Abstract
1. Although high concentrations of insulin affect both synthesis and degradation of skeletal-muscle protein, it is not known to what extent these effects occur with physiological concentrations. The effects of a physiological concentration of insulin (100 mu units/ml) on muscle protein synthesis, measured with [3H]tyrosine, and on muscle protein degradation, measured by tyrosine release in the presence of cycloheximide, were studied in mouse soleus and extensor digitorum longus muscles in vitro. 2. Insulin significantly stimualated protein synthesis in both muscles, but an inhibition of degradation was seen only in the extensor digitorum longus. 3. Starvation for 24 h decreased the rate of protein synthesis and increased the rate of breakdown in the extensor digitorum longus. Sensitivity to insulin-stimulation of proteins synthesis in the soleus was increased by starvation. 4. ;a 20%-surface-area full-skin-thickness dorsal scald injury produced a fall in total protein content in soleus and extensor digitorum muscles, maximal on the third day after injury. Soleus muscles 2 days after injury showed an impairment of protein synthesis; degradation was unaffected and neither synthesis nor degradation in vitro was significantly affected in the extensor digitorum longus. 5. The advantages and limitations of studies of protein metabolism in vitro are discussed.
Full text
PDF

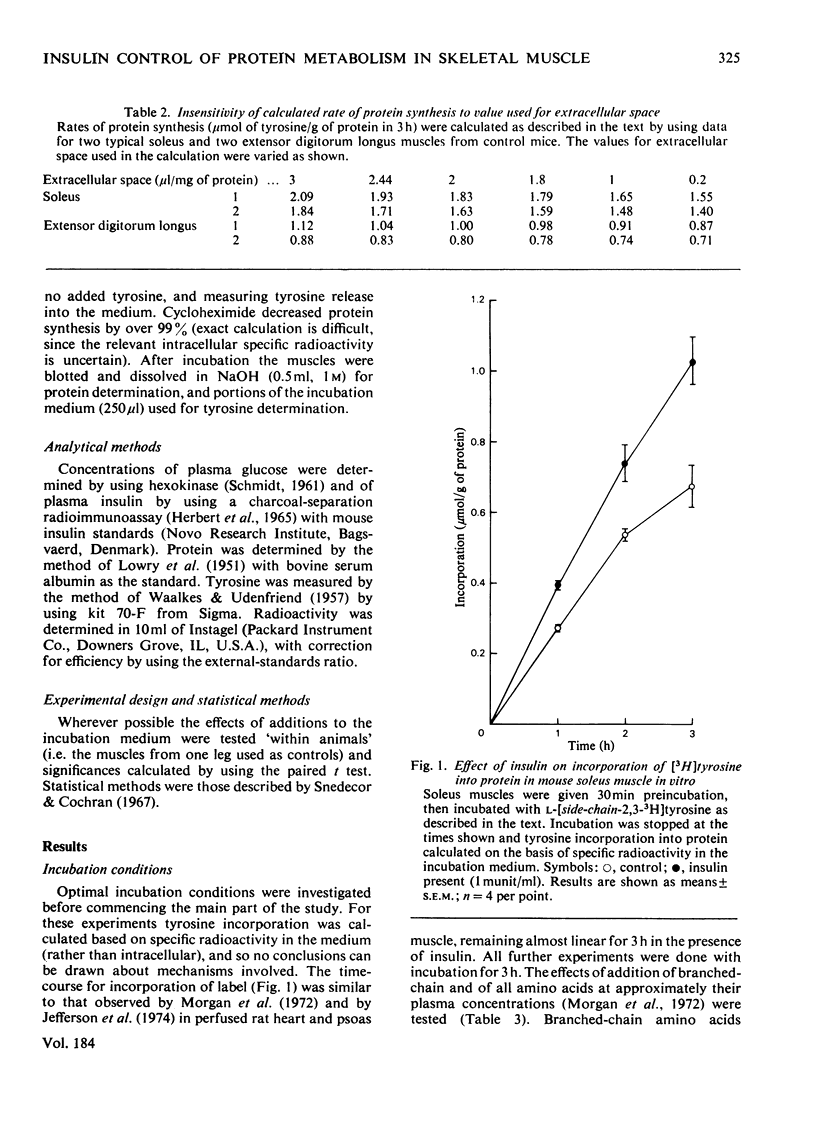




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adolfsson S., Arvill A., Ahrén K. Stimulation by insulin of accumulation and incorporation into protein of L-[3H]proline in the intact levator ani muscle from the rat. Biochim Biophys Acta. 1967 Feb 1;135(1):176–178. doi: 10.1016/0005-2736(67)90024-7. [DOI] [PubMed] [Google Scholar]
- Bilmazes C., Kien C. L., Rohrbaugh D. K., Uauy R., Burke J. F., Munro H. N., Young V. R. Quantitative contribution by skeletal muscle to elevated rates of whole-body protein breakdown in burned children as measured by N tau-methylhistidine output. Metabolism. 1978 Jun;27(6):671–676. doi: 10.1016/0026-0495(78)90004-5. [DOI] [PubMed] [Google Scholar]
- Brennan M. F., Tweedle D., Moore F. D. Letter: Body nitrogen after trauma. Lancet. 1975 Jan 4;1(7897):38–39. doi: 10.1016/s0140-6736(75)92399-5. [DOI] [PubMed] [Google Scholar]
- Chain E. B., Sender P. M. Protein synthesis by perfused hearts from normal and insulin-deficient rats. Effect of insulin in the presence of glucose and after depletion of glucose, glucose 6-phosphate and glycogen. Biochem J. 1973 Mar;132(3):593–601. doi: 10.1042/bj1320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudry I. H., Gould M. K. Kinetics of glucose uptake in isolated soleus muscle. Biochim Biophys Acta. 1969 May 6;177(3):527–536. doi: 10.1016/0304-4165(69)90315-8. [DOI] [PubMed] [Google Scholar]
- Crane C. W., Picou D., Smith R., Waterlow J. C. Protein turnover in patients before and after elective orthopaedic operations. Br J Surg. 1977 Feb;64(2):129–133. doi: 10.1002/bjs.1800640212. [DOI] [PubMed] [Google Scholar]
- Cuendet G. S., Loten E. G., Jeanrenaud B., Renold A. E. Decreased basal, noninsulin-stimulated glucose uptake and metabolism by skeletal soleus muscle isolated from obese-hyperglycemic (ob/ob) mice. J Clin Invest. 1976 Nov;58(5):1078–1088. doi: 10.1172/JCI108559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel P. M. The metabolic homoeostatic role of muscle and its function as a store of protein. Lancet. 1977 Aug 27;2(8035):446–448. doi: 10.1016/s0140-6736(77)90622-5. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J. Protein turnover and amino acid metabolism in the regulation of gluconeogenesis. Fed Proc. 1974 Apr;33(4):1092–1097. [PubMed] [Google Scholar]
- Fern E. B., Garlick P. J. The specific radioactivity of the precursor pool for estimates of the rate of protein synthesis. Biochem J. 1973 Aug;134(4):1127–1130. doi: 10.1042/bj1341127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt J. P., Blackburn G. L. The matabolic fuel regulatory system: implications for protein-sparing therapies during caloric deprivation and disease. Am J Clin Nutr. 1974 Feb;27(2):175–187. doi: 10.1093/ajcn/27.2.175. [DOI] [PubMed] [Google Scholar]
- Frayn K. N., Le Marchand-Brustel Y., Freychet P. Studies on the mechanism of insulin resistance after injury in the mouse. Diabetologia. 1978 May;14(5):337–341. doi: 10.1007/BF01223026. [DOI] [PubMed] [Google Scholar]
- Frayn K. N., Maycock P. F. Apparent effect of octan-1-ol on glycogen metabolism in muscle in vitro. Horm Metab Res. 1979 Mar;11(3):255–255. doi: 10.1055/s-0028-1095774. [DOI] [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Goldberg A. L., Goodman H. M. Relationship between cortisone and muscle work in determining muscle size. J Physiol. 1969 Feb;200(3):667–675. doi: 10.1113/jphysiol.1969.sp008715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L. Protein synthesis in tonic and phasic skeletal muscles. Nature. 1967 Dec 23;216(5121):1219–1220. doi: 10.1038/2161219a0. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Gardner J. D., Neville D. M., Jr Insulin action in isolated rat thymocytes. I. Binding of 125 I-insulin and stimulation of -aminoisobutyric acid transport. J Biol Chem. 1972 Nov 10;247(21):6919–6926. [PubMed] [Google Scholar]
- Goldspink D. F. The effects of denervation on protein turnover of rat skeletal muscle. Biochem J. 1976 Apr 15;156(1):71–80. doi: 10.1042/bj1560071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink D. F. The influence of passive stretch on the growth and protein turnover of the denervated extensor digitorum longus muscle. Biochem J. 1978 Aug 15;174(2):595–602. doi: 10.1042/bj1740595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S., Reddy W. J. Insulin and protein synthesis in muscle. Arch Biochem Biophys. 1970 Sep;140(1):181–189. doi: 10.1016/0003-9861(70)90021-4. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Hinton P., Allison S. P. Insulin and glucose to reduce catabolic response to burns. Lancet. 1971 Jun 12;1(7711):1247–1247. doi: 10.1016/s0140-6736(71)91767-3. [DOI] [PubMed] [Google Scholar]
- Hoffmann C. C., Carroll K. F., Goldrick R. B. Studies on lipid and carbohydrate metabolism in the rat. Effects of diet on body composition, plasma glucose and insulin concentrations, insulin secretion in vitro and tolerance to intravenous glucose and intravenous glucose and intravenous insulin. Aust J Exp Biol Med Sci. 1972 Jun;50(3):267–287. [PubMed] [Google Scholar]
- Hoover-Plow J. L., Clifford A. J. The effect of surgical trauma on muscle protein turnover in rats. Biochem J. 1978 Oct 15;176(1):137–142. doi: 10.1042/bj1760137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson L. S., Li J. B., Rannels S. R. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. J Biol Chem. 1977 Feb 25;252(4):1476–1483. [PubMed] [Google Scholar]
- Jefferson L. S., Rannels D. E., Munger B. L., Morgan H. E. Insulin in the regulation of protein turnover in heart and skeletal muscle. Fed Proc. 1974 Apr;33(4):1098–1104. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurent G. J., Sparrow M. P., Bates P. C., Millward D. J. Turnover of muscle protein in the fowl (Gallus domesticus). Rates of protein synthesis in fast and slow skeletal, cardiac and smooth muscle of the adult fowl. Biochem J. 1978 Nov 15;176(2):393–401. doi: 10.1042/bj1760393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. B., Goldberg A. L. Effects of food deprivation on protein synthesis and degradation in rat skeletal muscles. Am J Physiol. 1976 Aug;231(2):441–448. doi: 10.1152/ajplegacy.1976.231.2.441. [DOI] [PubMed] [Google Scholar]
- Lundholm K., Scherstén T. Protein synthesis in human skeletal muscle tissue: influence of insulin and amino acids. Eur J Clin Invest. 1977 Dec;7(6):531–536. doi: 10.1111/j.1365-2362.1977.tb01647.x. [DOI] [PubMed] [Google Scholar]
- MANCHESTER K. L., RANDLE P. J., YOUNG F. G. An insulin assay based on the incorporation of labelled glycine into protein of isolated rat diaphragm. J Endocrinol. 1959 Dec;19:259–262. doi: 10.1677/joe.0.0190259. [DOI] [PubMed] [Google Scholar]
- MANCHESTER K. L., YOUNG F. G. Hormones and protein biosynthesis in isolated rat diaphragm. J Endocrinol. 1959 Jul;18:381–394. doi: 10.1677/joe.0.0180381. [DOI] [PubMed] [Google Scholar]
- Maizels E. Z., Ruderman N. B., Goodman M. N., Lau D. Effect of acetoacetate on glucose metabolism in the soleus and extensor digitorum longus muscles of the rat. Biochem J. 1977 Mar 15;162(3):557–568. doi: 10.1042/bj1620557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M., Shafrir E., Kaiser N., Milholland R. J., Rosen F. Interaction of glucocorticoid hormones with rat skeletal muscle: catabolic effects and hormone binding. Metabolism. 1976 Feb;25(2):157–167. doi: 10.1016/0026-0495(76)90046-9. [DOI] [PubMed] [Google Scholar]
- Millward D. J., Waterlow J. C. Effect of nutrition on protein turnover in skeletal muscle. Fed Proc. 1978 Jul;37(9):2283–2290. [PubMed] [Google Scholar]
- O'Keefe S. J., Sender P. M., James W. P. "Catabolic" loss of body nitrogen in response to surgery. Lancet. 1974 Nov 2;2(7888):1035–1038. doi: 10.1016/s0140-6736(74)92149-7. [DOI] [PubMed] [Google Scholar]
- Pozefsky T., Felig P., Tobin J. D., Soeldner J. S., Cahill G. F., Jr Amino acid balance across tissues of the forearm in postabsorptive man. Effects of insulin at two dose levels. J Clin Invest. 1969 Dec;48(12):2273–2282. doi: 10.1172/JCI106193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannels D. E., Kao R., Morgan H. E. Effect of insulin on protein turnover in heart muscle. J Biol Chem. 1975 Mar 10;250(5):1694–1701. [PubMed] [Google Scholar]
- Ruderman N. B. Muscle amino acid metabolism and gluconeogenesis. Annu Rev Med. 1975;26:245–258. doi: 10.1146/annurev.me.26.020175.001333. [DOI] [PubMed] [Google Scholar]
- Röthig H. J., Stiller N., Dahlmann B., Reinauer H. Insulin effect on proteolytic activities in rat skeletal muscle. Horm Metab Res. 1978 Mar;10(2):101–104. doi: 10.1055/s-0028-1093452. [DOI] [PubMed] [Google Scholar]
- SCHMIDT F. H. [Enzymatic determination of glucose and fructose simultaneously]. Klin Wochenschr. 1961 Dec 1;39:1244–1247. doi: 10.1007/BF01506150. [DOI] [PubMed] [Google Scholar]
- SRETER F. A., WOO G. CELL WATER, SODIUM, AND POTASSIUM IN RED AND WHITE MAMMALIAN MUSCLES. Am J Physiol. 1963 Dec;205:1290–1294. doi: 10.1152/ajplegacy.1963.205.6.1290. [DOI] [PubMed] [Google Scholar]
- Short F. A. Protein synthesis by red and white muscle in vitro: effect of insulin and animal age. Am J Physiol. 1969 Jul;217(1):307–309. doi: 10.1152/ajplegacy.1969.217.1.307. [DOI] [PubMed] [Google Scholar]
- Tomas F. M., Munro H. N., Young V. R. Effect of glucocorticoid administration on the rate of muscle protein breakdown in vivo in rats, as measured by urinary excretion of N tau-methylhistidine. Biochem J. 1979 Jan 15;178(1):139–146. doi: 10.1042/bj1780139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner L. V., Garlick P. J. The effect of unilateral phrenicectomy on the rate of protein synthesis in rat diaphragm in vivo. Biochim Biophys Acta. 1974 Apr 27;349(1):109–113. doi: 10.1016/0005-2787(74)90013-6. [DOI] [PubMed] [Google Scholar]
- WAALKES T. P., UDENFRIEND S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med. 1957 Nov;50(5):733–736. [PubMed] [Google Scholar]
- WOOL I. G., MANCHESTER K. L. Insulin and incorporation of amino-acids into protein of rat tissues. Nature. 1962 Jan 27;193:345–346. doi: 10.1038/193345a0. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Farrell R., Kerr A., Smith R. Muscle-protein catabolism after injury in man, as measured by urinary excretion of 3-methylhistidine. Clin Sci Mol Med. 1977 May;52(5):527–533. doi: 10.1042/cs0520527. [DOI] [PubMed] [Google Scholar]
- Young V. R., Munro H. N. Ntau-methylhistidine (3-methylhistidine) and muscle protein turnover: an overview. Fed Proc. 1978 Jul;37(9):2291–2300. [PubMed] [Google Scholar]


