Abstract
Seasonal bird migration may provide energy benefits associated with moving to areas with less physiologically challenging climates or increased food availability, but migratory movements themselves may carry high costs. However, time-dynamic energy profiles of free-living migrants—especially small-bodied songbirds—are challenging to measure. Here we quantify energy output and thermoregulatory costs in partially migratory common blackbirds using implanted heart rate and temperature loggers paired with automated radio telemetry and energetic modelling. Our results show that blackbirds save considerable energy in preparation for migration by decreasing heart rate and body temperature 28 days before departure, potentially dwarfing the energy costs of migratory flights. Yet, in warmer wintering areas, migrants do not appear to decrease total daily energy expenditure despite a substantially reduced cost of thermoregulation. These findings indicate differential metabolic programmes across different wintering strategies despite equivalent overall energy expenditure, suggesting that the maintenance of migration is associated with differences in energy allocation rather than with total energy expenditure.
Subject terms: Behavioural ecology, Animal migration, Ecophysiology
The energetic costs of seasonal bird migration are challenging to measure. Here bio-loggers paired with radio telemetry and energetic modelling show that blackbirds offset the energetic cost of migration by decreasing heart rate and body temperature 28 days before departure but do not decrease total daily energy expenditure in warmer wintering areas.
Main
Seasonal bird migration is an impressive and widespread phenomenon1 that evolves primarily to capitalize on ubiquitous environmental seasonality2,3. In temperate environments, the onset of winter brings a decrease in available energy supplies4, along with an increase in the energy cost of thermoregulation. Thus, the net energy expenditure required to ensure winter survival increases relative to other seasons5,6 and, for some species, favours escape to milder regions through migration. Although active travel during migration can be energetically costly7, theory predicts that migration confers other benefits, such as milder weather conditions, greater food availability or reduced predation1,8,9. Many of these benefits may directly offset the metabolic demands of migration itself, while others might necessitate alternative life history strategies to overcome energy deficits. However, the specifics of if, when and how migrants realize the presumed energy benefits of their mobile lifestyle remain unknown because it was previously impossible to quantify the dynamic energy consumption of free-living migratory individuals over multiple seasons.
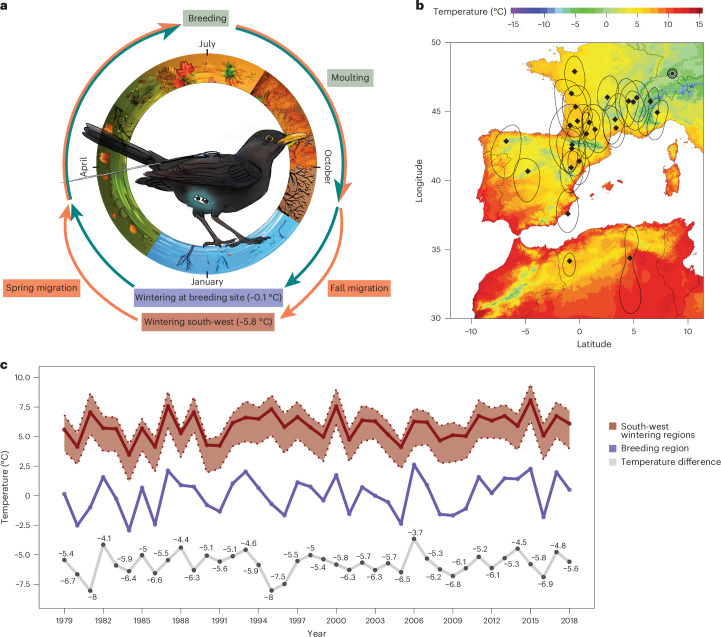
The common blackbird (Turdus merula) is a wide-ranging species across Europe and has populations with varying proportions of migratory individuals10. Blackbirds from our study population share a common breeding area in southern Germany, from which roughly 25% of birds migrate to winter in southern Europe each year (‘migrants’), 66% remain on the breeding grounds year-round (‘residents’) and a further 9% leave mid-winter when declining temperatures lead to ground frost and continuous snowfall covers the ground, which starkly decreases food availability (‘winter escapees’)10,11 (Fig. 1a). In recent years warming temperatures12 and increasing urbanization13 have led to a decrease in migratory propensity in the species which opens various questions about drivers and energetic consequences of migration. Migrants winter on average 793 km (median, minimum 275 km, maximum 1,717 km) south-west of the breeding site14 (Fig. 1b) and over 39 consecutive years have experienced on average ~5.7 °C warmer ambient temperatures (Ta) (t = −21.56, d.f. 60.65, P < 0.01) than their resident counterparts throughout the non-breeding season (Fig. 1c).
Fig. 1. Study system and temperature conditions.
a, Illustration of the experimental setup with a common blackbird (Turdus merula) carrying a radio transmitter backpack and an implanted fH and temperature logger. The surrounding seasonal cycle highlights the main phases during the year for both wintering strategies. b, Temperature map for south-west Europe with known breeding and wintering sites of previously studied migratory blackbirds (N = 25) of the same population as the birds in the current study. The temperature gradient represents the mean Ta during December and January in south-west Europe. The black triple circle depicts the breeding site and single black diamonds and black outlines (25% kernel utilization distribution) represent the centroid of wintering sites estimated by using geolocators of blackbirds from the same breeding area from a previous study14. c, Comparison of temperatures between wintering sites and breeding site during winter. The mean Ta during winter (3 December to 17 January) at wintering sites (red, including the lower 25th and upper 75th quantiles) and at the breeding site (blue) over 39 years. The grey line underneath represents the mean temperature difference and calculated value between both location types.
In this study, we aim to assess phenotype-specific differences in metabolic programmes. First, we examined whether overall heart rate (fH), a proxy for energy expenditure15,16, differs between migrant and resident blackbirds. Second, we investigated whether energy or thermoregulatory dynamics differ among phenotypes (that is, migrants versus residents). Lastly, we quantified if those differences imply differential energy allocation among organismal processes. Previous work in blackbirds has shown that fH negatively correlated with Ta (ref. 17). Therefore, we presumed warmer Ta on wintering sites to reduce total energy expenditure (that is, fH) of migrant birds via reduced metabolic costs of thermoregulation18. We, thus, predicted that migrants would on average exhibit ~7% lower fH than residents during winter based on a previously estimated relationship between fH and Ta in resident individuals17. Conversely, we expected migrants to bear increased energy costs of previously unquantified magnitude due to migration itself.
To measure the relative energy costs and benefits of migratory versus sedentary lifestyles, we compared individual blackbirds’ fH and core body temperature (Tb) throughout fall, winter and spring. We measured fH and Tb at 30 min intervals spanning the entire non-breeding season (starting before fall migration and ending after spring migration) for individual resident (N = 54) and migrant (N = 19) blackbirds using surgically implanted miniature bio-loggers (Star-Oddi, DST micro-HRT, 8.3 × 25.4 mm, 3.3 g; Fig. 1a). Finally, we quantified differences in fH and Tb and modelled expected thermoregulatory energy expenditure among wintering strategies across seasons (Fig. 2), as well as during eight individual-specific periods representing key migratory stages (Fig. 3).
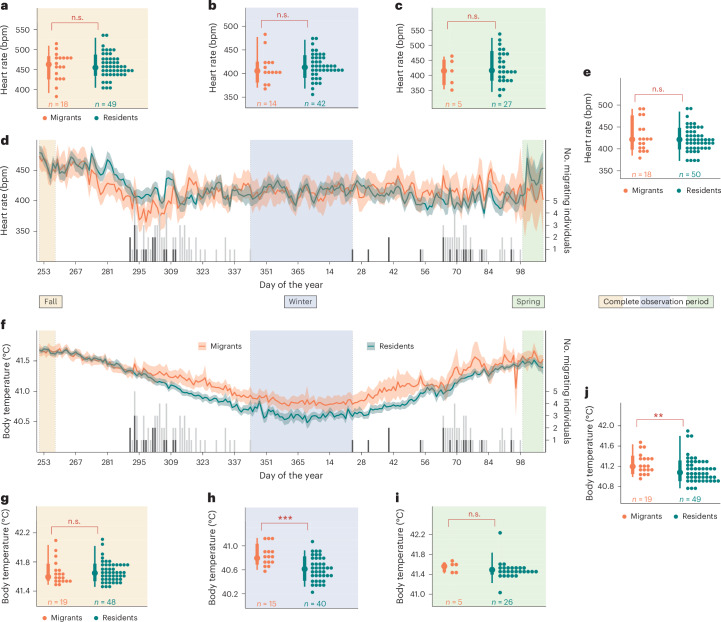
Fig. 2. Temporal comparison of fH and Tb between overwintering strategies with depicted individual migration events.
a–j, The mean fH (a–e) and Tb (f–j) over time (d and f) and during distinct time periods (a, b, c, e and g–j) are displayed for both wintering strategies with 95% confidence intervals. The black/grey histograms mark the number of individuals migrating each night: the black bars depict the number of individuals on their first night of migration and the grey bars show the number of individuals on subsequent migration nights (right y axis). The ochre time frame in the first week of the experiment highlights the fall period that precedes the initial departures by at least 30 days. The middle blue area between the last fall migration event and the first spring migration marks the core winter period, while the green marked period defines the spring period, which starts with the return of the last migrant to the breeding area. The dots mark individual means in fall for a and g, winter for b and h, spring for c and i and the whole timeframe for e and j, next to the coloured bars showing distribution within each wintering strategy (mean, and 75% and 25% percentiles). Sample sizes are shown below each group. Significant differences, derived from a linear mixed model with Bonferroni correction (Supplementary Table 1 and Supplementary Results) are indicated by asterisks: ***P < 0.001, **P < 0.01, *P < 0.05 and ‘non-significant (n.s.)’ where P > 0.05.
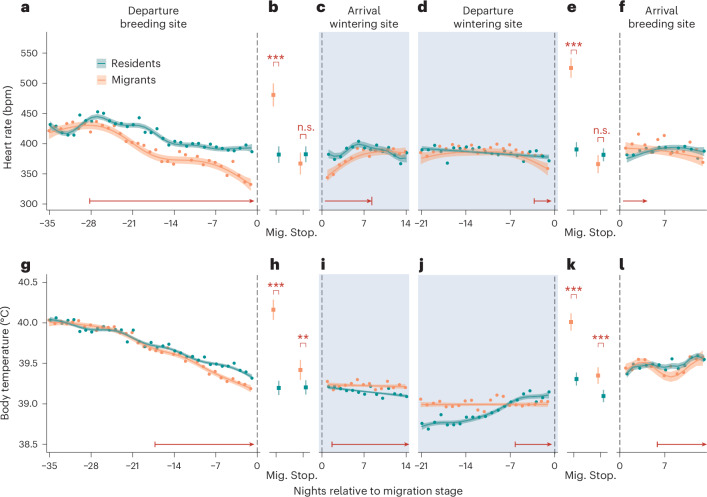
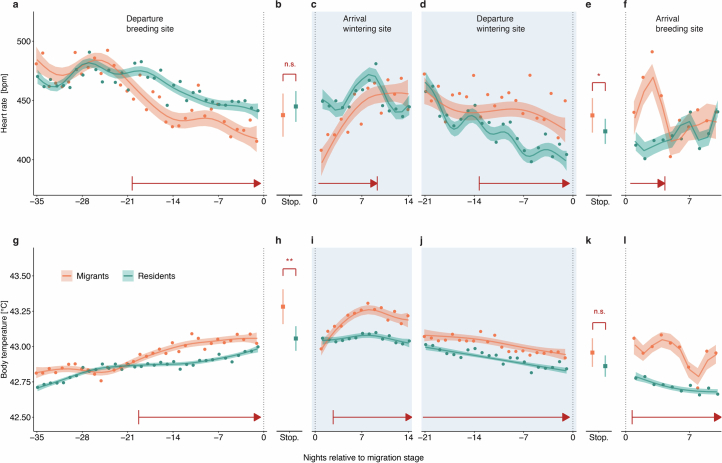
Fig. 3. fH and Tb in different stages relative to migration.
a–f, Mean fH (a–e) and Tb (g–l) in seperate chronological stages in relation to migration, are shown as points during night across all migrants (orange) and all residents (dark green) centred on departure date from breeding site relative to initial departure (a and g), migration (Mig.) and stopover (Stop.) (b and h) in fall, centred on arrival date in wintering site (c and i), centred on departure date from wintering site relative to spring departure (d and j), migration and stopover in spring (e and k) and centred on arrival date on breeding site (f and l). For all measurements over time (a, c, d, f, g and i–l), the vertical dashed line marks the point of reference, while each single point represents the mean value across each overwintering strategy, with migrants centred and residents correspondingly assigned (Methods). The coloured solid line shows predicted fH and Tb values for each strategy derived from a GAMM, including individual measurements for each bird (Supplementary Tables 2–7 and Supplementary Results). Correspondingly coloured ribbons show the 95% confidence interval of those predictions. The blue-marked periods highlight the time when migratory birds reside in their final wintering grounds. The horizontal red arrows mark the first and last times when measures significantly differ between strategies. For migration stage-centred comparisons via linear mixed models (b, e, h and k), the means are shown as coloured squares with standard error bars. Bonferroni corrected statistical significance levels: ***P < 0.001, **P < 0.01, *P < 0.05 and ‘non-significant (n.s.)’ where P > 0.05.
Equality of overall energetics
Overall, fH did not differ between residents and migrants (estimate (EST) of 0.94, standard error (SE) of 6.82, Z = 0.14, P = 0.891; Fig. 2e and Supplementary Table 1), even during winter (EST of −9.66, SE of 7.89, Z = −1.22, P = 0.221; Fig. 2b and Supplementary Table 1), when differences in Ta are assumed to be most pronounced (Fig. 1c). Thus, wintering in warmer locations apparently did not change migrants’ overall energy expenditure relative to residents. Moreover, migratory blackbirds exhibited a slightly (but significantly) higher Tb than residents (EST of 0.11, SE of 0.04, Z = 2.92, P = 0.003; Fig. 2j and Supplementary Table 1), particularly while occupying warmer wintering sites (EST of 0.18, SE of 0.04, Z = 3.98, P < 0.001; Fig. 2h and Supplementary Table 1). The magnitude of the difference in Tb between migrants and residents was almost the same as the ~0.14 °C warmer Tb we expected based on an earlier study17.
Given that the difference between Tb and Ta was larger for residents than for migrants and considering that heat loss intensifies with greater Tb − Ta differences19, the actual energy expenditure on thermoregulation probably varies between the two strategies. On the other hand (and unsurprisingly), migratory movements themselves incurred energetic costs (as expressed by fH) for migratory individuals that resident birds did not experience. Together, these findings imply that while overall energy expenditure apparently does not vary between the two strategies, allocation of energy to specific organismal processes probably vary between migrants and residents during various phases of migration and overwintering (for example, migration preparation, stopover and arrival).
Metabolic dynamics and cost of migration
Migratory travel itself can be energetically expensive7,20 and often requires special adjustments in physiological processes with changes in the physical makeup, for example, size and weight, of organs and tissue21,22. Migrants’ putative thermoregulatory savings may be offset by the increased expense of migration itself or mediated by changes in the functional organ size and performance22. However, during several key periods of the non-breeding phase, migrant blackbirds displayed individualized metabolic dynamics apparently aimed at offsetting migration costs.
Starting 28 days before fall migration departure, future migrants nocturnally decreased fH relative to residents (EST of −12.67, SE of 7.57, F = 1.96; Fig 3a and Supplementary Table 2). This cumulative fH reduction, as evidenced by the mean across each strategy, amplified as departure approached (up to a maximum of −19.5% in beats per minute) and suggests substantial metabolic downregulations and energy-saving in advance of migration23,24. Migrants also concurrently reduced Tb for 17 days before spring departure (EST of −0.04, SE of 0.03, F = 1.96; Fig. 3g and Supplementary Table 2). This suggests a potential mechanism for pre-departure energy conservation: migrants lower their Tb setpoint25, allowing nocturnal Tb to decrease more in the lead-up-to-fall migration than in other phases. By reducing the energy expended on thermoregulation26–28, migrants are able to allocate energy to other processes, such as fat accumulation for fuel storage29,30 and the increase of flight muscles31,32, both important components of preparation for migration. Differences in heart size between strategies (which would result in difference in stroke volume33,34 and haematocrit values35) could, in principle, increase during the pre-migration phase, potentially decoupling fH from oxygen consumption and, thus, energy expenditure. However, our data show that the combined decreases in fH and Tb specifically occured during nocturnal periods and were not observed during the day (EST of −0.07, SE of −0.08, F = −12.29; Supplementary Table 2 and Extended Data Fig. 1a,g). This suggests that the metabolic rate reduction is a strategic adaptation for night-time energy conservation rather than a general increase in heart efficiency. If heart size and stroke volume changes were primary factors, we would have also expected to see these effects during the day, which we did not. Additionally, the near disappearance of this difference in the following spring (EST of −56, SE of 60.05, F = 27.13; Fig. 3d and Supplementary Table 5) underscores the likelihood that the observed nocturnal fH and Tb reductions are non-morphological pre-migratory adaptations, rather than changes in heart and cell physiology. Our findings show that the decision to migrate during fall precedes departure and requires physiological preparation well in advance of any movements, rather than an acute response triggered by environmental conditions, as had been previously suggested36,37. Similarly, in fall (30 days before the earliest migratory activity) and spring (after the arrival of all migrants), when all birds were in the shared breeding grounds, we observed no significant differences in overall Tb or fH, indicating comparable metabolic and thermoregulatory expenses in both strategies (Fig. 2a,g and Supplementary Table 1). We also observed no differences among strategies in the thermoregulatory response to changes in Ta during fall season (Extended Data Fig. 2 and Supplementary Table 10). Together, these findings imply that the migratory strategy is not a simple function of individuals’ inherent metabolic and thermoregulatory capabilities.
Extended Data Fig. 1. Comparison of heart rate and body temperature between strategies relative to migration stages during day.
a-f, Mean heart rate and g-l, body temperature are shown as points during day across all migrants (orange) and all residents (dark green) centred on departure date from breeding site relative to initial departure (a,g), stopover (b,h) in fall, centred on arrival date in wintering site (c,i), centred on departure date from wintering site relative to spring departure (d,j), stopover in spring (e,k), centred on arrival date on breeding site (f,l). For all measurements over time (a,c,d,f,g,i,j,k,l), each single point represents the mean value across each overwintering strategy, with migrants centred and residents correspondingly assigned. The coloured solid line shows predicted heart rate and body temperature values for each strategy derived from a generalised additive mixed model, including individual measurements for each bird. Correspondingly coloured ribbons show the 95% confidence interval of those predictions. Blue-marked periods highlight the time when migratory birds reside in their final wintering grounds. Horizontal red arrows mark the first and last times when measures significantly differ between strategies. For migration stage-centered comparisons via linear mixed models (b,e,h,k), means are shown as colored squares with standard error bars (SEM). Bonferroni corrected statistical significance levels: *** = p < 0.001, ** = p < 0.01, * = p < 0.05, and ‘n.s.’ = p > 0.05.
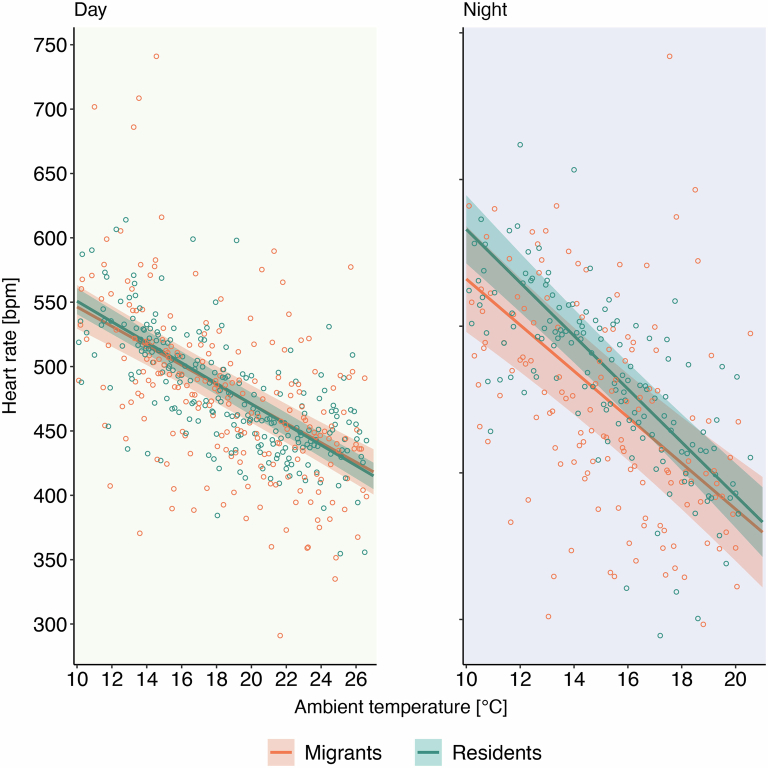
Extended Data Fig. 2. Visualization of reactions in HRT to different ambient temperatures for both wintering strategies.
Mean heart rate of resident and migratory blackbirds in relation to ambient temperature during day and night. Plotted circles are fH mean values for all occurring temperatures during fall (1st Sep.–7th Sep.). Lines are predicted values of the calculated linear mixed model (Supplementary Table 10) with respective 95% confidence intervals as ribbons around them.
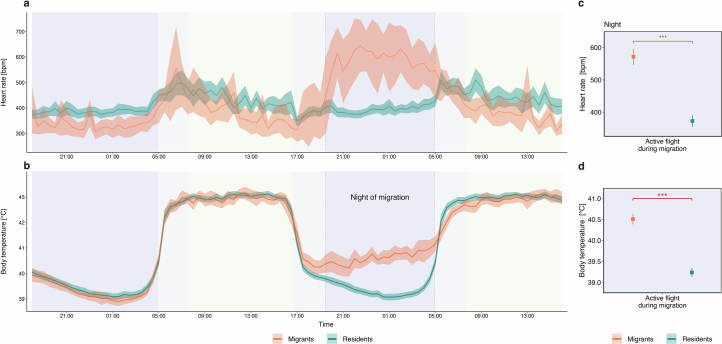
On nights with active migration, migrants exhibited a significantly higher fH compared with residents. Specifically, there was an increase of at least 25.9% (99 bpm) during fall migration and an even greater 36.4% increase (135 bpm) during spring migration (fall: EST of 98.75, SE of 11.29, Z = 8.78, P < 0.001, Fig. 3b and Supplementary Table 3; spring: EST of 135.30, SE of 9.60, Z = 14.09, P < 0.001, Fig. 3e and Supplementary Table 6). This increase in fH was complemented by a 0.7–0.9 °C elevation in Tb for migrants (fall: EST of 0.97, SE of 0.08, Z = 12.63, P < 0.001, Fig. 3h and Supplementary Table 3; spring: EST of 0.70, SE of 0.06, Z = 10.95, P < 0.001, Fig. 3k and Supplementary Table 6). The energy costs of actual flight were probably even higher because blackbirds rarely migrated continuously through an entire night and actively travelled on average during only four nights (Supplementary Table 9). When considering periods of active travel only, migrants showed 53.2% (199 bpm) higher fH (EST of 199.41, SE of 14.46, t = 13.79, P < 0.001; Supplementary Table 8 and Extended Data Fig. 3c), accompanied by a 1.23 °C higher Tb (EST of 1.23, SE of 10.07, t = 18.71, P < 0.001; Supplementary Table 8 and Extended Data Fig. 3d) compared with resident birds at the same time. Interestingly, migrants’ nocturnal Tb during active flight was intermediate between resting Tb (for example, during sleep) and non-migratory diurnal Tb (Extended Data Fig. 3b,d). We hypothesize that elevated Tb arises from increased muscle activity during flight38 rather than any adjustment to the ‘normal’ daily cycle of Tb setpoint regulation17.
Extended Data Fig. 3. Heart rate and body temperature relative to the initial migratory departure.
a, Mean heart rate and b, body temperature in 30-minute intervals over 2 consecutive hours with 95% confidence interval bands for migrants and residents relative to the initial migratory departure of the migrants. Data of resident birds have been individually aligned to birds of the same sex and at the same date and time. The light blue periods mark night-time for all fall migrants, orange periods mark daytime, and light grey periods in between are estimated dusk and dawn phases, depending on the exact departure date. c, Comparison of mean predicted heart rate and (d) mean body temperature via linear mixed model between migrants and residents with 95% confidence interval bars. Significant differences, derived from a linear mixed model and Bonferroni corrected (see Supplementary Data Table 1 and Supplementary Results) are indicated by asterisks: *** = p < 0.001, ** = p < 0.01, * = p < 0.05, and ‘n.s.’ = p > 0.05. Analysed data include only active flight periods during migration nights from initial departure up to final arrival returning at the breeding site.
Immediately upon departing the breeding grounds, migrants demonstrated thermoregulatory advantages that did not translate to detectable differences in fH. On stopovers, migrants already exhibited slightly higher Tb compared with their resident counterparts (EST of 0.22, SE of 0.08, Z = 2.84, P = 0.005; Fig. 3h and Supplementary Table 3), potentially due to milder conditions (Fig. 1b and Supplementary Table 9). After final arrival, we observed that the nocturnal Tb of migrants remained more consistent, while Tb of resident birds continued to decrease seasonally at the same time17, resulting in the lower winter Tb for residents (Fig. 3i). We found no significant differences in fH between the two groups during fall stopovers (EST of −15.37, SE of 10.89, Z = −1.41, P = 0.16; Fig. 3b and Supplementary Table 3), consistent with the patterns observed during winter (Fig. 2b and Fig. 2d).
Upon arrival at wintering sites, migrants exhibited temporarily lower fH for up to eight days (EST of −36.27, SE of 11.96, F = 1.96; Fig. 3c and Supplementary Table 4), indicating a short recovery phase39 following completion of fall migration. A similar tendency could be seen already during earlier stopovers; however, the effect was not statistically significant (EST of −15.37, SE of 10.89, Z = −1.41, P = 0.16; Supplementary Table 3). It should be noted that incorporating numerous consecutive stopover nights40 (Supplementary Table 9) could diminish any potential signal of recovery periods after active flights (Fig. 3b).
In contrast to fall, we found little evidence of pre-migratory metabolic adjustments during the lead-up spring migration. Migrants did exhibit a lower fH 3 days before spring departure (EST of −10.64, SE of 7.29, F = 1.96; Fig. 3d and Supplementary Table 5). However, the magnitude of this reduction was relatively modest (9%), in comparison with fall (Fig. 3a). Notably, we did not observe any evidence of the nocturnal thermoregulatory downregulation, as was the case during fall. Although the Tb of the migrant birds was marginally lower than that of the resident birds 6 days before departure (EST of −0.06, SE of 0.03, F = 1.96; Fig. 3j and Supplementary Table 5), this difference was attributed to the seasonally increasing Tb of the resident birds during this period, probably caused by a greater change in Ta at the more northern breeding site17. It is possible that spring Ta was simply too high or that the preparation of the reproductive system41,42 already started, which, in turn, did not allow downregulation of Tb during the night as observed during the fall, suggesting intrinsic differences between spring and fall pre-migratory programmes.
The pronounced differences in pre-migratory metabolic dynamics between fall and spring migration suggest that these periods involve different metabolic preparations and mechanisms. This notion complements existing evidence suggesting different drivers and strategies employed between the two seasonal journeys (for example, variations in migration speed and the rationale for timely arrival during these seasons43–45). Furthermore, our findings have important implications for understanding the potential influence of environmental changes on the energy balance and, ultimately, wintering decisions of migratory species10,12. For example, environmental factors may differentially affect aspects of fall and spring migrations.
Differences in thermoregulation costs
To estimate differences in energy spent on thermoregulation between the strategies, we parameterized a blackbird-specific individual-based biophysical model of endothermic thermoregulation using only observed Tb and observed or interpolated Ta46 (the model does not use fH; Methods, Extended Data Fig. 4 and Supplementary Table 11). This model predicted that resident blackbirds in substantially colder winter environments incurred markedly higher metabolic costs of thermoregulation than migrants (Fig. 4) despite maintaining a lower Tb (Fig. 2h). This finding was robust to substantial variation in assumptions about Ta, which could arise from uncertainty about wintering locations, micro-climatic buffering or behavioural compensation. Thus, migrants may realize a thermoregulatory benefit of higher Ta during winter, which apparently does not extend to the overall metabolic rate. Instead, the warmer Ta experienced by migrants could provide other organismal temperature-related benefits, such as a more reactive immune system47 or greater predator avoidance capabilities48. It is important to note that our current model assumes no strategy-specific morphological differences between migrants and residents that would affect insulative capacity. If present, such differences could change the estimated difference in estimated thermoregulatory energy expense. Previous work has found no difference in flight-related morphology (wing aspect ratio and tail length) between migratory strategies49 but whether internal morphology differs remains unstudied.
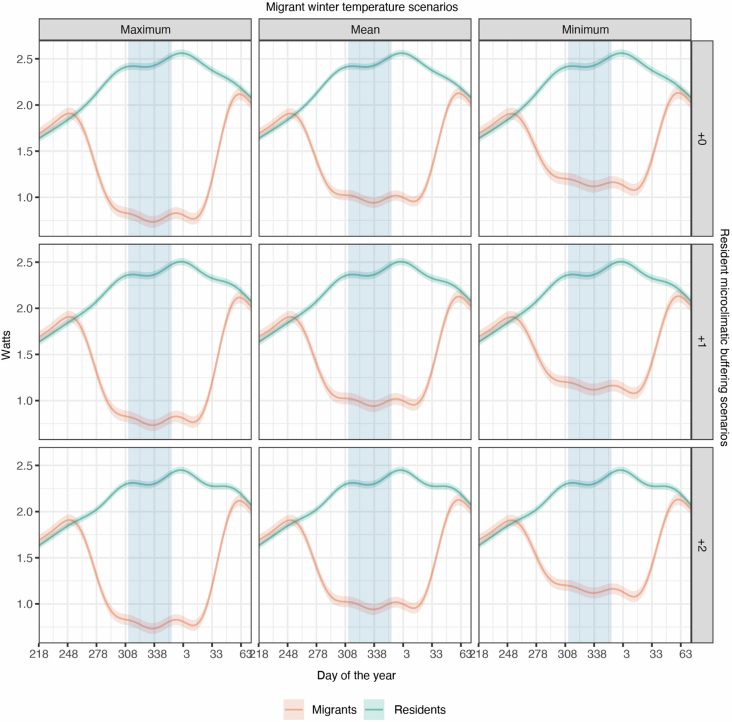
Extended Data Fig. 4. Alternative thermoregulatory scenarios.
Energetic expenditures on thermoregulation over time across migrant and resident blackbirds. To quantify the sensitivity of our findings to alternative Ta timeseries, we considered alternatives for both overwintering residents as well as migrants. For migrants we considered Ta timeseries comprised of the mean Ta across the winter range (top middle, primary result in main text) but also considered 25% and 75% temperature quantiles from across the range on each day. On the breeding grounds, the ambient temperature is better estimated but does not include the potential for buffering via the disproportionate use of warmer micro-climates. Thus, we considered two extreme alternative scenarios wherein we inflated the Ta for wintering residents (but not for migrants) by one and two degrees (rows). Blue shaded area denotes the core winter period.
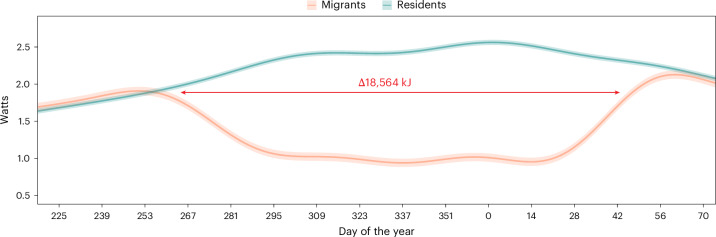
Fig. 4. Thermoregulatory simulation model.
Thermoregulatory simulation showing differential energy expenditures for migrant and resident blackbirds. The predicted energy expense of thermoregulation (lines) and 95% confidence intervals (ribbons) for both migrant and resident phenotypes derived from GAMM. Thermoregulatory metabolism is estimated on the basis of observed Ta and Tb (Methods). The periods, where confidence intervals do not overlap, indicate significantly different energetic expense of thermoregulation.
Energy in the life history of migration
According to our metabolic simulation model, on average, residents expended 18,564 kJ more energy on thermoregulation than migrants, an approximately 1.75-fold difference in allocation despite equivalent total energy expenditures. Thus, assuming approximately equal total energy expended between migrants and residents, as implied by our fH results, these ‘savings’ represent a potential energy surplus available to migrant blackbirds.
A portion of energy saved on thermoregulation could potentially be used to offset any increased costs associated with migration. However, it is unlikely that the additional costs of undertaking migration rise to this magnitude for several reasons. First, the attenuation of pre-migratory fH and lowering of minimum Tb setpoint are expected to offset the net cost of fall and spring migration, at least to some extent. Second, the relatively limited number of active migration nights (range 1–9) during fall or spring migration (Supplementary Table 9) implies a comparatively minimal metabolic cost of migratory flights themselves, in line with findings in other thrush species7. In fact, we calculated that the energy cost of active migration constitutes only 6.7% (1.7–12.2%) of the estimated energy savings from reduced thermoregulatory needs in warmer environments50 (Methods: ‘Biophysical models’, Supplementary Table 12). Instead, we propose that the observed differences in energy budgets may offer a novel explanation for the persistence of different wintering strategies, as their annual routines inevitably impose divergent pressures on individual fitness components.
Morphological differences which decouple fH from true metabolic rate could conceivably account for this discrepancy and would potentially reduce or eliminate this surplus. However, for that to be the case, resident birds would need to exhibit increased metabolic efficiency per heartbeat (that is, greater stroke volume) during winter. Seasonal changes in morphology and physiology, such as increased heart size and size of pectoralis muscles, have been observed in association with avian migration22, as well as cold-induced thermogenesis51. Thus, future work comparing morphological plasticity, especially heart size, would be especially useful.
The pace of life theory52 proposes that traditional life history trade-offs53 are mediated by physiological mechanisms. The extended pace of life theory further suggests that behavioural strategies should predictably covary with life history strategies54. In the context of migration, understanding how a behavioural phenotype is linked to specific life history trade-offs has been hindered by the challenges in accurately estimating fitness components of migratory animals55. While not well estimated, preliminary findings suggest that survival rates may be lower during migrations1 but may enhance or equalize survival probability during other seasons or on an annual basis14,56. Alternatively (or additionally), migrants may trade off survival costs with other fitness components, for example, by increasing fecundity57. Counterbalancing lower migration period survival with elevated survival during other periods and/or higher fecundity might be necessary to ensure equivalent long-term relative fitness among strategies. Interestingly, migratory blackbirds have previously been shown to exhibit higher annual survival than residents14, but strategy-specific differences in, for example, clutch size remain unknown. Thus, migratory blackbirds may use the putative surplus energy arising from lower thermoregulatory burdens to better regulate body condition and, thus, reduce overall intrinsic mortality risk.
Previous hypotheses to explain the emergence and maintenance of partial migration focus on intraspecific competition, positing either a frequency-dependent evolutionary stable strategy, a conditional frequency-dependent strategy (fitness contingent on individual traits)58,59 and/or density-dependent effects of seasonal resource fluctuations60,61. While competition-based theories do not exclude the possibility that individuals modulate fitness components to achieve equivalent overall fitness62, these trade-offs are typically viewed as secondary consequences and not ultimate explanations. More recent work has framed the evolution of bird migration as an explicitly individual phenomenon to follow environmental seasonality and, in this way, escape from harsh winter conditions (rather than density-dependent optimal resource tracking)3,63. In this framing, winter residency in seasonal environments requires as much explanation as migration because overwintering residents must contend with the many challenges posed by winter3. Frequency- and/or density-dependent explanations are only partly satisfactory because they only consider changes in resource distributions and ignore other facets of environmental dynamism that accompany winter. Our findings present a competition-free, individual-based mechanism for modulating energy allocations across non-breeding strategies which could result in concurrent variance in fitness components.
Overall, we did not find differences in total energy spent between migrants and residents. However, different thermoregulatory contexts apparently drive varying energy budgets offset by other currently unknown costs. An individual choice of residency over migration suggests a bi-modal distribution of life history strategies in the species. The exact physiological mechanisms by which individuals ‘choose’ one strategy over the other still need to be determined, but our data at least help to solve the debate whether energy trade-offs are involved in such decisions. These insights emphasise the importance of incorporating field-based energetic measurements in the re-evaluation, refinement and potential rejection of long-standing dogma in the field.
Methods
Study area and captures
We captured a total of 118 adult common blackbirds (Turdus merula) in a mixed forest in southern Germany (47.7801° N, 9.0203° E) over three consecutive years (2016–2018). This population is partially migratory—about 26% of all individuals migrate in autumn (female 36%, male 16%)11. Adult blackbirds (both sexes) were caught with mist nets, fitted with an aluminium leg ring, and transported in cloth cages (height 30 cm, width 26.5 cm, length 49.5 cm) to the Max Planck Institute of Animal Behaviour, Radolfzell (~10 min drive). At the institute, we surgically implanted internal fH and Tb loggers (‘Surgery’ section) and affixed external radio transmitters. Birds were then returned to their original capture location and released.
Surgery
We placed birds on a 40 °C heating pad to prevent hypothermia and then anaesthetized them with isoflurane (CPH Pharma CP 1 ml ml−1, %5). We continuously controlled the bird’s Tb and monitored its breathing frequency. We injected 2 ml of ringer solution into the femur tibia joint to avoid extensive dehydration. After carefully removing some abdominal feathers, we made a 10 mm abdominal incision in the skin and tissue layer beneath the sternum. Star-Oddi DST micro-HRT/temperature data loggers (Star-Oddi, dimensions 8.3 mm × 25.4 mm, weight 3.3 g), which were gas sterilized with ethylene-oxide at 38 °C before (done at Osypka AG), were inserted after which skin and tissue were stitched separately with an absorbent suture. We then monitored the recovery of the bird in hand and, after ensuring its well-being and normal behaviour, we attached a backpack with a radio transmitter (≤2.6 g; produced by Sparrow Systems, the Swiss Ornithological Institute or Holohil Systems) via a leg-loop harness to the bird. The mean weight of a blackbird is about 90 g. Thus, external radio tags, in combination with an implanted fH and Tb logger, add approximately 6.56% (5.9 g) to total body mass. The weight of the transmitter varied from ~1.8 g to 2.6 g, with heavier birds receiving the heavier tags to mitigate the relative burden. Besides the weight, the aerodynamic effects of external tags could notably impact bird activities. Due to their location within the body, the implanted loggers probably have reduced aerodynamic influence, contributing to lesser negative impacts on the birds. To provide some recovery time after surgery and to transport the bird back to the catching site, we placed birds back in a cloth cage where water and food were available ad libitum. In 2015, we conducted a pilot study with five blackbirds kept in aviaries to test their response to implanted loggers. We verified the physical health after this type of surgery and observed that wound healing was not affected after a short recovery phase. Furthermore, during the main study, recapture and migratory return rates were not lower for birds with implanted loggers compared with only radiotagged birds from previous years. The return rates for birds with implanted loggers were 90%, compared with 43% for the control group, and recapture rates were 80% versus 23%, respectively. The experimental setup may have influenced these findings, which required extensive recapturing efforts to retrieve the loggers and continuous monitoring, making direct comparisons challenging.
Data collection
The attached radio transmitter backpacks enabled us to determine the status (presence/absence and alive/dead), non-breeding strategy (migrant versus resident) and the timing of departures and arrivals of individuals at the breeding site. To this end, we deployed six automated receiver units (ARU, Sparrow Systems) at selected locations in the study site64, where each ARU searched for up to 60 frequencies chosen within a maximum time frame of 240 s. The ARUs were connected to H antennas, mounted at 3–12 m. A total of 24 h ARU monitoring allowed us to precisely determine departure and arrival events via an initial rapid increase in the signal strength of the radio transmitters, followed by a steady decline during fall or a sudden reappearance accompanied by an increase in signal strength and continuous presence afterwards. We later used visual controls of ARU data sightings and manual handheld tracking to ensure the absence or presence of an individual within a 2.5 km radius. Manual tracking was performed using a handheld H antenna (Andreas Wagener Telemetry Systems) and a Yaesu VR 500 receiver (Vertex Standard USA). We also used car-mounted Yagi-antennas (AF Antronics) and an airplane equipped with two H antennas and two Biotrack receivers (Lotek) to ensure the departure of an individual within a 20 km radius of the study site. All post-breeding departures between 2 September and 24 November were included in our analysis. Later departures were classified as ‘winter migration’ or irruptive migration11 and excluded from this study.
The implanted data loggers were programed to start recording on 1 September at 1:00. They recorded fH at 600 Hz and core Tb every 30 min, including a measure of the signal-to-noise ratio (quality index, QI) of the electrocardiogram (ECG). Additionally, raw ECG measurements were saved every 60 h for later verification of data quality (‘Pre-processing of fH and Tb data’ section).
Recapture
We attempted to recapture all birds for data extraction during the following spring. We used the telemetry-derived positions of the birds (either on-site throughout the winter or whose return was recorded by the ARUs) to precisely target recapture using mist nets. After surgical extraction of the data loggers (using the same protocols as for implantation), the birds were released at their capture site. The data on the loggers were downloaded using the Mercury program (Star-Oddi).
Sample sizes
We implanted 118 loggers from 2016 to 2018 and were able to recapture 83 birds from 2017 to 2019. From that, we get a total of 890,689 measurements (see Supplementary Table 1 for the exact distribution of the measurements).
Pre-processing of fH and Tb data
Although Tb measurements were pre-calibrated to ±0.2 °C during production, the quality of the collected fH measurements depends on the individual-specific signal-to-noise ratio and varies considerably between the loggers. Since the QI, a measure of the signal-to-noise ratio provided by the logger algorithm is based on all previously taken measurements in each logger, it is not comparable between loggers and therefore requires individual filtering. We used the raw ECG data saved every 60 h to include only reliable measurements with known uncertainty. We manually calculated the correct beats per minute for these measurements via the raw ECG trace plots and compared this with the one internally calculated by the logger algorithm. We then individually estimated the assigned error rate for each logger and QI’s. We filtered all data accordingly to include only the QI with a known error rate.
Furthermore, a manual calculation of all ECGs allowed us to determine the maximum and minimum plausible fH that can be observed and verified in the field. After final filtering, we excluded 12 loggers due to insufficient data quality. We expected only measurements with a QI error rate of less than 15% and exhibiting values within the known range of reasonable fH. The final data set for analysis included 510,654 and 597,321 measurements of fH and Tb, respectively.
Classification of migration
We used the known breeding site departure and arrival dates for migratory birds recorded via ARU radio telemetry11 to train a gradient-boosted machine-learning model (R package ‘gbm’65) based on fH, Tb, individual logger identification, individually scaled temperature and fH, the difference to the mean fH and Tb and proportional temperature increase. The model classified all nightly measurements between departure and arrival as migration or stationary phases. Afterwards, we visually classified all measurements by ourselves and compared our manual classification with the one via the machine-learning model. Both classifications matched by 97.7% (model building AUC 0.966, classification AUC 0.977). We then used these data to predict arrival on and departure from the wintering sites as well as stopover periods, which were not observable via ARU radio telemetry.
Definition of seasons and individual key migratory stages
In addition to comparing the fH and Tb of the two migratory phenotypes, we also defined three main calendar seasons for a more focused analysis.
We defined the first 7 days of measurement (1–7 September) as fall, where all individuals of both strategies are in the same location, have finished breeding but are still relatively far away (30 days) from the first recorded departure of a migratory blackbird (on 11 October). We conservatively defined winter as the 46 days between the last fall and first spring migration events detected for our blackbirds (3 December until 17 January). During this time, birds of the two overwintering phenotypes are spatially separated and reside at their respective wintering sites.
The arrival of the last migratory blackbird at the breeding site (2 April) marks the start of our definition of the post-migration spring season. It spans 8 days until April 10, when the sample size of migratory birds becomes less than five, owing to recapture and battery depletion.
Because we observed high individual variance in the phenology of migratory events (for example, departure and arrival timing, duration and so on (Fig. 2d,f)), for some analyses, we standardized Tb and fH on the migration-relevant transition events (rather than calendar dates) for eight stages of the life cycle. The first period is the fall pre-migration phase (35 days before fall departure), followed by fall migration and stopover periods, which mark the time between initial departure and last arrival before the core winter season starts. The very last fall migration starts the winter arrival (first 14 days after arrival in the wintering site), which turns into the core winter from the calendar-based analysis (Fig. 2d,f, ‘Winter’ area). The following year, the return migration period starts with a spring pre-migration phase (21 days before spring departure), followed by spring migration, spring stopover and finally, spring arrival (first 14 days after arriving back at the breeding site).
Previous work shows that physiological responses to environmental conditions and seasonal adaptations can differ day and night. As blackbirds, to the best of our knowledge, migrate only at night, we also separated the analysis for day and night (Fig. 3 and Extended Data Fig. 1)
Weather data
For Fig. 1b,c, we obtained monthly mean temperature data in a 2.5 min spatial resolution from the ‘WorldClim’ dataset (R package ‘geodata’66). The temperature data of our study population’s 25 known wintering areas based on previous tracking using geolocators of the same population12 were annotated using the Env-DATA System on Movebank67. We used the hourly ‘ECMWF ERA5 SL’ temperature (2 m above ground) accounting for atmospheric conditions and the inverse distance weighted between the weather stations. To compare the conditions between breeding and wintering areas, the annual average and the corresponding 25% and 75% quantiles were calculated in December and February, as these are the general periods when both phenotypes (migrants and residents) are spatially separated in their respective wintering areas. Since the environmental data were available at hourly resolution, but physiological measurements were taken every half hour, we linearly interpolated the Ta. We used these extracted Ta to estimate strategy-specific thermoregulatory energy expenditures (‘Biophysical models’ section). To assign the estimated Ta to the respective migratory birds based on their progress towards their wintering grounds, we divided the migration period for each bird by the number of migration nights it undertook, segmenting the journey accordingly. With each migratory night, the experienced Ta then converges linearly towards the respective temperature mean of the wintering sites or the 25/75% quantile. During spring migration, the temperatures experienced gradually adjusted to the temperatures of the breeding site in the same way.
Statistics
To test for differences in fH and Tb for resident and migratory blackbirds in different calendar periods, we used a linear mixed model (R package ‘lme4’68) with individual measurements of fH/Tb on a resolution of 30 min as a response variable and wintering strategy, calendar season, day phase and sex as predictors. The birds’ identification and date were included as random factors.
To analyse energetic differences at various migration stages (‘Definition of seasons and individual key migratory stages’ section), we assigned each single fH and Tb measurement of resident birds to simultaneous single measurements of migratory individuals of the same sex based on the real-time timestamp. By distributing all measurements of resident birds (N = 54) from the same sex equally among the migratory birds (N = 19), every single measurement from a resident was only referenced once to a specific measurement of a migrant bird. This assigned each single measurement of a resident a ‘stage of migration’, corresponding to the reference migrant measurement, allowing us to directly compare the physiological data of residents and migratory blackbirds in relation to the departure and arrival events of the migrants. Since each resident measurement was assigned only once, the dataset contains unique occurrences of each measurement, thereby avoiding any pseudoreplication. We performed migration stage-centred analysis with generalized additive mixed models (GAMM, R package ‘mgcv’69), including fH/Tb measurements again as the response variable. Each migration stage was analysed in a separate model, and the days before and after arrival and departure events have been used as a smoothing factor. Wintering strategy and sex were included as predictors. In both analyses, we eliminated temporal autocorrelation, following the established procedure of randomly discarding 30% of the data from each individual17,70. In addition, the birds’ identification and date were included as random factors to account for individual-specific variation and repeated measurements. We used a post hoc test with a Bonferroni correction to calculate pairwise comparisons in each season.
Biophysical models
To estimate strategy-specific thermoregulatory energy expenditures, we used an instantiation of the endotherm model contained in the ‘nichemapr’ package46. This model, based on Porter and Kearny (2009)71, estimates the dynamic metabolic expenditure of a homoeostatic endotherm based on taxon-specific morphological parameters and typified behavioural responses to thermal fluctuations. The performance of this model has been widely validated against empirical measurement, including in birds72–75. We fitted models based on observed and interpolated Ta (‘Weather data’ section) and the bio-logger-recorded Tb. Species-specific functional trait values can be found in Supplementary Table 11. Thus, we produced dynamic metabolic models for thermoregulation for all 73 individual blackbirds in our dataset. To capture potential uncertainty in Ta during winter for both residents and migrants, we considered alternative Ta timeseries for each. It is possible that resident individuals’ experienced Ta was slightly higher than weather-station observations due to micro-climatic buffering. Thus, we considered scenarios wherein we added 1 °C and 2 °C to the observed temperatures for the resident birds during the period when migrants were off-site (the most conservative possible difference). Similarly, because over-winter Ta was estimated from geolocator-based estimates of winter range from previously studied birds of the same population14, we also considered the minimum and maximum temperatures possible within the migrants’ possible range to bracket the warmest and coldest possible Ta timeseries. We compared all combinations of these scenarios to evaluate the sensitivity of our results to the specific temperature timeseries.
To quantify the differences in the energy expense of thermoregulation between migrants and residents, we fit a hierarchical GAMM for thermoregulatory expenditure (output from the ‘nichemapr’ model) as a function of Julian day interacted with migratory strategy using the ‘mgcv’ package in R (ref. 69). We used a thin plate smoothing term and included a random intercept by individual year to account for individual differences in metabolic rate (for example, body size variation). This allowed us to directly model thermoregulatory metabolic expense as an individual-based timeseries dependent on migratory strategy.
Energy expenditure of migratory flights
To estimate the energy expenditure for individual migratory journeys, we applied an allometric equation derived from Bishop and Butler (2015)50, y = 52.6M0.74, where y represents the power required for flight in watts (J s−1) and M is the body mass in kilograms.
Using this equation, we calculated the power required for each bird’s flight and multiplied the power by the total flight duration in seconds to obtain the total energy expenditure in joules (Supplementary Table 12).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Supplementary Results.
Supplementary Tables 1–12 are as one workbook with multiple tabs. The titles and descriptions are within the tabs themselves.
Acknowledgements
We thank A. Schmidt, A. Meltzer, M. Borho, L. Kettemer and D. Hägele for their help as field assistants in collecting the data. L. Keicher performed surgeries. Á. Bjarnason helped understand the data and developed loggers. Y. Pei provided the blackbird artwork in Fig. 1a. We also thank D. Dent, J. W. Arnold, R. Shipley, K. Morelle and A. Koelzsch for discussions and comments on the manuscript. This work was funded by the Max Planck Gesellschaft and was approved by the responsible ethic commission and ministry in Germany: Regierungspräsidium Freiburg, 35-9185.81/G-16/115, 35-9185.81/G-13/29 and 35-9185.81/G-09/08 (receiving authors: N.L., T.V., D.Z., M.W. and J.P.).
Extended data
Author contributions
Conceptualization: N.L. and J.P. Methodology: N.L., S.W.Y., D.Z., M.W. and J.P. Investigation: N.L., S.W.Y., T.V., M.W. and J.P. Data curation: N.L. Formal analysis: N.L. and S.W.Y. Visualization: N.L. and S.W.Y. Funding acquisition: M.W. and J.P. Project administration: N.L. and J.P. Supervision: J.P. Writing—original draft: N.L., S.W.Y. and J.P. Writing—review and editing: N.L., S.W.Y., T.V., M.W. and J.P.
Peer review
Peer review information
Nature Ecology & Evolution thanks Andrew McKechnie and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Funding
Open access funding provided by Max Planck Society.
Data availability
The datasets supporting the conclusions of this article are available in the figshare data repository at 10.6084/m9.figshare.24799596.
Code availability
The code for the biophysical models in this article is available as a GitHub repository at https://github.com/syanco/blackbird_metabolics. All other analyses used standard software and scripts as described in Methods and Supplementary Information.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Nils Linek, Scott W. Yanco.
Extended data
is available for this paper at 10.1038/s41559-024-02545-y.
Supplementary information
The online version contains supplementary material available at 10.1038/s41559-024-02545-y.
References
- 1.Newton, I. The Migration Ecology of Birds (Academic Press, 2007).
- 2.Somveille, M., Rodrigues, A. S. L. & Manica, A. Energy efficiency drives the global seasonal distribution of birds. Nat. Ecol. Evol.2, 962–969 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Winger, B. M., Auteri, G. G., Pegan, T. M. & Weeks, B. C. A long winter for the Red Queen: rethinking the evolution of seasonal migration. Biol. Rev. Camb. Philos. Soc.94, 737–752 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Newton, I. & Dale, L. Relationship between migration and latitude among West European birds. J. Anim. Ecol.65, 137 (1996). [Google Scholar]
- 5.Petit, M., Clavijo-Baquet, S. & Vézina, F. Increasing winter maximal metabolic rate improves intrawinter survival in small birds. Physiol. Biochem. Zool.90, 166–177 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Swanson, D. L., Agin, T. J., Zhang, Y., Oboikovitz, P. & Dubay, S. Metabolic flexibility in response to within-season temperature variability in house sparrows. Integr. Org. Biol.2, obaa039 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wikelski, M. et al. Costs of migration in free-flying songbirds. Nature423, 704 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Alerstam, T. & Hedenström, A. The development of bird migration theory. J. Avian Biol.29, 343–369 (1998). [Google Scholar]
- 9.Berthold, P. Control of Bird Migration (Springer, 1996).
- 10.Linek, N. et al. A partial migrant relies upon a range-wide cue set but uses population-specific weighting for migratory timing. Mov. Ecol.9, 1–14 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fudickar, A. M., Schmidt, A., Hau, M., Quetting, M. & Partecke, J. Female-biased obligate strategies in a partially migratory population. J. Anim. Ecol.82, 863–871 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Pulido, F. & Berthold, P. Current selection for lower migratory activity will drive the evolution of residency in a migratory bird population. Proc. Natl Acad. Sci. USA107, 7341–7346 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Partecke, J. & Gwinner, E. Increased sedentariness in European blackbirds following urbanization: a consequence of local adaptation? Ecology88, 882–890 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Zúñiga, D. et al. Migration confers winter survival benefits in a partially migratory songbird. eLife6, e28123 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butler, P. J., Green, J. A., Boyd, I. L. & Speakman, J. R. Measuring metabolic rate in the field: the pros and cons of the doubly labelled water and heart rate methods. Funct. Ecol.18, 168–183 (2004). [Google Scholar]
- 16.Halsey, L. G. et al. Flexibility, variability and constraint in energy management patterns across vertebrate taxa revealed by long-term heart rate measurements. Funct. Ecol.33, 260–272 (2019). [Google Scholar]
- 17.Linek, N. et al. A songbird adjusts its heart rate and body temperature in response to season and fluctuating daily conditions. Philos. Trans. R. Soc. B376, 20200213 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinrich, B. Why have some animals evolved to regulate a high body temperature? Am. Nat.111, 623–640 (1977). [Google Scholar]
- 19.Scholander, P. F., Walters, V., HOCK, R. & Irving, L. Body insulation of some arctic and tropical mammals and birds. Biol. Bull.99, 225–236 (1950). [DOI] [PubMed] [Google Scholar]
- 20.McWilliams, S. R., Guglielmo, C., Pierce, B. & Klaassen, M. Flying, fasting, and feeding in birds during migration: a nutritional and physiological ecology perspective. J. Avian Biol.35, 377–393 (2004). [Google Scholar]
- 21.Guglielmo, C. G. Move that fatty acid: fuel selection and transport in migratory birds and bats. Integr. Comp. Biol.50, 336–345 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Piersma, T. & van Gils, J. A. The Flexible Phenotype: A Body-Centred Integration of Ecology, Physiology, and Behaviour (Oxford Univ. Press, 2011).
- 23.Green, J. A. The heart rate method for estimating metabolic rate: review and recommendations. Comp. Biochem. Physiol. A158, 287–304 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Steiger, S. S., Kelley, J. P., Cochran, W. W. & Wikelski, M. Low metabolism and inactive lifestyle of a tropical rain forest bird investigated via heart-rate telemetry. Physiol. Biochem. Zool.82, 580–589 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Ruf, T. & Geiser, F. Daily torpor and hibernation in birds and mammals. Biol. Rev.90, 891–926 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geiser, F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu. Rev. Physiol.66, 239–274 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Romano, A. B., Hunt, A., Welbergen, J. A. & Turbill, C. Nocturnal torpor by superb fairy-wrens: a key mechanism for reducing winter daily energy expenditure. Biol. Lett.15, 20190211 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wellbrock, A. H. J. et al. Cool birds: first evidence of energy-saving nocturnal torpor in free-living common swifts Apus apus resting in their nests. Biol. Lett.18, 20210675 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goymann, W., Spina, F., Ferri, A. & Fusani, L. Body fat influences departure from stopover sites in migratory birds: evidence from whole-island telemetry. Biol. Lett.6, 478–481 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenni, L. & Jenni-Eiermann, S. Fuel supply and metabolic constraints in migrating birds. J. Avian Biol.29, 521 (1998). [Google Scholar]
- 31.Driedzic, W. R., Crowe, H. L., Hicklin, P. W. & Sephton, D. H. Adaptations in pectoralis muscle, heart mass, and energy metabolism during premigratory fattening in semipalmated sandpipers (Calidris pusilla). Can. J. Zool.71, 1602–1608 (1993). [Google Scholar]
- 32.Jehl, J. R. Cyclical changes in body composition in the annual cycle and migration of the eared grebe Podiceps nigricollis. J. Avian Biol.28, 132–142 (1997). [Google Scholar]
- 33.Bishop, C. M. Heart mass and the maximum cardiac output of birds and mammals: implications for estimating the maximum aerobic power input of flying animals. Philos. Trans. R. Soc. Lond. Ser. B352, 447–456 (1997). [Google Scholar]
- 34.Battley, P. F. et al. Empirical evidence for differential organ reductions during trans-oceanic bird flight. Proc. R. Soc. Lond. Ser. B267, 191–195 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenni, L., Müller, S., Spina, F., Kvist, A. & Lindström, Å. Effect of endurance flight on haematocrit in migrating birds. J. Ornithol.147, 531–542 (2006). [Google Scholar]
- 36.Zúñiga, D. et al. Abrupt switch to migratory night flight in a wild migratory songbird. Sci. Rep.6, 34207 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramenofsky, M. & Wingfield, J. C. Regulation of migration. BioScience57, 135–143 (2007). [Google Scholar]
- 38.Guillemette, M., Polymeropoulos, E. T., Portugal, S. J. & Pelletier, D. It takes time to be cool: on the relationship between hyperthermia and body cooling in a migrating seaduck. Front. Physiol.8, 532 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferretti, A., Maggini, I. & Fusani, L. How to recover after sea crossing: the importance of small islands for passerines during spring migration. Ethol. Ecol. Evol.33, 307–320 (2021). [Google Scholar]
- 40.Hedenström, A. & Alerstam, T. Optimum fuel loads in migratory birds: distinguishing between time and energy minimization. J. Theor. Biol.189, 227–234 (1997). [DOI] [PubMed] [Google Scholar]
- 41.Dominoni, D., Quetting, M. & Partecke, J. Artificial light at night advances avian reproductive physiology. Proc. R. Soc. B280, 20123017 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bauchinger, U., Hof, T. V. & Biebach, H. Testicular development during long-distance spring migration. Horm. Behav.51, 295–305 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Kölzsch, A. et al. Towards a new understanding of migration timing: slower spring than autumn migration in geese reflects different decision rules for stopover use and departure. Oikos125, 1496–1507 (2016). [Google Scholar]
- 44.Tøttrup, A. P. et al. The annual cycle of a trans-equatorial Eurasian–African passerine migrant: different spatio-temporal strategies for autumn and spring migration. Proc. R. Soc. B279, 1008–1016 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nilsson, C., Klaassen, R. H. G. & Alerstam, T. Differences in speed and duration of bird migration between spring and autumn. Am. Nat.181, 837–845 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Kearney, M. R., Briscoe, N. J., Mathewson, P. D. & Porter, W. P. NicheMapR—an R package for biophysical modelling: the endotherm model. Ecography44, 1595–1605 (2021). [Google Scholar]
- 47.Thaxton, P. Influence of temperature on the immune response of birds. Poult. Sci.57, 1430–1440 (1978). [DOI] [PubMed] [Google Scholar]
- 48.Laurila, M. & Hohtola, E. The effect of ambient temperature and simulated predation risk on fasting-induced nocturnal hypothermia of pigeons in outdoor conditions. J. Therm. Biol.30, 392–399 (2005). [Google Scholar]
- 49.Fudickar, A. M. & Partecke, J. The flight apparatus of migratory and sedentary individuals of a partially migratory songbird species. PLoS ONE7, e51920 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bishop, C. M. & Butler, P. J. in Sturkie’s Avian Physiology (ed. Scanes, C. G.) 919–974 (Elsevier, 2015).
- 51.King, M. O. et al. Phenotypic flexibility of skeletal muscle and heart masses and expression of myostatin and tolloid-like proteinases in migrating passerine birds. J. Comp. Physiol. B185, 333–342 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Ricklefs, R. E. & Wikelski, M. The physiology/life-history nexus. Trends Ecol. Evol.17, 462–468 (2002). [Google Scholar]
- 53.Rose, M. & Mueller, L. The evolution of life histories. J. Evol. Biol.6, 304–306 (2002). [Google Scholar]
- 54.Dammhahn, M., Dingemanse, N. J., Niemelä, P. T. & Réale, D. Pace-of-life syndromes: a framework for the adaptive integration of behaviour, physiology and life history. Behav. Ecol. Sociobiol.72, 62 (2018). [Google Scholar]
- 55.Marra, P. P., Cohen, E. B., Loss, S. R., Rutter, J. E. & Tonra, C. M. A call for full annual cycle research in animal ecology. Biol. Lett.11, 20150552 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buechley, E. R. et al. Differential survival throughout the full annual cycle of a migratory bird presents a life-history trade-off. J. Anim. Ecol.90, 1228–1238 (2021). [DOI] [PubMed] [Google Scholar]
- 57.Jetz, W., Sekercioglu, C. H. & Böhning-Gaese, K. The worldwide variation in avian clutch size across species and space. PLoS Biol.6, e303 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lundberg, P. Partial bird migration and evolutionarily stable strategies. J. Theor. Biol.125, 351–360 (1987). [Google Scholar]
- 59.Lundberg, P. The evolution of partial migration in birds. Trends Ecol. Evol.3, 172–175 (1988). [DOI] [PubMed] [Google Scholar]
- 60.Griswold, C. K., Taylor, C. M. & Norris, D. R. The evolution of migration in a seasonal environment. Proc. Biol. Sci.277, 2711–2720 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taylor, C. M. & Norris, D. R. Predicting conditions for migration: effects of density dependence and habitat quality. Biol. Lett.3, 280–284 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adriaensen, F. & Dhondt, A. A. Population dynamics and partial migration of the European robin (Erithacus rubecula) in different habitats. J. Anim. Ecol.59, 1077 (1990). [Google Scholar]
- 63.Salewski, V. & Bruderer, B. The evolution of bird migration—a synthesis. Naturwissenschaften94, 268–279 (2007). [DOI] [PubMed] [Google Scholar]
- 64.Crofoot, M. C., Gilby, I. C., Wikelski, M. C. & Kays, R. W. Interaction location outweighs the competitive advantage of numerical superiority in Cebus capucinus intergroup contests. Proc. Natl Acad. Sci. USA105, 577–581 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ridgeway, G. et al. gbm: Generalized Boosted Regression Models (version 2.2.2). CRAN https://cran.r-project.org/package=gbm (2024).
- 66.Hijmans, R. J., Barbosa, M., Ghosh, A. & Mandel, A. geodata: download geographic data (version 0.6-2). CRAN https://cran.r-project.org/package=geodata (2023).
- 67.Kays, R. et al. The Movebank system for studying global animal movement and demography. Methods Ecol. Evol.13, 419–431 (2022). [Google Scholar]
- 68.Bates, D., Mächler, M., Bolker, B. M. & Walker, S. C. Fitting linear mixed-effects models using lme4. J. Stat. Softw.67, 1–48 (2015). [Google Scholar]
- 69.Wood, S. N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B73, 3–36 (2011). [Google Scholar]
- 70.Hawkes, L. A. et al. The trans-Himalayan flights of bar-headed geese (Anser indicus). Proc. Natl Acad. Sci. USA108, 9516–9519 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Porter, W. P. & Kearney, M. Size, shape, and the thermal niche of endotherms. Proc. Natl Acad. Sci. USA106, 19666–19672 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Conradie, S. R. et al. An evaluation of a biophysical model for predicting avian thermoregulation in the heat. J. Exp. Biol.226, jeb245066 (2023). [DOI] [PubMed] [Google Scholar]
- 73.Fitzpatrick, M. J., Mathewson, P. D. & Porter, W. P. Validation of a mechanistic model for non-invasive study of ecological energetics in an endangered wading bird with counter-current heat exchange in its legs. PLoS ONE10, e0136677 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mathewson, P. D. et al. Field data confirm the ability of a biophysical model to predict wild primate body temperature. J. Therm. Biol.94, 102754 (2020). [DOI] [PubMed] [Google Scholar]
- 75.Mathewson, P. D. et al. Experimental and modeled thermoregulatory costs of repeated sublethal oil exposure in the double-crested cormorant, Phalacrocorax auritus. Mar. Pollut. Bull.135, 216–223 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Results.
Supplementary Tables 1–12 are as one workbook with multiple tabs. The titles and descriptions are within the tabs themselves.
Data Availability Statement
The datasets supporting the conclusions of this article are available in the figshare data repository at 10.6084/m9.figshare.24799596.
The code for the biophysical models in this article is available as a GitHub repository at https://github.com/syanco/blackbird_metabolics. All other analyses used standard software and scripts as described in Methods and Supplementary Information.