Abstract
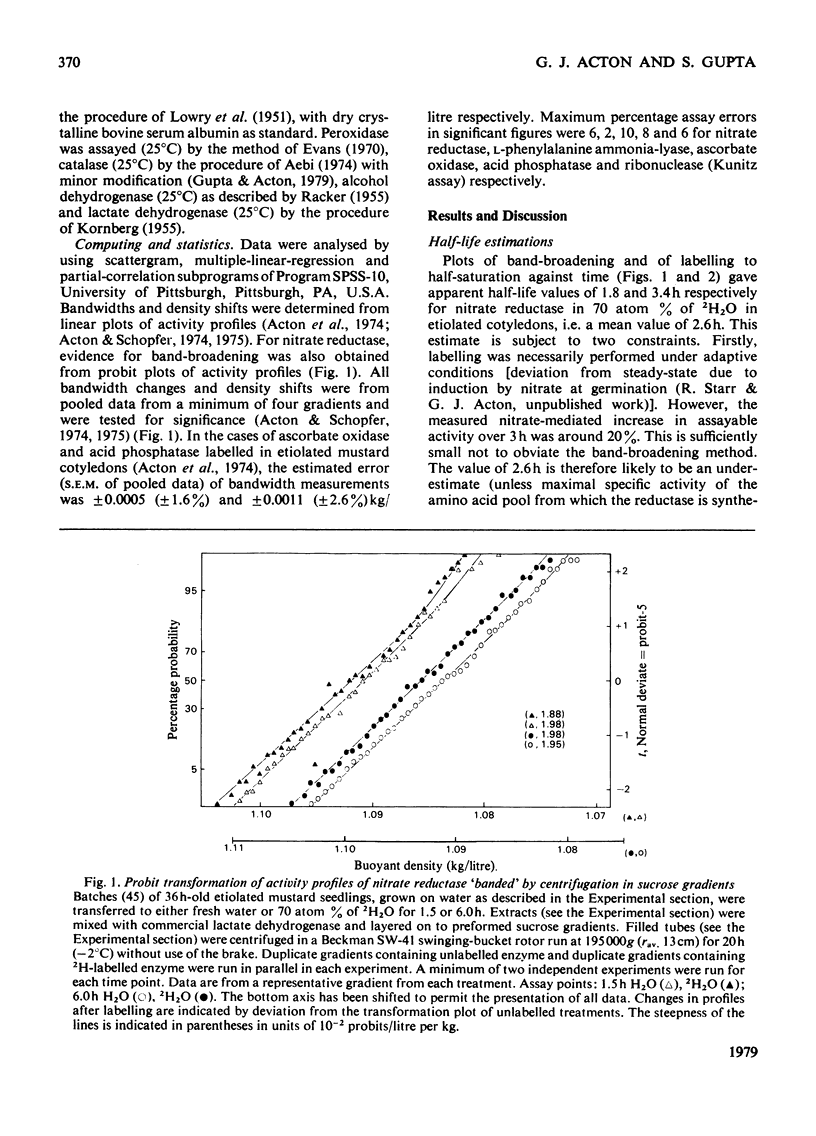
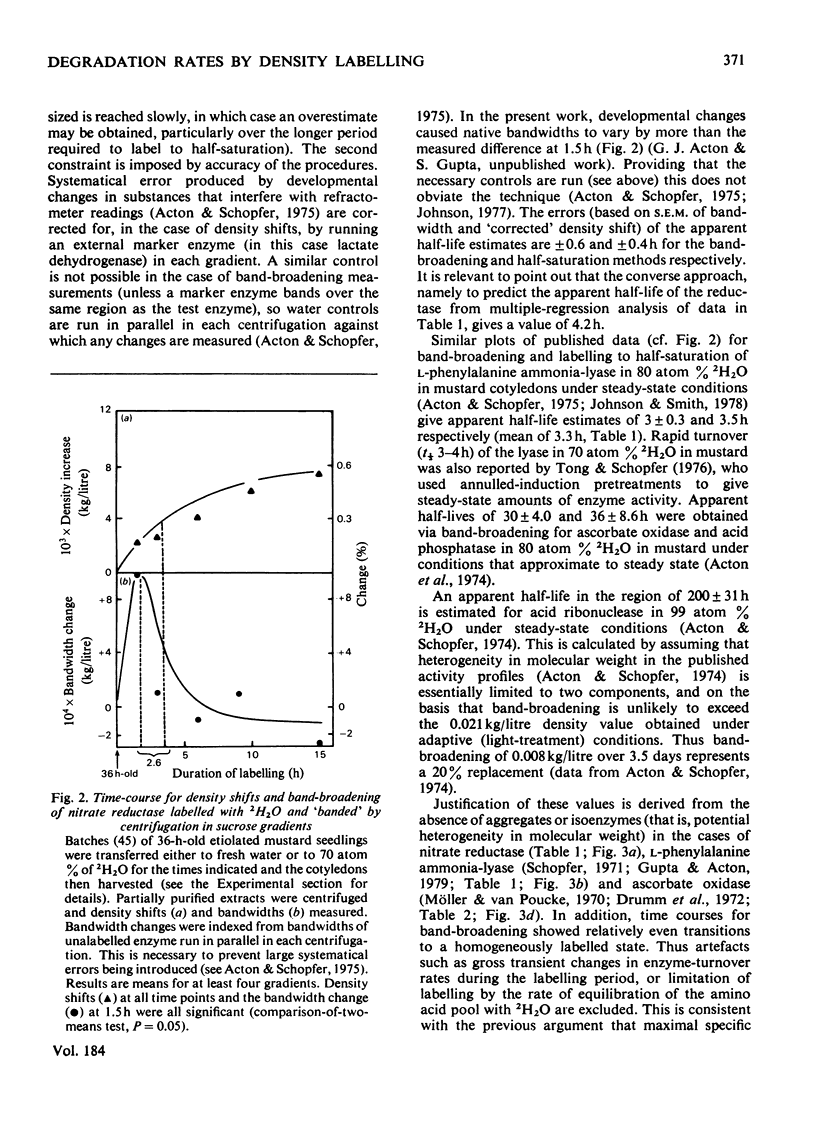
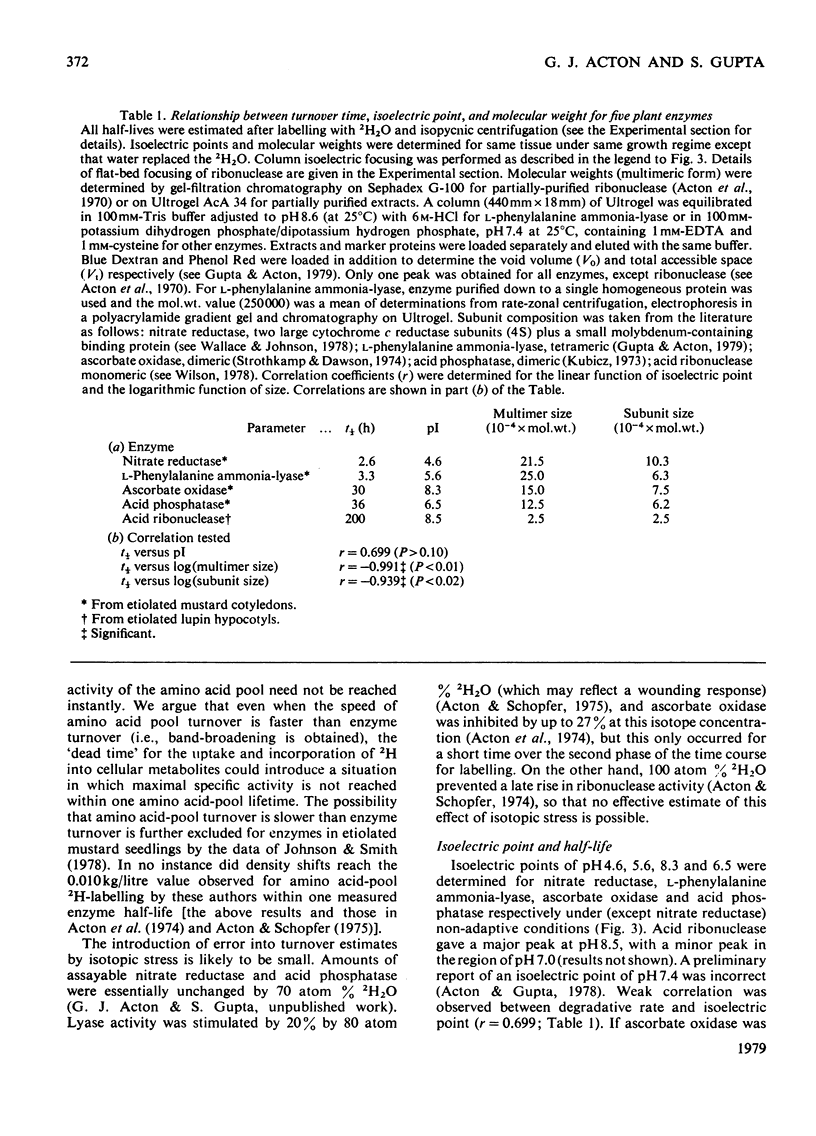
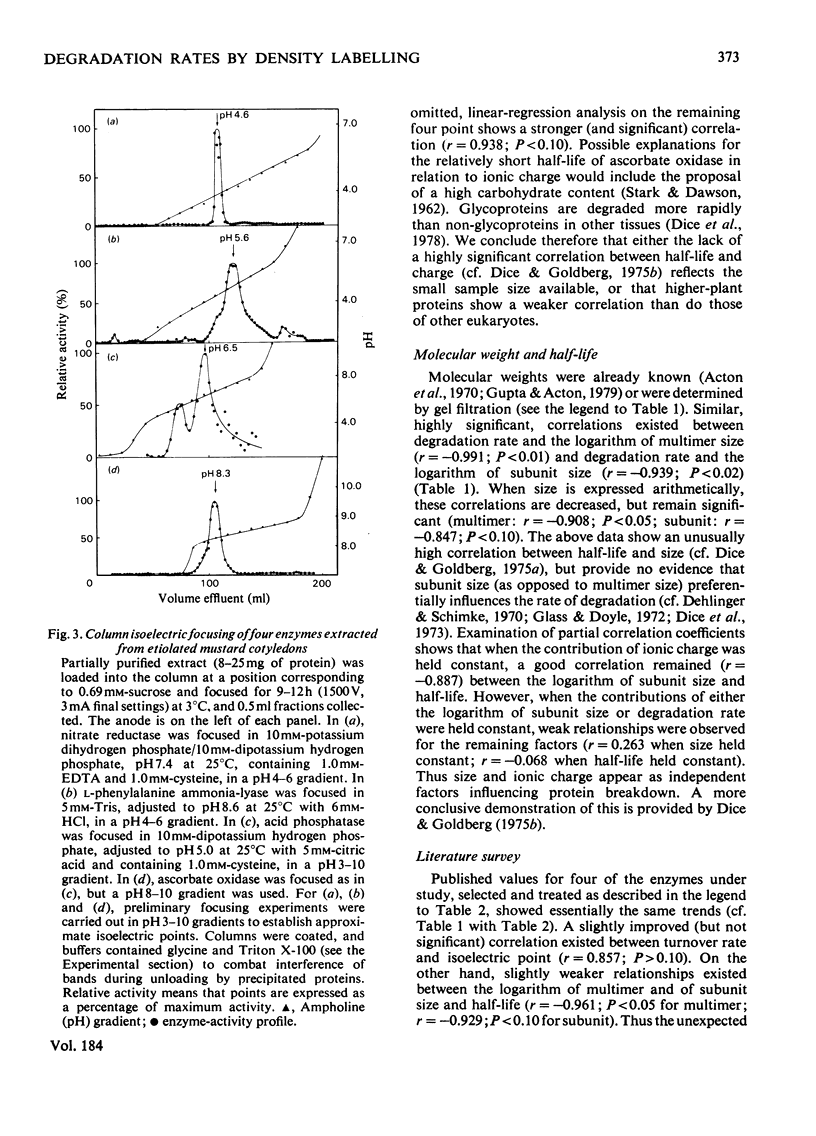
1. Half-lives of five plant enzymes were estimated by rate-labeling with 2H2O on the assumption that loss of catalytic activity is equivalent to protein degradation. 2. This involved measuring band-broadening of activity profiles after isopycnic centrifugation. 3. Isoelectric points were determined by isoelectric focusing, and molecular weights were estimated by gel filtration. 4. The conclusion is drawn from the experimental evidence presented that a weak correlation exists between rates of degradation and isoelectric points (r = 0.699; P greater than 0.10; not significant). 5. A highly significant relationship exists between the logarithm of subunit size and half-life (r = -0.939; P greater than 0.02). 6. A literature survey confirmed the trends observed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acton G. J., Lewington R. J., Myers A. The effect of light upon ribonuclease activity of etiolated Lupinus albus hypocotyls. Biochim Biophys Acta. 1970 Mar 19;204(1):144–155. doi: 10.1016/0005-2787(70)90497-1. [DOI] [PubMed] [Google Scholar]
- Acton G. J., Schopfer P. Control over activation or synthesis of phenylalanine ammonia-lyase by phytochrome in mustard (Sinapis alba L.)? A contribution to eliminate some misconceptions. Biochim Biophys Acta. 1975 Oct 9;404(2):231–242. doi: 10.1016/0304-4165(75)90329-3. [DOI] [PubMed] [Google Scholar]
- Acton G. J., Schopfer P. Phytochrome-induced synthesis of ribonuclease de novo in lupin hypocotyl sections. Biochem J. 1974 Sep;142(3):449–455. doi: 10.1042/bj1420449a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias I. M., Doyle D., Schimke R. T. Studies on the synthesis and degradation of proteins of the endoplasmic reticulum of rat liver. J Biol Chem. 1969 Jun 25;244(12):3303–3315. [PubMed] [Google Scholar]
- Aslam M., Oaks A. Comparative studies on the induction and inactivation of nitrate reductase in corn roots and leaves. Plant Physiol. 1976 Apr;57(4):572–576. doi: 10.1104/pp.57.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attridge T. H., Johnson C. B., Smith H. Density-labelling evidence for the phytochrome-mediated activation of phenylalanine ammonia-lyase in mustard cotyledons. Biochim Biophys Acta. 1974 May 24;343(3):440–451. doi: 10.1016/0304-4165(74)90261-x. [DOI] [PubMed] [Google Scholar]
- Betsche T., Gerhardt B. Apparent Catalase Synthesis in Sunflower Cotyledons during the Change in Microbody Function: A Mathematical Approach for the Quantitative Evaluation of Density-labeling Data. Plant Physiol. 1978 Oct;62(4):590–597. doi: 10.1104/pp.62.4.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz B., Schäfer E., Hahlbrock K. Light-induced phenylalanine ammonia-lyase in cell-suspension cultures of Petroselinum hortense. Quantitative comparison of rates of synthesis and degradation. Arch Biochem Biophys. 1978 Sep;190(1):126–135. doi: 10.1016/0003-9861(78)90259-x. [DOI] [PubMed] [Google Scholar]
- Boudet A., Humphrey T. J., Davies D. D. The measurement of protein turnover by density labelling. Biochem J. 1975 Nov;152(2):409–416. doi: 10.1042/bj1520409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNN F. J., DAWSON C. R. On the nature of ascorbic acid oxidase. J Biol Chem. 1951 Apr;189(2):485–497. [PubMed] [Google Scholar]
- Davies D. D., Humphrey T. J. Amino Acid recycling in relation to protein turnover. Plant Physiol. 1978 Jan;61(1):54–58. doi: 10.1104/pp.61.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehlinger P. J., Schimke R. T. Effect of size on the relative rate of degradation of rat liver soluble proteins. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1473–1480. doi: 10.1016/0006-291x(70)90034-3. [DOI] [PubMed] [Google Scholar]
- Dehlinger P. J., Schimke R. T. Size distribution of membrane proteins of rat liver and their relative rates of degradation. J Biol Chem. 1971 Apr 25;246(8):2574–2583. [PubMed] [Google Scholar]
- Dice J. F., Dehlinger P. J., Schimke R. T. Studies on the correlation between size and relative degradation rate of soluble proteins. J Biol Chem. 1973 Jun 25;248(12):4220–4228. [PubMed] [Google Scholar]
- Dice J. F., Goldberg A. L. A statistical analysis of the relationship between degradative rates and molecular weights of proteins. Arch Biochem Biophys. 1975 Sep;170(1):213–219. doi: 10.1016/0003-9861(75)90112-5. [DOI] [PubMed] [Google Scholar]
- Dice J. F., Goldberg A. L. Relationship between in vivo degradative rates and isoelectric points of proteins. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3893–3897. doi: 10.1073/pnas.72.10.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dice J. F., Hess E. J., Goldberg A. L. Studies on the relationship between the degradative rates of proteins in vivo and their isoelectric points. Biochem J. 1979 Feb 15;178(2):305–312. doi: 10.1042/bj1780305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dice J. F., Walker C. D., Byrne B., Cardiel A. General characteristics of protein degradation in diabetes and starvation. Proc Natl Acad Sci U S A. 1978 May;75(5):2093–2097. doi: 10.1073/pnas.75.5.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchesne M., Fritig B., Hirth L. Phenylalanine ammonia-lyase in tobacco mosaic virus-infected hypersensitive tobacco. Density-labelling evidence of de novo synthesis. Biochim Biophys Acta. 1977 Dec 8;485(2):465–481. doi: 10.1016/0005-2744(77)90182-6. [DOI] [PubMed] [Google Scholar]
- Evans J. J. Spectral similarities and kinetic differences of two tomato plant peroxidase isoenzymes. Plant Physiol. 1970 Jan;45(1):66–69. doi: 10.1104/pp.45.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filner P., Varner J. E. A test for de novo synthesis of enzymes: density labeling with H2O18 of barley alpha-amylase induced by gibberellic acid. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1520–1526. doi: 10.1073/pnas.58.4.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B., Esra G., Cain M. J., Rossman S., Johnson C. Myelomonocytic leukemia in an orangutan. Vet Pathol. 1978 Sep;15(5):667–670. doi: 10.1177/030098587801500510. [DOI] [PubMed] [Google Scholar]
- Glass R. D., Doyle D. On the measurement of protein turnover in animal cells. J Biol Chem. 1972 Aug 25;247(16):5234–5242. [PubMed] [Google Scholar]
- Goldberg A. L., Dice J. F. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem. 1974;43(0):835–869. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Havir E. A., Hanson K. R. L-phenylalanine ammonia-lyase (maize and potato). Evidence that the enzyme is composed of four subunits. Biochemistry. 1973 Apr 10;12(8):1583–1591. doi: 10.1021/bi00732a019. [DOI] [PubMed] [Google Scholar]
- Havir E. A. l-Phenylalanine Ammonia-Lyase (Maize): Evidence for a Common Catalytic Site for l-Phenylalanine and l-Tyrosine. Plant Physiol. 1971 Aug;48(2):130–136. doi: 10.1104/pp.48.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer Y. M., Filner P. Regulation of the nitrate assimilation pathway in cultured tobacco cells. 3. The nitrate uptake system. Biochim Biophys Acta. 1971 Feb 23;230(2):362–372. doi: 10.1016/0304-4165(71)90223-6. [DOI] [PubMed] [Google Scholar]
- Kubicz A. Acid phosphatase 3 from potato tubers, molecular weight and subunit structure. Acta Biochim Pol. 1973;20(3):223–229. [PubMed] [Google Scholar]
- Kubicz A., Morawiecka B., Kruzel M. Heterogeneity of acid phosphatase from potato tubers. Acta Biochim Pol. 1974;21(2):113–117. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee M. H., Dawson C. R. Ascorbate oxidase. Further studies on the purification of the enzyme. J Biol Chem. 1973 Oct 10;248(19):6596–6602. [PubMed] [Google Scholar]
- Marchesini A., Capelletti P., Canonica L., Danieli B., Tollari S. Evidence about the catecholoxidase activity of the enzyme ascorbate oxidase extracted from Cucurbita pepo medullosa. Biochim Biophys Acta. 1977 Oct 13;484(2):290–300. doi: 10.1016/0005-2744(77)90085-7. [DOI] [PubMed] [Google Scholar]
- Momany F. A., Aguanno J. J., Larrabee A. R. Correlation of degradative rates of proteins with a parameter calculated from amino acid composition and subunit size. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3093–3097. doi: 10.1073/pnas.73.9.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Makino N., Ogura Y. Purification and properties of ascorbate oxidase from cucumber. J Biochem. 1968 Aug;64(2):189–195. doi: 10.1093/oxfordjournals.jbchem.a128879. [DOI] [PubMed] [Google Scholar]
- Naya J., Vigne J. L., De Castro F. T. The dynamic state of Tetrahymena pyriformis cytosol proteins during culture development. FEBS Lett. 1977 Apr 15;76(2):269–273. doi: 10.1016/0014-5793(77)80166-x. [DOI] [PubMed] [Google Scholar]
- Oaks A., Wallace W., Stevens D. Synthesis and turnover of nitrate reductase in corn roots. Plant Physiol. 1972 Dec;50(6):649–654. doi: 10.1104/pp.50.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin J. W. Distribution and development of nitrate reductase activity in germinating cotton seedlings. Plant Physiol. 1974 Mar;53(3):458–463. doi: 10.1104/pp.53.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radola B. J. Isoelectric focusing in layers of granulated gels. II. Preparative isoelectric focusing. Biochim Biophys Acta. 1975 Mar 28;386(1):181–195. doi: 10.1016/0005-2795(75)90258-5. [DOI] [PubMed] [Google Scholar]
- STARK G. R., DAWSON C. R. On the accessibility of sulfhydryl groups in ascorbic acid oxidase. J Biol Chem. 1962 Mar;237:712–716. [PubMed] [Google Scholar]
- Schrader L. E., Ritenour G. L., Eilrich G. L., Hageman R. H. Some characteristics of nitrate reductase from higher plants. Plant Physiol. 1968 Jun;43(6):930–940. doi: 10.1104/pp.43.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal H. L. Mechanism and regulation of protein turnover in animal cells. Curr Top Cell Regul. 1976;11:183–201. doi: 10.1016/b978-0-12-152811-9.50012-2. [DOI] [PubMed] [Google Scholar]
- Strothkamp K. G., Dawson C. R. Concerning the quaternary structure of ascorbate oxidase. Biochemistry. 1974 Jan 29;13(3):434–440. doi: 10.1021/bi00700a006. [DOI] [PubMed] [Google Scholar]
- Tischner R. Light-mediated Activation of Nitrate Reductase in Synchronous Chlorella. Plant Physiol. 1978 Aug;62(2):284–286. doi: 10.1104/pp.62.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W. F., Schopfer P. Phytochrome-mediated de novo synthesis of phenylalanine ammonia-lyase: An approach using pre-induced mustard seedlings. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4017–4021. doi: 10.1073/pnas.73.11.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis R. L., Jordan W. R., Huffaker R. C. Evidence for an Inactivating System of Nitrate Reductase in Hordeum vulgare L. during Darkness That Requires Protein Synthesis. Plant Physiol. 1969 Aug;44(8):1150–1156. doi: 10.1104/pp.44.8.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara K., Fujimoto S., Taniguchi T. Studies on violet-colored acid phosphatase of sweet potato. I. Purification and some physical properties. J Biochem. 1974 Mar;75(3):627–638. doi: 10.1093/oxfordjournals.jbchem.a130431. [DOI] [PubMed] [Google Scholar]
- WILLS E. D. Enzyme inhibition by suramin and the measurement of the isoelectric points of some enzymes. Biochem J. 1952 Jan;50(3):421–425. doi: 10.1042/bj0500421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace W., Johnson C. B. Nitrate Reductase and Soluble Cytochrome c Reductase(s) in Higher Plants. Plant Physiol. 1978 May;61(5):748–752. doi: 10.1104/pp.61.5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmann E., Schopfer P. Phytochrome-mediated de Novo Synthesis of Phenylalanine Ammonia-Lyase in Cell Suspension Cultures of Parsley. Plant Physiol. 1975 May;55(5):822–827. doi: 10.1104/pp.55.5.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells G. N., Hageman R. H. Specificity for nicotinamide adenine dinucleotide by nitrate reductase from leaves. Plant Physiol. 1974 Aug;54(2):136–141. doi: 10.1104/pp.54.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. M. Plant Nucleases: V. Survey of Corn Ribonuclease II Isoenzymes. Plant Physiol. 1978 May;61(5):861–863. doi: 10.1104/pp.61.5.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielke H. R., Filner P. Synthesis and turnover of nitrate reductase induced by nitrate in cultured tobacco cells. J Biol Chem. 1971 Mar 25;246(6):1772–1779. [PubMed] [Google Scholar]


