Abstract
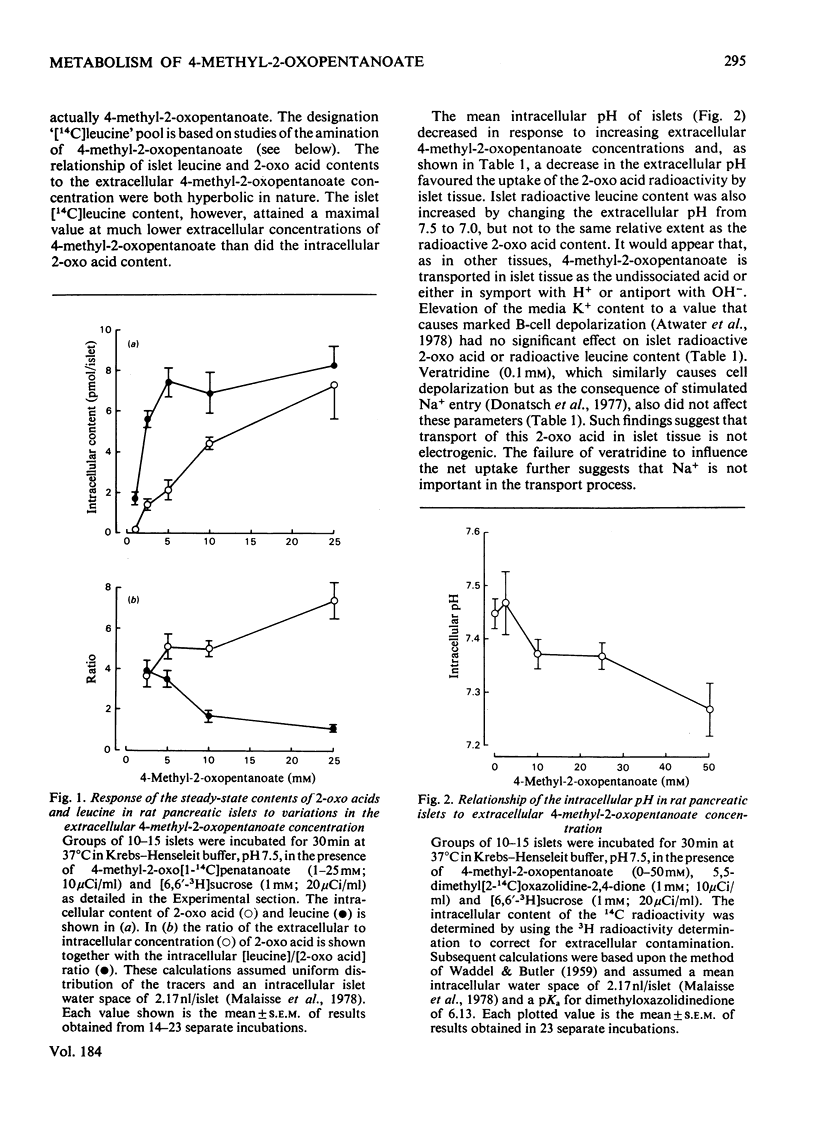
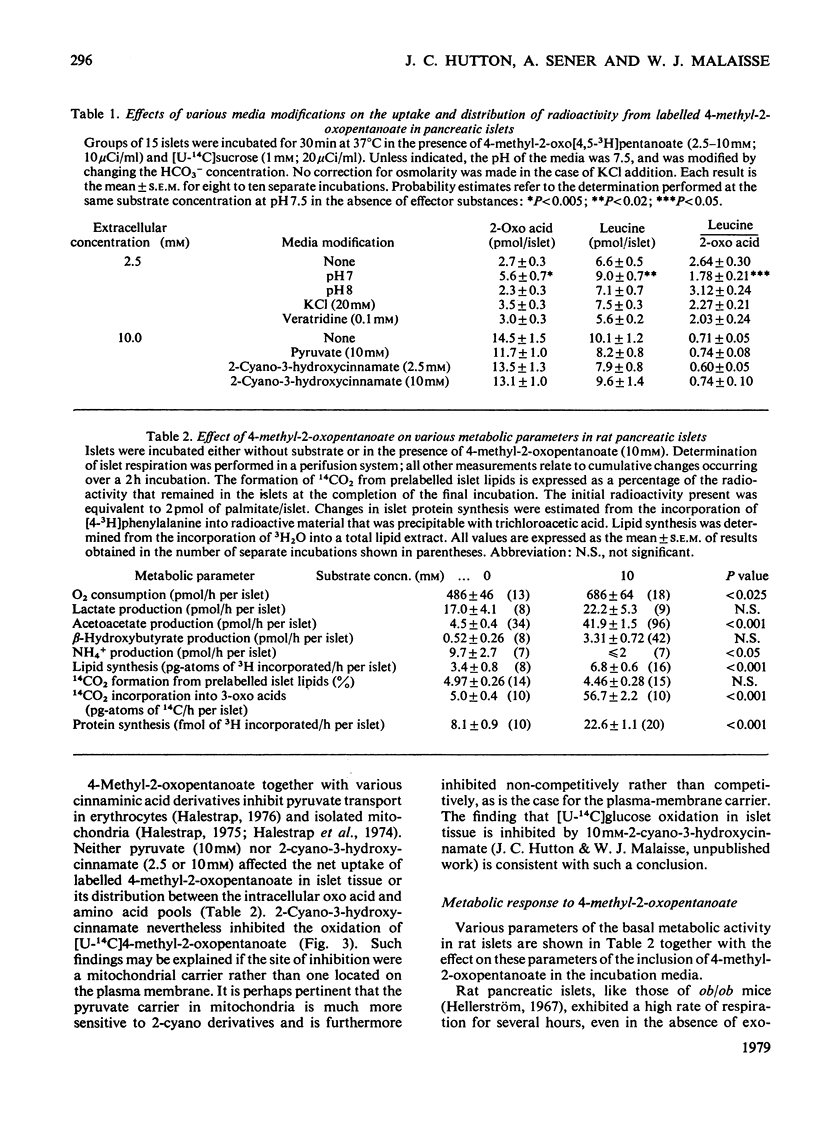
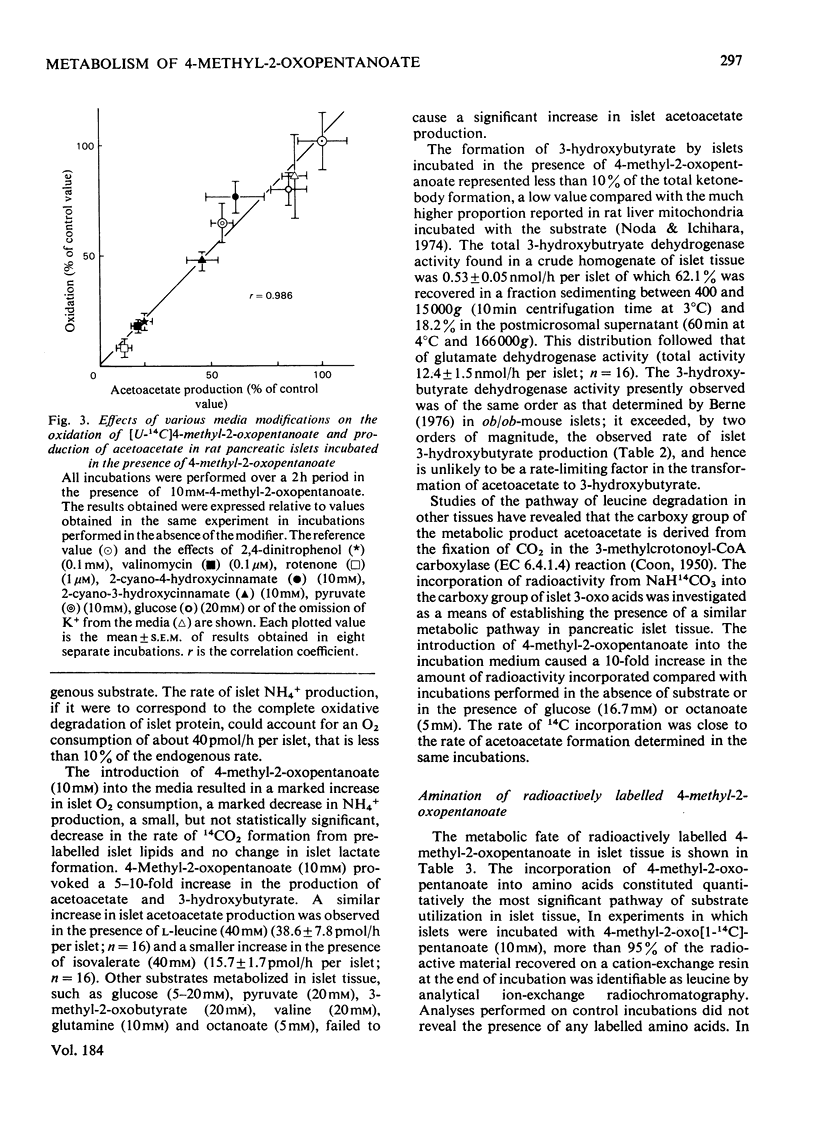
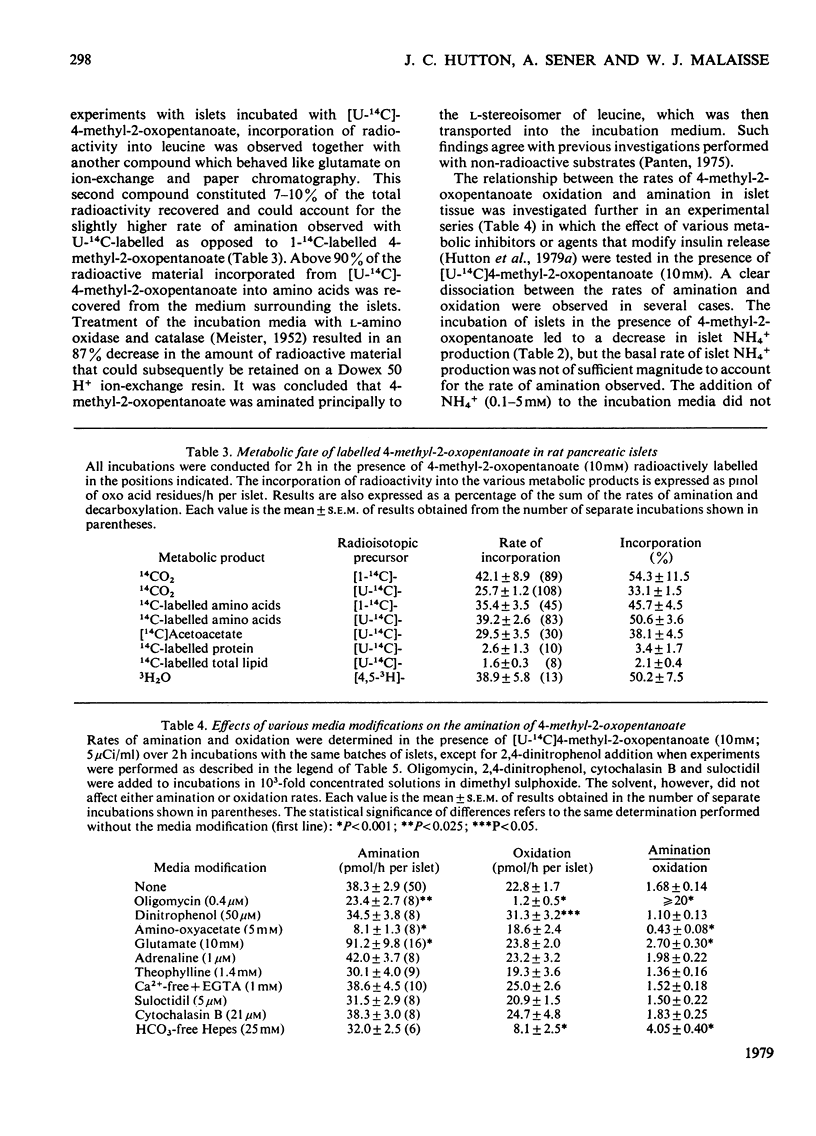
1. Radioactively labelled 4-methyl-2-oxopentanoate was taken up by isolated pancreatic islets in a concentration- and pH-dependent manner and led to the intracellular accumulation of labelled amino acid and to a decrease in the intracellular pH. Uptake of 4-methyl-2-oxopentanoate did not appear to be either electrogenic or Na+-dependent. The islet content of 2-oxo acid radioactivity was not affected by either 2-cyano-3-hydroxy-cinnamate (10mM) or pyruvate (10mM), although both these substances inhibited the oxidation of [U-14C]4-methyl-2-oxopentanoate by islet tissue. 2. 4-Methyl-2-oxopentanoate markedly stimulated islet-cell respiration, ketone-body formation and biosynthetic activity. The metabolism of endogenous nutrients by islets appeared to be little affected by the compound. 3. Studies with the 3H- and 14C-labelled substrate revealed that 4-methyl-2-oxopentanoate was incorporated by islets into CO2, water, acetoacetate, L-leucine and to a lesser extent into islet protein and lipid. Carbon atoms C-2, C-3 and C-4 of the acetoacetate produced were derived from the carbon skeleton of the 4-methyl-2-oxopentanoate, but the acetoacetate carboxy group was derived from the incorporation of CO2. These results, and consideration of the relative rates of 14CO2 and acetoacetate formation from 1-14C-labelled as opposed to U-14C-labelled 4-methyl-2-oxopentanoate, led to the conclusion that the pathway of catabolism of this 2-oxo acid in pancreatic islets is identical with that described in other tissues. The amination of 4-methyl-2-oxopentanoate by islets was attributed to the presence of a branched-chain amino acid aminotransferase (EC 2.6.1.42) activity in the tissue. Although glutamate dehydrogenase activity was demonstrated in islet tissue, the reductive amination of 2-oxoacids did not seem to be of importance in the formation of leucine from 4-methyl-2-oxopentanoate. 4. The results of experiments with respiratory inhibitors and uncouplers, and the finding that 14CO2 production and islet respiration were linked in a 1:1 stoicheiometry suggested that 4-methyl-2-oxopentanoate catabolism was coupled to mitochondrial oxidative phosphorylation. The catabolism of 4-methyl-2-oxopentanoate in islet tissue appeared to be regulated at the level of the initial 2-oxo acid dehydrogenase (EC 1.2.1.25) reaction.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atwater I., Ribalet B., Rojas E. Cyclic changes in potential and resistance of the beta-cell membrane induced by glucose in islets of Langerhans from mouse. J Physiol. 1978 May;278:117–139. doi: 10.1113/jphysiol.1978.sp012296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berne C. Determination of D-3-hydroxybutyrate dehydrogenase in mouse pancreatic islets with a photokinetic technique using bacterial luciferase. Enzyme. 1976;21(2):127–136. doi: 10.1159/000458851. [DOI] [PubMed] [Google Scholar]
- COON M. J. The metabolic fate of the isopropyl group of leucine. J Biol Chem. 1950 Nov;187(1):71–82. [PubMed] [Google Scholar]
- Chaykin S. Assay of nicotinamide deamidase. Determination of ammonia by the indophenol reaction. Anal Biochem. 1969 Oct 1;31(1):375–382. doi: 10.1016/0003-2697(69)90278-4. [DOI] [PubMed] [Google Scholar]
- Donatsch P., Lowe D. A., Richardson B. P., Taylor P. The functional significance of sodium channels in pancreatic beta-cell membranes. J Physiol. 1977 May;267(2):357–376. doi: 10.1113/jphysiol.1977.sp011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gylfe E. Amino acid content as indicator of membrane permeability in pancreatic beta-cells. Horm Res. 1974;5(6):339–343. doi: 10.1159/000178648. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P., Brand M. D., Denton R. M. Inhibition of mitochondrial pyruvate transport by phenylpyruvate and alpha-ketoisocaproate. Biochim Biophys Acta. 1974 Oct 10;367(1):102–108. doi: 10.1016/0005-2736(74)90140-0. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P. The mitochondrial pyruvate carrier. Kinetics and specificity for substrates and inhibitors. Biochem J. 1975 Apr;148(1):85–96. doi: 10.1042/bj1480085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P. Transport of pyruvate nad lactate into human erythrocytes. Evidence for the involvement of the chloride carrier and a chloride-independent carrier. Biochem J. 1976 May 15;156(2):193–207. doi: 10.1042/bj1560193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerström C. Effects of carbohydrates on the oxygen consumption of isolated pancreatic islets of mice. Endocrinology. 1967 Jul;81(1):105–112. doi: 10.1210/endo-81-1-105. [DOI] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. B. The intracellular pH of mammalian pancreatic -cells. Endocrinology. 1972 Jan;90(1):335–337. doi: 10.1210/endo-90-1-335. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Sener A., Malaisse W. J. The stimulus--secretion coupling 4-methyl-2-oxopentanoate-induced insulin release. Biochem J. 1979 Nov 15;184(2):303–311. doi: 10.1042/bj1840303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara A., Koyama E. Transaminase of branched chain amino acids. I. Branched chain amino acids-alpha-ketoglutarate transaminase. J Biochem. 1966 Feb;59(2):160–169. doi: 10.1093/oxfordjournals.jbchem.a128277. [DOI] [PubMed] [Google Scholar]
- Johnson W. A., Connelly J. L. Cellular localization and characterization of bovine liver branched-chain -keto acid dehydrogenases. Biochemistry. 1972 May 9;11(10):1967–1973. doi: 10.1021/bi00760a036. [DOI] [PubMed] [Google Scholar]
- Jungas R. L. Fatty acid synthesis in adipose tissue incubated in tritiated water. Biochemistry. 1968 Oct;7(10):3708–3717. doi: 10.1021/bi00850a050. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Eggleston L. V. Metabolism of acetoacetate in animal tissues. 1. Biochem J. 1945;39(5):408–419. [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Lund P. Aspects of the regulation of the metabolism of branched-chain amino acids. Adv Enzyme Regul. 1976;15:375–394. doi: 10.1016/0065-2571(77)90026-7. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Lenzen S. Effects of alpha-ketocarboxylic acids and 4-pentenoic acid on insulin secretion from the perfused rat pancreas. Biochem Pharmacol. 1978 May 1;27(9):1321–1324. doi: 10.1016/0006-2952(78)90114-4. [DOI] [PubMed] [Google Scholar]
- MEISTER A. Enzymatic preparation of alpha-keto acids. J Biol Chem. 1952 May;197(1):309–317. [PubMed] [Google Scholar]
- Malaisse W. J., Boschero A. C., Kawazu S., Hutton J. C. The stimulus secretion coupling of glucose-induced insulin release. XXVII. Effect of glucose on K+ fluxes in isolated islets. Pflugers Arch. 1978 Mar 20;373(3):237–242. doi: 10.1007/BF00580830. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Brisson G., Malaisse-Lagae F. The stimulus-secretion coupling of glucose-induced insulin release. I. Interaction of epinephrine and alkaline earth cations. J Lab Clin Med. 1970 Dec;76(6):895–902. [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Walker M. O., Lacy P. E. The stimulus-secretion coupling of glucose-induced insulin release. V. The participation of a microtubular-microfilamentous system. Diabetes. 1971 May;20(5):257–265. doi: 10.2337/diab.20.5.257. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Sener A., Mahy M. The stimulus-secretion coupling of glucose-induced insulin release. Sorbitol metabolism in isolated islets. Eur J Biochem. 1974 Sep 1;47(2):365–370. doi: 10.1111/j.1432-1033.1974.tb03701.x. [DOI] [PubMed] [Google Scholar]
- Noda C., Ichihara A. Control of ketogenesis from amino acids. II. Ketone bodies formation from alpha-ketoisocaproate, the keto-analogue of leucine, by rat liver mitochondria. J Biochem. 1974 Nov;76(5):1123–1130. [PubMed] [Google Scholar]
- Panten U. Effects of alpha-ketomonocarboxylic acids upon insulin secretion and metabolism of isolated pancreatic islets. Naunyn Schmiedebergs Arch Pharmacol. 1975;291(4):405–420. doi: 10.1007/BF00501798. [DOI] [PubMed] [Google Scholar]
- Panten U., Kriegstein E. v., Poser W., Schönborn J., Hasselblatt A. Effects of L-leucine and alpha-ketoisocaproic acid upon insulin secretion and metabolism of isolated pancreatic islets. FEBS Lett. 1972 Feb 1;20(2):225–228. doi: 10.1016/0014-5793(72)80801-9. [DOI] [PubMed] [Google Scholar]
- Sener A., Kawazu S., Hutton J. C., Boschero A. C., Devis G., Somers G., Herchuelz A., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. Effect of exogenous pyruvate on islet function. Biochem J. 1978 Oct 15;176(1):217–232. doi: 10.1042/bj1760217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sener A., Malaisse W. J. Measurement of lactic acid in nanomolar amounts. Reliability of such a method as an index of glycolysis in pancreatic islets. Biochem Med. 1976 Feb;15(1):34–41. doi: 10.1016/0006-2944(76)90072-7. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]


