Abstract
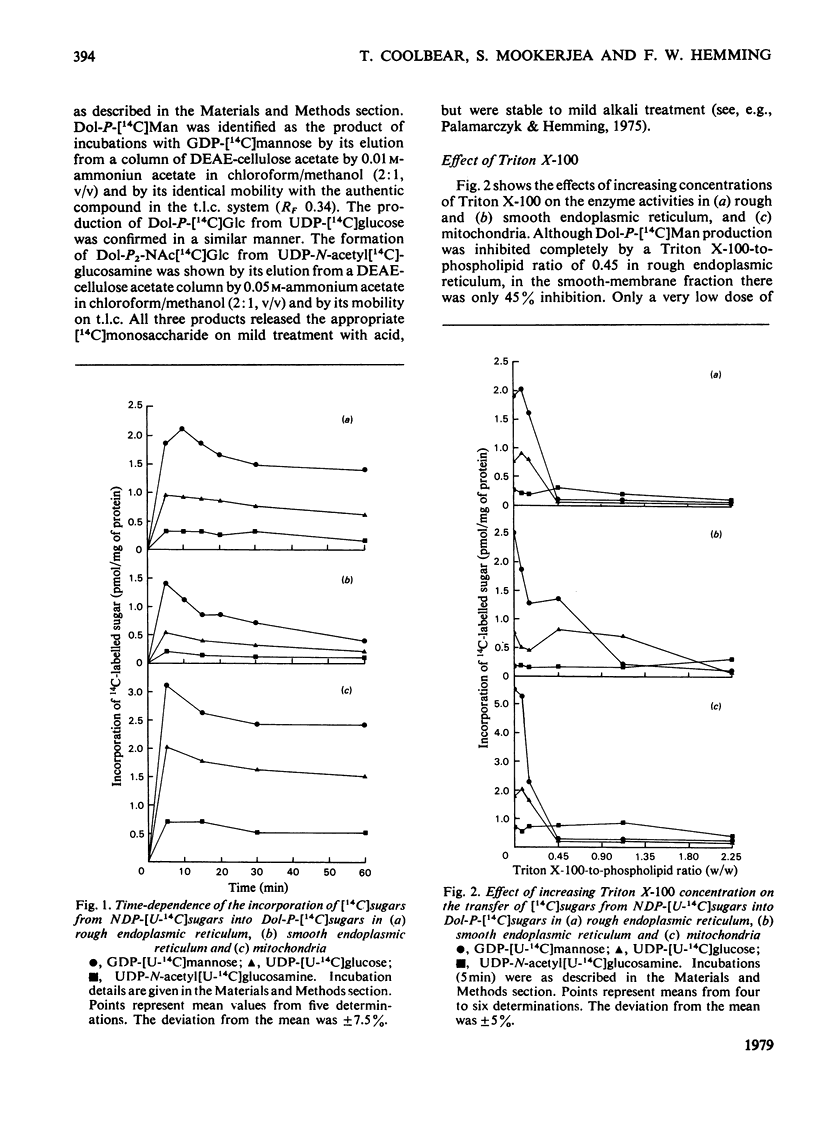
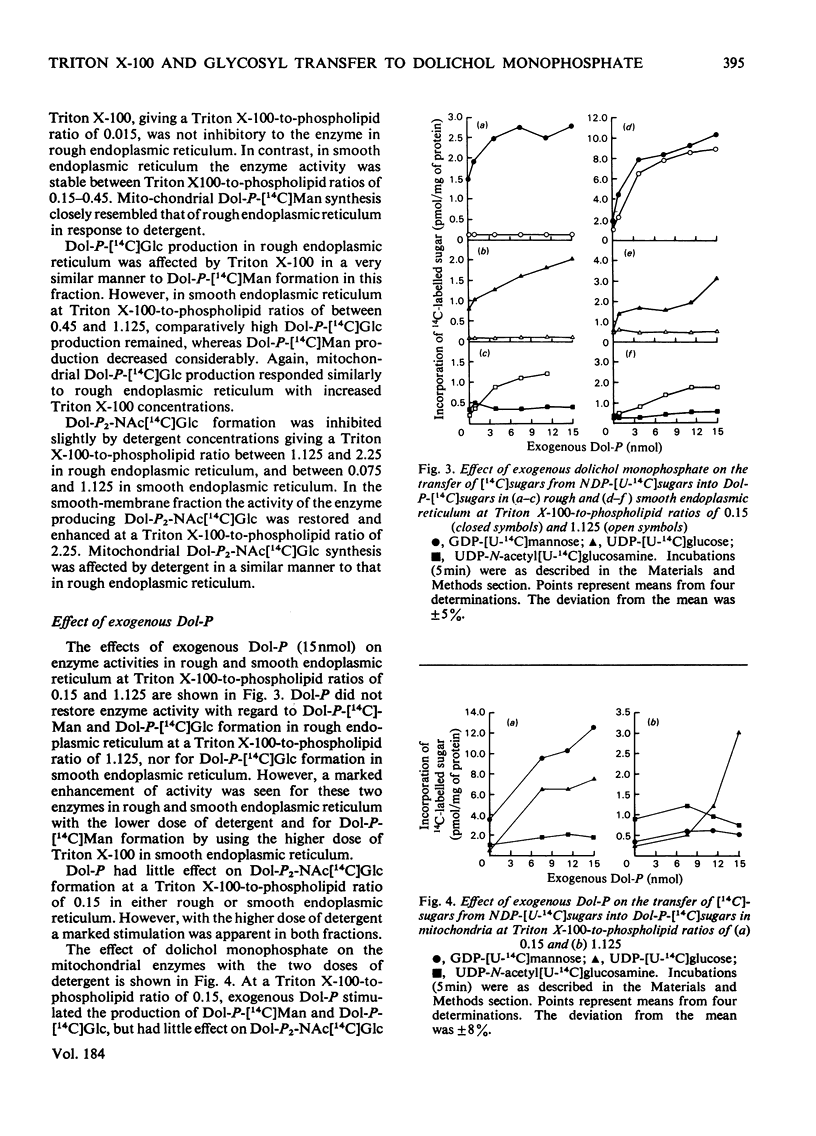
Triton X-100 and exogenous dolichol monophosphate have been used to investigate the nature of enzymes responsible for the transfer of mannose, glucose and N-acetylglucosamine phosphate from nucleotide donors to dolichol monophosphate in vesicles derived from rough and smooth endoplasmic reticulum and mitochondria. Mitochondria were shown to contain the highest specific activities of these enzymes. The responses of the glycosyltransferases to increasing concentrations of Triton X-100 and the effect on these responses of exogenous dolichol monophosphate suggest that the enzymes for mannose and glucose transfer are less hydrophobic, and therefore less intrinsic, in the membrane than the enzyme for N-acetylglucosamine phosphate transfer. In smooth vesicles the results are consistent with mannosyl- and glucosyl-transferases being located at both inner and outer faces of the membrane. In rough vesicles and in mitochondria mannosyl- and glucosyl-transferases were confirmed at the outer face. There is, however, only one site of N-acetylglucosamine phosphate transfer, this being more hydrophobically located in the membrane than the other sites of glycosyl transfer. Mitochondrial enzyme activity closely resembled that of rough endoplasmic reticulum in response to Triton X-100 and exogenous dolichol monophosphate, and is probably associated with the outer membrane.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Behrens N. H., Parodi A. J., Leloir L. F. Glucose transfer from dolichol monophosphate glucose: the product formed with endogenous microsomal acceptor. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2857–2860. doi: 10.1073/pnas.68.11.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccam J. F., Jackson J. J., Eylar E. H. The biosynthesis of mannose-containing glycoproteins: a possible lipid intermediate. Biochem Biophys Res Commun. 1969 May 22;35(4):505–511. doi: 10.1016/0006-291x(69)90375-1. [DOI] [PubMed] [Google Scholar]
- Chen W. W., Lennarz W. J. Participation of a trisaccharide-lipid in glycosylation of oviduct membrane glycoproteins. J Biol Chem. 1976 Dec 25;251(24):7802–7809. [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DERVARTANIAN D. V., VEEGER C. STUDIES ON SUCCINATE DEHYDROGENASE. I. SPECTRAL PROPERTIES OF THE PURIFIED ENZYME AND FORMATION OF ENZYME-COMPETITIVE INHIBITOR COMPLEXES. Biochim Biophys Acta. 1964 Nov 22;92:233–247. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Heifetz A., Elbein A. D. Biosynthesis of Man-beta-GlcNAc-GlcNAc-pyrophosphoryl-polyprenol by a solubilized enzyme from aorta. Biochem Biophys Res Commun. 1977 Mar 7;75(1):20–28. doi: 10.1016/0006-291x(77)91283-9. [DOI] [PubMed] [Google Scholar]
- Heifetz A., Elbein A. D. Solubilization and properties of mannose and N-acetylglucosamine transferases involved in formation of polyprenyl-sugar intermediates. J Biol Chem. 1977 May 10;252(9):3057–3063. [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Lehle L., Tanner W. Biosynthesis and characterization of large dolichyl diphosphate-linked oligosaccharides in Saccharomyces cerevisiae. Biochim Biophys Acta. 1978 Mar 1;539(2):218–229. doi: 10.1016/0304-4165(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Nilsson O. S., Dallner G. Enzyme and phospholipid asymmetry in liver microsomal membranes. J Cell Biol. 1977 Mar;72(3):568–583. doi: 10.1083/jcb.72.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson O. S., De Tomás M. E., Peterson E., Bergman A., Dallner G., Hemming F. W. Mannosylation of endogenous proteins of rough and smooth endoplasmic reticulum and of Golgi membranes. Eur J Biochem. 1978 Sep 1;89(2):619–628. doi: 10.1111/j.1432-1033.1978.tb12566.x. [DOI] [PubMed] [Google Scholar]
- Palamarczyk G., Hemming F. W. The formation of mono-N-acetylhexosamine derivatives of dolichol diphosphate by pig liver microsomal fractions. Biochem J. 1975 May;148(2):245–251. doi: 10.1042/bj1480245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi A. J., Behrens N. H., Leloir L. F., Carminatti H. The role of polyprenol-bound saccharides as intermediates in glycoprotein synthesis in liver. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3268–3272. doi: 10.1073/pnas.69.11.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuvers F., Habets-Willems C., Reinking A., Boer P. Glycolipid intermediates involved in the transfer of N-acetylglucosamine to endogenous proteins in a yeast membrane preparation. Biochim Biophys Acta. 1977 Mar 25;486(3):541–552. doi: 10.1016/0005-2760(77)90104-7. [DOI] [PubMed] [Google Scholar]
- Schachter H., Jabbal I., Hudgin R. L., Pinteric L., McGuire E. J., Roseman S. Intracellular localization of liver sugar nucleotide glycoprotein glycosyltransferases in a Golgi-rich fraction. J Biol Chem. 1970 Mar 10;245(5):1090–1100. [PubMed] [Google Scholar]
- Tetas M., Chao H., Molnar J. Incorporation of carbohydrates into endogenous acceptors of liver microsomal fractions. Arch Biochem Biophys. 1970 May;138(1):135–146. doi: 10.1016/0003-9861(70)90292-4. [DOI] [PubMed] [Google Scholar]
- WEINBACH E. C. A procedure for isolating stable mitochondria from rat liver and kidney. Anal Biochem. 1961 Aug;2:335–343. doi: 10.1016/0003-2697(61)90006-9. [DOI] [PubMed] [Google Scholar]
- Walter H., Hasselbach W. Properties of the calcium-independent ATPase of the membranes of the sarcoplasmic reticulum delipidated by the nonionic detergent Triton X-100. Eur J Biochem. 1973 Jul 2;36(1):110–119. doi: 10.1111/j.1432-1033.1973.tb02890.x. [DOI] [PubMed] [Google Scholar]
- Wolfe L. S., Breckenridge W. C., Skelton P. P. Involvement of mannosyl-phosphoryl-dolichols in mannose transfer to brain glycoproteins. J Neurochem. 1974 Jul;23(1):175–185. doi: 10.1111/j.1471-4159.1974.tb06932.x. [DOI] [PubMed] [Google Scholar]


