outer membranes via a stalk, as an intermediate to forming a partially collapsed (WLM) inner NT, i.e., the inner and outer NTs will occasionally connect and disconnect via a stalk, until the connection leads to a partial collapse (WLM) of the inner NT. The latter then undergoes rupture. The outer membrane subsequently fissions via the mechanism in Fig. 12 A. This stalk-intermediate pathway has several stages at which either membrane may form transient pores, making it a potentially leaky pathway. This more thorough investigation thus agrees with the prediction of (26).
Comparison between SCFT and particle-based simulations
SCFT and the string method have allowed us to obtain a detailed picture of many membrane remodeling processes, including free-energy barriers that would be computationally prohibitive for direct particle-based simulations. This approach, however, merely relaxes the transformation pathway from an initial path into the nearest local MFEP. It may therefore fail to find even close-by (in configuration space) pathways with lower barriers. This is particularly important for cases like double-membrane remodeling, where there are many degrees of freedom, and it is practically impossible to examine every way that a membrane could rearrange. Combining our calculations with particle-based simulations can reveal transformation pathways that we may have missed, in addition to allowing us to validate our calculations, by verifying that the predicted evolution actually occurs.
SCFT and the particle-based simulations also differ in that the evolution of the particle-based simulations is locally mass conserving, unlike the SCFT evolution, which is governed by model A dynamics (77). It is possible to carry out dynamic SCFT calculations using model B; however, the implementation is more complicated and usually (when considering only (meta)stable states) the outcome is the same. For nonequilibrium behavior, as in the string method, the two models may in general produce different dynamics.
It would take prohibitively long to carry out simulations by simply initializing a sNT or dNT and waiting for the previously described processes to occur. A more productive approach is to use the fields from SCFT to initialize a starting configuration of the particle-based model and allow the system to evolve. We initialized SOMA simulations at key locations, such as metastable configurations or saddle-points on the free-energy landscape, and allowed them to relax. The setup and an example of subsequent evolution of the SOMA simulations are shown in Videos S5 and S6, respectively.
In each case, the system evolved by “descending” the pathway predicted by the string method. Side-by-side comparisons between SCFT and particle-based simulation are shown in Fig. 13, and further discussed below. The consistency suggests that the pathways are realistic, and are not sensitive to either fluctuations or the particular dynamical model employed.
Figure 13.
Comparison of configurations from SCFT calculations and particle-based simulations. Simulations were initialized using fields derived from SCFT strings (see Video S5) and, in each case, they either remained in a metastable state or followed the string derived from SCFT along the direction of decreasing free-energy. (A) The sNT partially collapses into a WLM. (B) Initialized with a cylindrically hemifused state, the inner tube disconnects from the sNT-dNT-junction by either (left) the mechanism in Fig. 9A or (right) Fig. 9B. (C) Starting from an HD, the diaphragm unzips, descending the curve in Fig. 9. The system then proceeds via one of the pathways in (D) and (E), which correspond to the blue and pink curves in Fig. 6, respectively. For pathway (E), side-by-side trajectories are shown in Video S6.
A sNT partially collapses into a WLM, as illustrated in Fig. 13 A. This corresponds to the sNT pathway discussed earlier (Fig. 1). Unlike in the SCFT calculations, we do not observe the fission of the WLM connecting the two capped sNTs. In the SCFT calculations there is a constant tension along the NT, set by the chemical potential. By contrast, the particle-based simulations are conducted in the canonical ensemble, where we fix the number of lipids rather than the chemical potential. Partially collapsing the NT reduces the tension in the membrane resulting in insufficient tension to break the WLM.
If the system is initialized in a cylindrically hemifused state, it remains metastable for a short time, then the inner NT disconnects from the outer one via either of the predicted mechanisms, i.e., the transformation pathways illustrated in Fig. 10, A or B and corresponding to the dark and light green data in Fig. 11. The comparison between particle-based simulation and SCFT for these transformation pathways is shown in Fig. 13 B.
A partially zipped HD further unzips, decreasing the diaphragm that connects the inner and outer NTs, as illustrated in Fig. 13 C. This mechanism corresponds to the data shown in Fig. 9, along the direction of decreasing free-energy and decreasing α. The system remains metastable for a short time and then proceeds by the transformation pathways shown in Fig. 13, D or E. These pathways are the reverse of the blue and pink curves in Fig. 6, respectively. The HD may radially shrink, as in Fig. 13 D, collapsing into a stalk that connects the inner and outer membranes. Alternatively, the diaphragm may disconnect, as in Fig. 13 E, resulting in a partially collapsed (WLM) inner NT. This observation is consistent with our prediction that the stalk or HD is metastable and can act as an intermediate state toward the partial collapse of the inner NT into a WLM, along the pathway to fission.
The comparisons provide validation that the pathways that we have found are not artifacts of the additional approximations in SCFT. Another aspect of the kinetics is the transition rate. As discussed above, the barriers are too large to simply wait for each process to occur in particle-based simulations. A more promising approach is to combine the free-energy barrier and a kinetic coefficient to obtain the Kramers rate (78). We have calculated the former using SCFT. Although we present some kinetic information in the supporting material, obtaining the kinetic coefficients requires modifications to our particle-based simulation approach. One strategy is to use forward-flux sampling (79). This method breaks each process into many small subprocesses, calculates the time (thus rate) for the first subprocess, and the success probability (thus free-energy barrier) to carry out each subsequent subprocess. This is outside of the current scope and is left for future work.
Discussion
Fusion and fission are indispensable and ubiquitous elements of intracellular membrane dynamics (80). Both control the membrane-barrier function and, hence, there has long been the question how to transform membrane topology without full-scale rupture and leakage (6,81). First, a highly curved lipid stalk was discovered and established, experimentally and theoretically, as the initial intermediate of leakless membrane fusion. Then, in 2003, Kozlovsky and Kozlov were the first to adapt this model to fission (4). In their study, the authors used elastic theory to analyze the transition from a constricted neck to a hypothetical HF intermediate (in the analogy to the fusion process, i.e., resembling a partially collapsed [WLM] NT). The work introduced the notion of critical curvature, at which the elastic energy of the neck becomes comparable with that of the WLM-like HF intermediate, thus favoring the transition. The HF intermediate was assumed to be unstable so that the HF-to-full-fission transition happened spontaneously.
Since then, membrane fission has been modeled using different phenomenological, mean field, and CG approaches. The use of CG simulations pointed to the metastable character of the HF intermediate (9,10), a discovery with mechanistic implications for the energy transduction by proteins during this type of membrane remodeling. Indeed, WLM-like HF intermediates were several times detected in protein-driven membrane transformations. Mattila et al. produced a truncated dynamin GTPase that mimicked the protein’s activated state during its GTPase cycle, usually linked to the protein’s fission activity (7). Upon incubation of the truncated protein with large unilamellar vesicles, the authors detected HF intermediates beneath the protein scaffold. Similarly, Mizuno et al. detected protein-coated cylindrical lipid micelles upon membrane incubation with endophilin molecules (82). However, in none of these cases a bona fide fission reaction was reconstructed, as the protein coats stabilized the HF intermediate, precluding the characterization of topological transitions along the fission pathway.
The data presented here as well as in (26) provide additional experimental evidence that membrane fission proceeds through the formation of a metastable WLM-like HF intermediate, which then resolves into full fission (Fig. 1). The HF intermediate has the expected geometry, with its thickness being similar to that of a lipid bilayer. Although not discussed therein, the statistics presented in (26) confirmed the earlier theoretical predictions about the geometry of membrane fission, as membrane remodeling proceeds only upon reaching the critical radius value in the range of the bilayer thickness (4).
To achieve fission in the pure lipid system we used membrane tension. In the cell, tension is often related to membrane dynamics, action of motor proteins, and cytoskeleton acting against adhesion and friction forces (83). The balance between the membrane-pulling forces and friction drag constitutes the constriction-by-friction mechanism of membrane fission where local membrane tension increases dramatically causing membrane HF and/or pore formation (18,20). Here, we reconstructed such dynamically elevated tension. The tension-driven constriction followed by HF was detected by cryo-EM. Both short HF intermediates and metastable WLMs were detected (Fig. 1 B). We found that HF becomes spontaneous at values of membrane tension that are at least one order of magnitude higher than that reported for cellular plasma membrane ( mN/m, (84)). High lateral membrane tensions, required to produce such a high membrane curvature, have been a long-known cause of poration and lysis of planar lipid bilayers (11). Importantly, using SCFT modeling, we showed that the HF pathway would be more likely than pore nucleation even at such high membrane tension (Fig. 1). High membrane tension also favors the formation and extension of the metastable WLM (Fig. 1).
Membrane fission, however, is not limited to single membranes. One of the best-known examples of double-membrane fission in cells is that of the mitochondrion. While the protein machinery involved in this process has been extensively explored, surprisingly little is known about the free-energy landscape of double-membrane remodeling itself (27,27,85,86,87,88,89,90,91). By direct extrapolation from single-membrane remodeling, we can expect two outcomes upon double-membrane constriction: sequential fission of the inner and then the outer membranes following the single-membrane pathway, or the fusion of the outer membrane to the inner one due to curvature stress. Consistent with previous work (26), we find the latter pathway for low tensions, mN/m, already associated with curvature instabilities in single-membrane systems. At higher tensions, however, the sequential fission pathway is the likely outcome of constriction.
The details of the membrane rearrangements were further analyzed by SCFT. The first step in the reaction is the formation of a close contact area between the membranes. Next, a stalk and then an HD is nucleated and, in some cases, leads to formation of an extended HD encircling the dNT, i.e., the CH state. Intriguingly, such restricted HF may result in a quick exchange of lipids, such as cardiolipin, between the outer and inner membrane of the mitochondrion (92). HF may resolve into full fusion (81,93); however, this transformation from dNT to sNT does not occur in our simulations. Instead, in the absence of cofactors, the HD rather decays via redetachment of the inner NT from the outer one. This process is accompanied by the formation of several pores. Such poration may result in depolarization of the mitochondrial inner membrane, a process well known to complement mitochondrial fission (87,89).
Of note, lipid rearrangements in fission and fusion are closely related both in vivo and via the pathways studied in this work. While we have been focused on the fission of membrane tubes, we can also consider the antipode transformation by simply looking at our MFEPs in the “reverse” (decreasing α). Barriers to some steps in the fusion process can be inferred from the free-energy profiles (Figs. 6, 9, and 11), and are partially illustrated in Fig. 12. Some reverse barriers, however, are omitted as they are difficult to obtain from our calculations. For example, as currently implemented, since the NTs are under tension, they simply retract as far as they can from one another upon fission. To reverse the process, one would need to fix the capped NTs at a set distance. In cells, this process is mediated by GTP-powered docking proteins (85). Based on the low barriers, once the membranes are brought together, the fusion process would readily follow the reversed path of fission. This observation might be of importance for mitochondrial fusion and fission known to be synchronized and colocalized in time and space (89,90). Analysis of mitochondrial remodeling indicate that fusion may trigger fission, but fusion is not affected by fission (90). Interestingly, the barriers in the fusion direction, shown in Fig. 12, suggest that the complex pathway may occur more readily when membranes are fusing, particularly under low tension.
The free-energy barriers reported here are valid for homogeneous lipid systems under high lateral tension, such as the one produced by the hydrodynamic flow in our cryo-EM experiments. While cellular membranes are at considerably lower tension on average, local spikes of tension during fast membrane transformations can lead to extreme membrane constriction and instabilities similar to those described here (18,20). Biological systems may also employ a variety of strategies to decrease the free-energy barriers and select the preferred transformation pathway. Among these strategies are protein catalysis of membrane remodeling. A great example of such catalyzers are the members of the dynamin superfamily of GTPases that transform the energy of GTP into membrane curvature stress both in fusion and in fission. Interestingly, many fusion and fission dynamins colocalize at the mitochondrial fission site (94). Another factor that may alter the outcome of the remodeling pathway is membrane asymmetry. Biological membranes present compositional asymmetry both between leaflets and between different membranes (95,95). Such asymmetry confers distinct mechanical properties onto each membrane, thus potentially shifting the free-energy barriers for the transformations we predicted in our model system. Finally, lipid and protein redistribution along the curvature gradients may also help remodeling (96,97,98).
Each one of the cited factors may alter the way that membranes rearrange during fission and fusion, possibly giving rise to new transformation pathways. However, as the most energetically expensive transformation steps involve locally constricting the (outer) membrane, it seems likely that a simple mechanical constriction can drastically lower the free-energy barriers, while leaving the details of the rearrangements mostly intact. The linearity of the required free energy with constriction radius implies that a constant force is required regardless of the membrane radius.
Future work should therefore explore the effect of constriction by cellular factors on the favorability of competing transformation pathways in membrane remodeling, particularly in fission and fusion of double-membrane systems. The main contribution from constriction proteins, such as dynamin, is likely simply a way to apply the required constriction force, but there may also be specific effects due to the particular membrane interactions, for example, splaying of headgroups due to the insertion of dynamin’s PH domains between the headgroups. Furthermore, although zipping of the HD around the double tube, to produce the cylindrically hemifused state, is prohibitively expensive, perhaps it may be possible with the aid of constriction.
Finally, it should be noted that our SCFT implementation used in the majority of this work leads to each membrane having the same tension. This is not generally the case, as each membrane, and indeed leaflet, may be attached to a different reservoir. It does, however, create concentric tubes, which are in close contact, as seen in the experiments, and which we could expect from external sources of constriction, such as helical constriction proteins around the outer tube. We have briefly explored, in the supporting material, an approach to calculating corrections due to different membrane tensions.
Conclusion
In this study, we have combined SCFT and the string method to identify optimal fission pathways in single- and double-membrane tubes. We have further validated these pathways using cryo-EM experiments, demonstrating the robustness of our approach.
Our results suggest that the free-energy barriers to single- and double-membrane fission are sensitive to membrane tension. In double-membrane tubes, there are several possible pathways, and the dominant pathway can be controlled by the membrane tension. We describe two competing pathways to fission. At very high tension, the more likely mechanism involves the partial collapses of the inner tube, into a WLM, which then ruptures, resulting in two capped tubes. This occurs without interaction with the outer membrane. The outer membrane then follows similarly. This pathway is nonleaky, i.e., the solvent inside the inner membrane, between the membranes and outside the outer membrane never mix. At lower, more physiologically relevant tension, however, the barrier to forming a WLM becomes prohibitive, and instead, the inner and outer membranes fuse, which then catalyzes fission via a more complicated pathway. This pathway is leaky as pores form close to the fusion sites.
In addition to these fission pathways, we explore a variety of other mechanisms by which double tubes may evolve. Except for the case of very high tensions, the free-energy barriers that we obtain (typically ) are larger than we would expect for biological processes that occur frequently. This is consistent with the need for constricting proteins to catalyze these processes in real biological systems. Further work is required to understand how these proteins affect the mechanism and free-energy barriers for the processes presented here.
Acknowledgments
We thank Vadim Frolov for many helpful discussions. Financial support has been provided by the Deutsche Forschungsgemeinschaft within CRC 1286 TP C06. A.V.S. was supported by grant PGC2018-099971-B-I00 funded by MCINAEI 10.13039501100011033 and “ERDF A way of making Europe” through the “European Union”, and by Basque Government Grant IT1625-22. The authors gratefully acknowledge the Gauss Centre for Supercomputing e.V. (www.gauss-centre.eu) for funding this research project by providing computing time through the John von Neumann Institute for Computing (NIC) on GCS Supercomputer JUWELS at the Jülich Supercomputing Centre (JSC). The authors are grateful to the Electron Microscopy and Crystallography platform of the CIC bioGUNE and the Basque Resource for Electron Microscopy for providing access to cryo-EM sample preparation and analysis equipment.
Author contributions
R.K.W.S., A.V.S., and M.M. designed the research. R.K.W.S. performed the theoretical research and analyzed data. I.S.-P. and A.V.S. performed the experimental research. A.V.S. analyzed the experimental data. R.K.W.S., A.V.S., and M.M. discussed the results and wrote the paper.
Declaration of interests
The authors declare no competing interests.
Editor: Padmini Rangamani.
Footnotes
Supporting material can be found online at https://doi.org/10.1016/j.bpj.2024.10.009.
Contributor Information
Russell K.W. Spencer, Email: russell.spencer@theorie.physik.uni-goettingen.de.
Anna V. Shnyrova, Email: anna.shnyrova@ehu.eus.
Marcus Müller, Email: mmueller@theorie.physik.uni-goettingen.de.
Supporting material
References
- 1.Renard H.-F., Johannes L., Morsomme P. Increasing Diversity of Biological Membrane Fission Mechanisms. Trends Cell Biol. 2018;28:274–286. doi: 10.1016/j.tcb.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Frolov V.A., Escalada A., et al. Shnyrova A.V. Geometry of membrane fission. Chem. Phys. Lipids. 2015;185:129–140. doi: 10.1016/j.chemphyslip.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Hochmuth R.M., Evans E.A. Extensional flow of erythrocyte membrane from cell body to elastic tether. I. Analysis. Biophys. J. 1982;39:71–81. doi: 10.1016/S0006-3495(82)84492-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kozlovsky Y., Kozlov M.M. Membrane Fission: Model for Intermediate Structures. Biophys. J. 2003;85:85–96. doi: 10.1016/S0006-3495(03)74457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bashkirov P.V., Akimov S.A., et al. Frolov V.A. GTPase cycle of dynamin is coupled to membrane squeeze and release, leading to spontaneous fission. Cell. 2008;135:1276–1286. doi: 10.1016/j.cell.2008.11.028. https://www.sciencedirect.com/science/article/pii/S0092867408015031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kozlov M.M., McMahon H.T., Chernomordik L.V. Protein-driven membrane stresses in fusion and fission. Trends Biochem. Sci. 2010;35:699–706. doi: 10.1016/j.tibs.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattila J.-P., Shnyrova A.V., et al. Frolov V.A. A hemi-fission intermediate links two mechanistically distinct stages of membrane fission. Nature. 2015;524:109–113. doi: 10.1038/nature14509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang G., Müller M. Rupturing the hemi-fission intermediate in membrane fission under tension: Reaction coordinates, kinetic pathways, and free-energy barriers. J. Chem. Phys. 2017;147 doi: 10.1063/1.4997575. [DOI] [PubMed] [Google Scholar]
- 9.Deserno M. Mesoscopic Membrane Physics: Concepts, Simulations, and Selected Applications. Macromol. Rapid Commun. 2009;30:752–771. doi: 10.1002/marc.200900090. [DOI] [PubMed] [Google Scholar]
- 10.Fuhrmans M., Müller M. Coarse-grained simulation of dynamin-mediated fission. Soft Matter. 2015;11:1464–1480. doi: 10.1039/c4sm02533d. [DOI] [PubMed] [Google Scholar]
- 11.Evans E., Heinrich V., et al. Rawicz W. Dynamic Tension Spectroscopy and Strength of Biomembranes. Biophys. J. 2003;85:2342–2350. doi: 10.1016/s0006-3495(03)74658-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marrink S.J., de Vries A.H., Mark A.E. Coarse Grained Model for Semiquantitative Lipid Simulations. J. Phys. Chem. B. 2004;108:750–760. [Google Scholar]
- 13.Marrink S.J., Risselada H.J., et al. de Vries A.H. The MARTINI Force Field: Coarse Grained Model for Biomolecular Simulations. J. Phys. Chem. B. 2007;111:7812–7824. doi: 10.1021/jp071097f. [DOI] [PubMed] [Google Scholar]
- 14.Sandre O., Moreaux L., Brochard-Wyart F. Dynamics of transient pores in stretched vesicles. Proc. Natl. Acad. Sci. USA. 1999;96:10591–10596. doi: 10.1073/pnas.96.19.10591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Müller M., Schick M. Structure and nucleation of pores in polymeric bilayers: A Monte Carlo simulation. J. Chem. Phys. 1996;105:8282–8292. [Google Scholar]
- 16.Morlot S., Galli V., et al. Roux A. Membrane Shape at the Edge of the Dynamin Helix Sets Location and Duration of the Fission Reaction. Cell. 2012;151:619–629. doi: 10.1016/j.cell.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shnyrova A.V., Bashkirov P.V., et al. Frolov V.A. Geometric Catalysis of Membrane Fission Driven by Flexible Dynamin Rings. Science. 2013;339:1433–1436. doi: 10.1126/science.1233920. https://science.sciencemag.org/content/339/6126/1433.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Espadas J., Pendin D., et al. Daga A. Dynamic constriction and fission of endoplasmic reticulum membranes by reticulon. Nat. Commun. 2019;10:5327. doi: 10.1038/s41467-019-13327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahecic D., Carlini L., et al. Manley S. Mitochondrial membrane tension governs fission. Cell Rep. 2021;35 doi: 10.1016/j.celrep.2021.108947. [DOI] [PubMed] [Google Scholar]
- 20.Simunovic M., Manneville J.-B., et al. Callan-Jones A. Friction Mediates Scission of Tubular Membranes Scaffolded by BAR Proteins. Cell. 2017;170:172–184.e11. doi: 10.1016/j.cell.2017.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tolpekina T.V., den Otter W.K., Briels W.J. Nucleation free energy of pore formation in an amphiphilic bilayer studied by molecular dynamics simulations. J. Chem. Phys. 2004;121:12060–12066. doi: 10.1063/1.1815296. [DOI] [PubMed] [Google Scholar]
- 22.Ting C.L., Appelö D., Wang Z.-G. Minimum Energy Path to Membrane Pore Formation and Rupture. Phys. Rev. Lett. 2011;106:309. doi: 10.1103/PhysRevLett.106.168101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu G.-B. Whole Cell Cryo-Electron Tomography Suggests Mitochondria Divide by Budding. Microsc. Microanal. 2014;20:1180–1187. doi: 10.1017/S1431927614001317. [DOI] [PubMed] [Google Scholar]
- 24.Mageswaran S.K., Grotjahn D.A., et al. Jensen G.J. Nanoscale details of mitochondrial fission revealed by cryo-electron tomography. bioRxiv. 2021 doi: 10.1101/2021.12.13.472487. Preprint at. [DOI] [Google Scholar]
- 25.Zhang L., Trushin S., et al. Trushina E. Altered brain energetics induces mitochondrial fission arrest in Alzheimer’s Disease. Sci. Rep. 2016;6 doi: 10.1038/srep18725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spencer R.K.W., Santos-Pérez I., et al. Müller M. Membrane fission via transmembrane contact. Nat. Commun. 2024;15:2793. doi: 10.1038/s41467-024-47122-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suen D.-F., Norris K.L., Youle R.J. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suhaili S.H., Karimian H., et al. Aguilar M.-I. Mitochondrial outer membrane permeabilization: a focus on the role of mitochondrial membrane structural organization. Biophys. Rev. 2017;9:443–457. doi: 10.1007/s12551-017-0308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gruhn T., Franke T., et al. Lipowsky R. Novel Method for Measuring the Adhesion Energy of Vesicles. Langmuir. 2007;23:5423–5429. doi: 10.1021/la063123r. [DOI] [PubMed] [Google Scholar]
- 30.Noguchi H. Membrane Simulation Models from Nanometer to Micrometer Scale. J. Phys. Soc. Jpn. 2009;78 [Google Scholar]
- 31.Kawamoto S., Klein M.L., Shinoda W. Coarse-grained molecular dynamics study of membrane fusion: Curvature effects on free energy barriers along the stalk mechanism. J. Chem. Phys. 2015;143 doi: 10.1063/1.4933087. [DOI] [PubMed] [Google Scholar]
- 32.Li Z.-L., Ding H.-M., Ma Y.-Q. Interaction of peptides with cell membranes: insights from molecular modeling. J. Phys. Condens. Matter. 2016;28 doi: 10.1088/0953-8984/28/8/083001. [DOI] [PubMed] [Google Scholar]
- 33.Ghosh R., Satarifard V., et al. Lipowsky R. Budding and Fission of Nanovesicles Induced by Membrane Adsorption of Small Solutes. ACS Nano. 2021;15:7237–7248. doi: 10.1021/acsnano.1c00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maragliano L., Fischer A., et al. Ciccotti G. String method in collective variables: Minimum free energy paths and isocommittor surfaces. J. Chem. Phys. 2006;125 doi: 10.1063/1.2212942. [DOI] [PubMed] [Google Scholar]
- 35.E W., Ren W., Vanden-Eijnden E. Simplified and improved string method for computing the minimum energy paths in barrier-crossing events. J. Chem. Phys. 2007;126 doi: 10.1063/1.2720838. [DOI] [PubMed] [Google Scholar]
- 36.Ting C.L., Wang Z.-G. Minimum free energy paths for a nanoparticle crossing the lipid membrane. Soft Matter. 2012;8:12066–12071. [Google Scholar]
- 37.Müller M., Smirnova Y.G., et al. Shi A.-C. Transition Path from Two Apposed Membranes to a Stalk Obtained by a Combination of Particle Simulations and String Method. Phys. Rev. Lett. 2012;108 doi: 10.1103/PhysRevLett.108.228103. [DOI] [PubMed] [Google Scholar]
- 38.Li J., Zhang H., et al. Shi A.-C. Emergence and stability of intermediate open vesicles in disk-to-vesicle transitions. Phys. Rev. 2013;88 doi: 10.1103/PhysRevE.88.012719. [DOI] [PubMed] [Google Scholar]
- 39.Kozlov M.M., Helfrich W. Effects of a cosurfactant on the stretching and bending elasticities of a surfactant monolayer. Langmuir. 1992;8:2792–2797. [Google Scholar]
- 40.Kozlovsky Y., Kozlov M.M. Stalk Model of Membrane Fusion: Solution of Energy Crisis. Biophys. J. 2002;82:882–895. doi: 10.1016/S0006-3495(02)75450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kozlovsky Y., Chernomordik L.V., Kozlov M.M. Lipid Intermediates in Membrane Fusion: Formation, Structure, and Decay of Hemifusion Diaphragm. Biophys. J. 2002;83:2634–2651. doi: 10.1016/S0006-3495(02)75274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Capraro B.R., Yoon Y., et al. Baumgart T. Curvature Sensing by the Epsin N-Terminal Homology Domain Measured on Cylindrical Lipid Membrane Tethers. J. Am. Chem. Soc. 2010;132:1200–1201. doi: 10.1021/ja907936c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bashkirov P.V., Chekashkina K.V., et al. Frolov V.A. Variation of lipid membrane composition caused by strong bending. Biochem. Moscow. Suppl. Ser. A. 2011;5:205–211. [Google Scholar]
- 44.Deserno M. Fluid lipid membranes: From differential geometry to curvature stresses. Chem. Phys. Lipids. 2015;185:11–45. doi: 10.1016/j.chemphyslip.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Ryham R.J., Klotz T.S., et al. Cohen F.S. Calculating Transition Energy Barriers and Characterizing Activation States for Steps of Fusion. Biophys. J. 2016;110:1110–1124. doi: 10.1016/j.bpj.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terzi M.M., Ergüder M.F., Deserno M. A consistent quadratic curvature-tilt theory for fluid lipid membranes. J. Chem. Phys. 2019;151 doi: 10.1063/1.5119683. [DOI] [PubMed] [Google Scholar]
- 47.Daoulas K.C., Müller M. Springer Berlin Heidelberg; Berlin, Heidelberg: 2010. Comparison of Simulations of Lipid Membranes with Membranes of Block Copolymers; pp. 43–85. [DOI] [Google Scholar]
- 48.Smirnova Y.G., Fuhrmans M., et al. Müller M. Free-energy calculation methods for collective phenomena in membranes. J. Phys. D Appl. Phys. 2015;48 [Google Scholar]
- 49.Smirnova Y.G., Risselada H.J., Müller M. Thermodynamically reversible paths of the first fusion intermediate reveal an important role for membrane anchors of fusion proteins. Proc. Natl. Acad. Sci. USA. 2019;116:2571–2576. doi: 10.1073/pnas.1818200116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katsov K., Müller M., Schick M. Field Theoretic Study of Bilayer Membrane Fusion. I. Hemifusion Mechanism. Biophys. J. 2004;87:3277–3290. doi: 10.1529/biophysj.103.038943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katsov K., Müller M., Schick M. Fusion of biological membranes. Pramana. 2005;64:1127–1134. [Google Scholar]
- 52.Katsov K., Müller M., Schick M. Field Theoretic Study of Bilayer Membrane Fusion: II. Mechanism of a Stalk-Hole Complex. Biophys. J. 2006;90:915–926. doi: 10.1529/biophysj.105.071092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Müller M., Katsov K., Schick M. Biological and synthetic membranes: What can be learned from a coarse-grained description? Phys. Rep. 2006;434:113–176. [Google Scholar]
- 54.Li J., Pastor K.A., et al. Zhou J. Elastic properties and line tension of self-assembled bilayer membranes. Phys. Rev. 2013;88 doi: 10.1103/PhysRevE.88.012718. [DOI] [PubMed] [Google Scholar]
- 55.Dehghan A., Pastor K.A., Shi A.-C. Line tension of multicomponent bilayer membranes. Phys. Rev. 2015;91 doi: 10.1103/PhysRevE.91.022713. [DOI] [PubMed] [Google Scholar]
- 56.Zhang P.-W., Shi A.-C. Application of self-consistent field theory to self-assembled bilayer membranes. Chin. Phys. B. 2015;24 [Google Scholar]
- 57.Ting C.L., Müller M. Membrane stress profiles from self-consistent field theory. J. Chem. Phys. 2017;146 doi: 10.1063/1.4977585. [DOI] [PubMed] [Google Scholar]
- 58.Cheng X., Lin L., et al. Shi A.-C. Nucleation of Ordered Phases in Block Copolymers. Phys. Rev. Lett. 2010;104 doi: 10.1103/PhysRevLett.104.148301. [DOI] [PubMed] [Google Scholar]
- 59.Ting C.L., Awasthi N., et al. Hub J.S. Metastable Prepores in Tension-Free Lipid Bilayers. Phys. Rev. Lett. 2018;120 doi: 10.1103/PhysRevLett.120.128103. [DOI] [PubMed] [Google Scholar]
- 60.Han Y., Xu Z., et al. Zhang L. Pathways connecting two opposed bilayers with a fusion pore: a molecularly-informed phase field approach. Soft Matter. 2020;16:366–374. doi: 10.1039/c9sm01983a. [DOI] [PubMed] [Google Scholar]
- 61.Schindelin J., Arganda-Carreras I., et al. Cardona A. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fredrickson G. Oxford University Press; USA: 2006. The Equilibrium Theory of Inhomogeneous Polymers (International Series of Monographs on Physics) [Google Scholar]
- 63.Gompper G., Schick M., editors. Volume 1. Polymer Melts and Mixtures; 2006. pp. 87–178. (Self-consistent Field Theory and its Applications, Soft Matter, Volume 1: Polymer Melts and Mixtures, Volume 1 of Soft Matter). [Google Scholar]
- 64.Spencer R.K.W., Smirnova Y.G., et al. Müller M. Transient pores in hemifusion diaphragms. Biophys. J. 2024;123:2455–2475. doi: 10.1016/j.bpj.2024.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ranjan A., Qin J., Morse D.C. Linear Response and Stability of Ordered Phases of Block Copolymer Melts. Macromolecules. 2008;41:942–954. [Google Scholar]
- 66.E W., Ren W., Vanden-Eijnden E. String method for the study of rare events. Phys. Rev. B. 2002;66 doi: 10.1021/jp0455430. [DOI] [PubMed] [Google Scholar]
- 67.Weinan E., Ren W., Vanden-Eijnden E. Finite Temperature String Method for the Study of Rare Events. J. Phys. Chem. B. 2005;109:6688–6693. doi: 10.1021/jp0455430. [DOI] [PubMed] [Google Scholar]
- 68.Vanden-Eijnden E. Towards a Theory of Transition Paths. J. Stat. Phys. 2006;123:503–523. [Google Scholar]
- 69.Maragliano L., Vanden-Eijnden E. On-the-fly string method for minimum free energy paths calculation. Chem. Phys. Lett. 2007;446:182–190. [Google Scholar]
- 70.E W., Vanden-Eijnden E. Transition-Path Theory and Path-Finding Algorithms for the Study of Rare Events. Annu. Rev. Phys. Chem. 2010;61:391–420. doi: 10.1146/annurev.physchem.040808.090412. [DOI] [PubMed] [Google Scholar]
- 71.Schneider L., Müller M. Multi-architecture Monte-Carlo (MC) simulation of soft coarse-grained polymeric materials: SOft coarse grained Monte-Carlo Acceleration (SOMA) Comput. Phys. Commun. 2019;235:463–476. [Google Scholar]
- 72.Kučerka N., Nieh M.-P., Katsaras J. Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature. Biochim. Biophys. Acta Biomembr. 2011;1808:2761–2771. doi: 10.1016/j.bbamem.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 73.Morris C.E., Homann U. Cell Surface Area Regulation and Membrane Tension. J. Membr. Biol. 2001;179:79–102. doi: 10.1007/s002320010040. [DOI] [PubMed] [Google Scholar]
- 74.Djakbarova U., Madraki Y., et al. Kural C. Dynamic interplay between cell membrane tension and clathrin-mediated endocytosis. Biol. Cell. 2021;113:344–373. doi: 10.1111/boc.202000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Müller M., Katsov K., Schick M. A New Mechanism of Model Membrane Fusion Determined from Monte Carlo Simulation. Biophys. J. 2003;85:1611–1623. doi: 10.1016/S0006-3495(03)74592-5. https://linkinghub.elsevier.com/retrieve/pii/S0006349503745925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Risselada H.J., Marelli G., et al. Müller M. Line-Tension Controlled Mechanism for Influenza Fusion. PLoS One. 2012;7 doi: 10.1371/journal.pone.0038302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Provatas N., Elder K. John Wiley & Sons, Ltd; 2010. Nonequilibrium Dynamics; pp. 33–56. chapter 4. [DOI] [Google Scholar]
- 78.Kramers H. Brownian motion in a field of force and the diffusion model of chemical reactions. Physica. 1940;7:284–304. [Google Scholar]
- 79.Allen R.J., Valeriani C., Rein Ten Wolde P. Forward flux sampling for rare event simulations. J. Phys. Condens. Matter. 2009;21 doi: 10.1088/0953-8984/21/46/463102. [DOI] [PubMed] [Google Scholar]
- 80.Wu L.-G., Hamid E., et al. Chiang H.-C. Exocytosis and Endocytosis: Modes, Functions, and Coupling Mechanisms. Annu. Rev. Physiol. 2014;76:301–331. doi: 10.1146/annurev-physiol-021113-170305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao W.-D., Hamid E., et al. Wu L.-G. Hemi-fused structure mediates and controls fusion and fission in live cells. Nature. 2016;534:548–552. doi: 10.1038/nature18598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mizuno N., Jao C.C., et al. Steven A.C. Multiple Modes of Endophilin-mediated Conversion of Lipid Vesicles into Coated Tubes Implications for Synaptic Endocytosis. J. Biol. Chem. 2010;285:23351–23358. doi: 10.1074/jbc.M110.143776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pontes B., Monzo P., Gauthier N.C. Membrane tension: A challenging but universal physical parameter in cell biology. Semin. Cell Dev. Biol. 2017;71:30–41. doi: 10.1016/j.semcdb.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 84.Sheetz M.P., Dai J. Modulation of membrane dynamics and cell motility by membrane tension. Trends Cell Biol. 1996;6:85–89. doi: 10.1016/0962-8924(96)80993-7. [DOI] [PubMed] [Google Scholar]
- 85.Chen H., Detmer S.A., et al. Chan D.C. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Otera H., Ishihara N., Mihara K. New insights into the function and regulation of mitochondrial fission. Biochim. Biophys. Acta. 2013;1833:1256–1268. doi: 10.1016/j.bbamcr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 87.Lee H., Yoon Y. Transient Contraction of Mitochondria Induces Depolarization through the Inner Membrane Dynamin OPA1 Protein. J. Biol. Chem. 2014;289:11862–11872. doi: 10.1074/jbc.M113.533299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee J.E., Westrate L.M., et al. Voeltz G.K. Multiple dynamin family members collaborate to drive mitochondrial division. Nature. 2016;540:139–143. doi: 10.1038/nature20555. https://www.nature.com/articles/nature20555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Abrisch R.G., Gumbin S.C., et al. Voeltz G.K. Fission and fusion machineries converge at ER contact sites to regulate mitochondrial morphology. J. Cell Biol. 2020;219 doi: 10.1083/jcb.201911122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chiu Y.-H., Lin S.-C.A., et al. Li C.-J. Molecular Machinery and Pathophysiology of Mitochondrial Dynamics. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.743892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Al Ojaimi M., Salah A., El-Hattab A.W. Mitochondrial Fission and Fusion: Molecular Mechanisms, Biological Functions, and Related Disorders. Membranes. 2022;12:893. doi: 10.3390/membranes12090893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Q., Tamura Y., et al. Sesaki H. Biosynthesis and roles of phospholipids in mitochondrial fusion, division and mitophagy. Cell. Mol. Life Sci. 2014;71:3767–3778. doi: 10.1007/s00018-014-1648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chernomordik L.V., Kozlov M.M. Membrane Hemifusion: Crossing a Chasm in Two Leaps. Cell. 2005;123:375–382. doi: 10.1016/j.cell.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 94.Praefcke G.J.K., McMahon H.T. The dynamin superfamily: universal membrane tubulation and fission molecules? Nat. Rev. Mol. Cell Biol. 2004;5:133–147. doi: 10.1038/nrm1313. [DOI] [PubMed] [Google Scholar]
- 95.Basu Ball W., Neff J.K., Gohil V.M. The role of nonbilayer phospholipids in mitochondrial structure and function. FEBS Lett. 2018;592:1273–1290. doi: 10.1002/1873-3468.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tian A., Baumgart T. Sorting of Lipids and Proteins in Membrane Curvature Gradients. Biophys. J. 2009;96:2676–2688. doi: 10.1016/j.bpj.2008.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu C.-H., Pedram K., et al. Cui B. Membrane curvature regulates the spatial distribution of bulky glycoproteins. Nat. Commun. 2022;13:3093. doi: 10.1038/s41467-022-30610-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bashkirov P.V., Kuzmin P.I., et al. Frolov V.A. Molecular Shape Solution for Mesoscopic Remodeling of Cellular Membranes. Annu. Rev. Biophys. 2022;51:473–497. doi: 10.1146/annurev-biophys-011422-100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.