Abstract
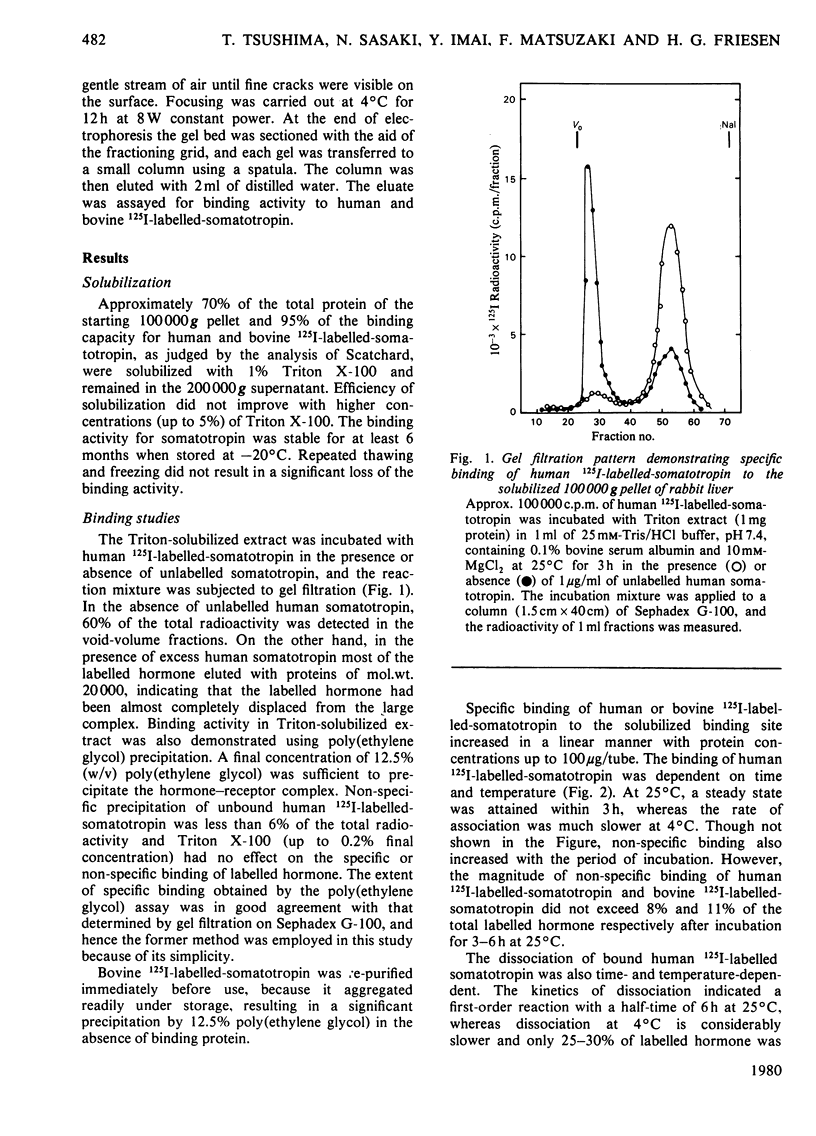
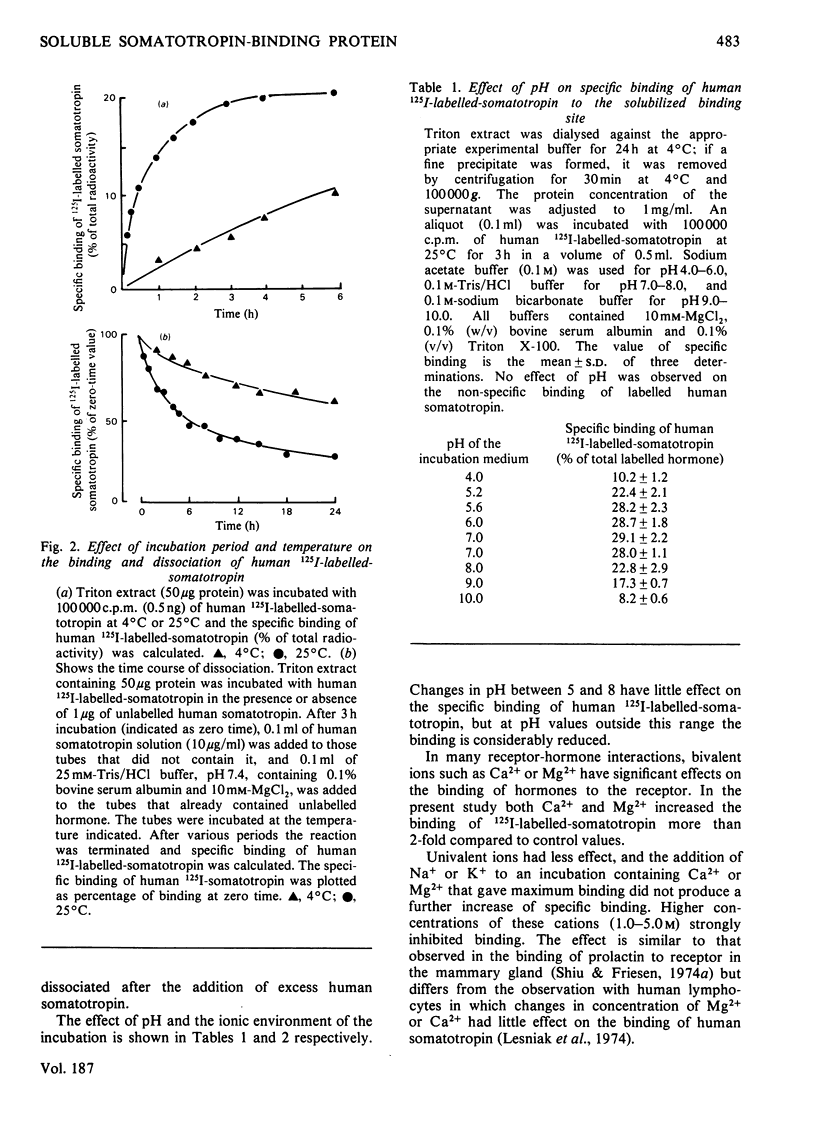
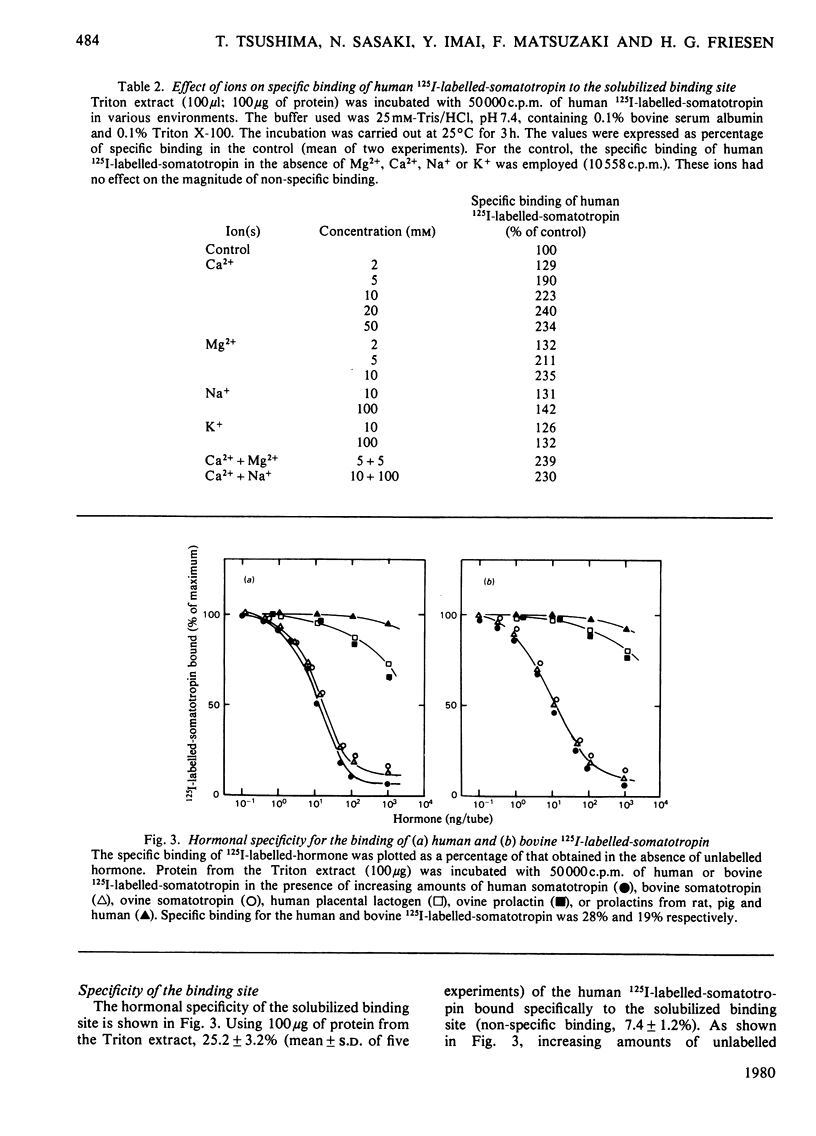
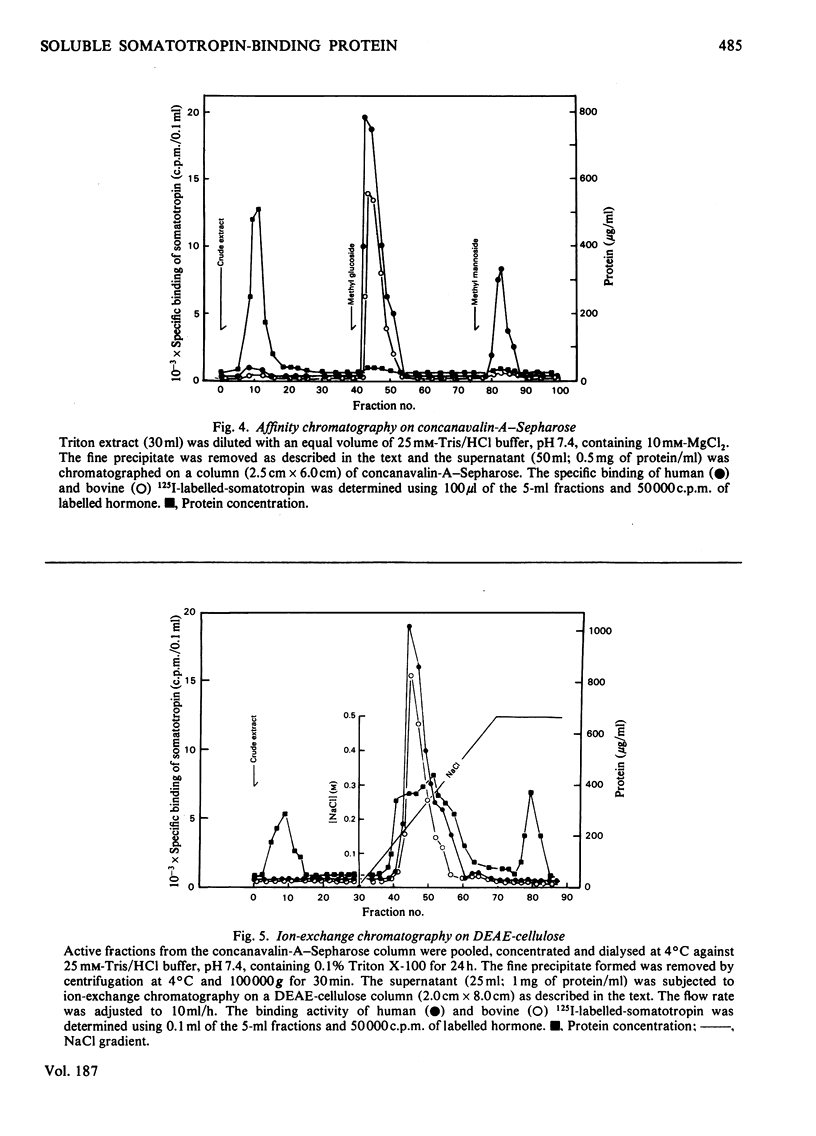
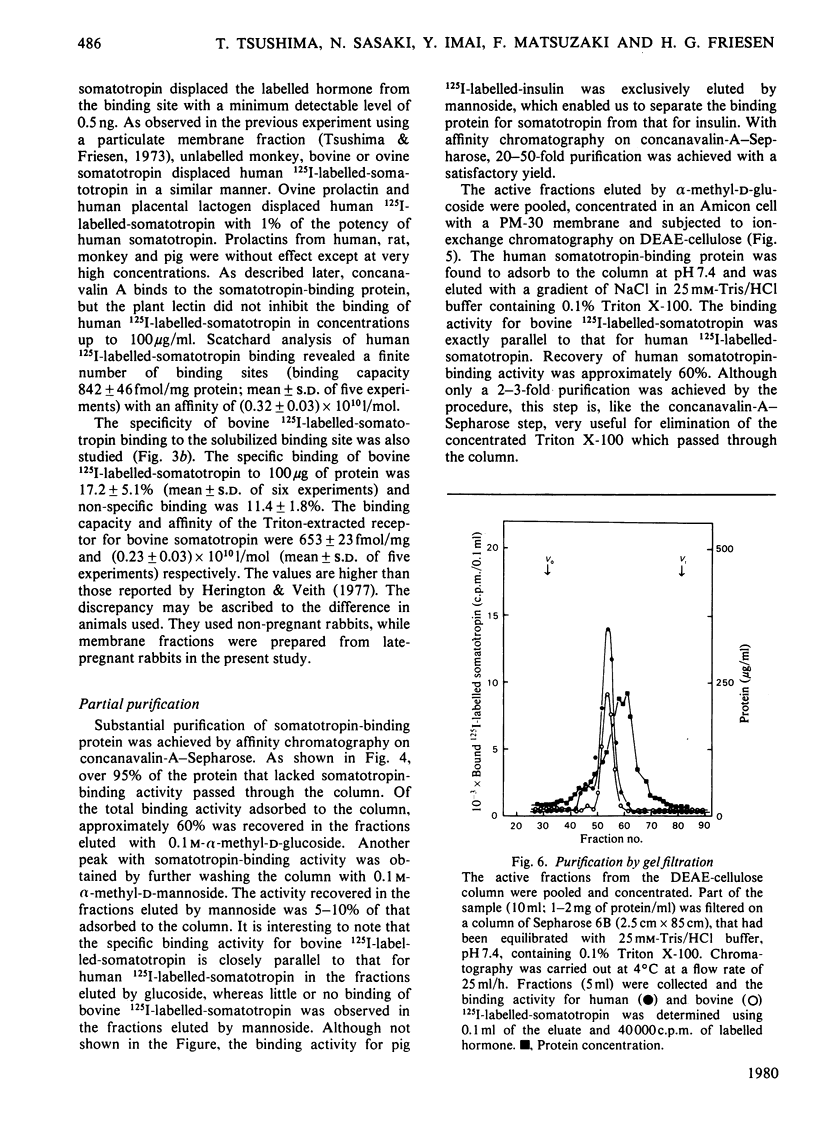
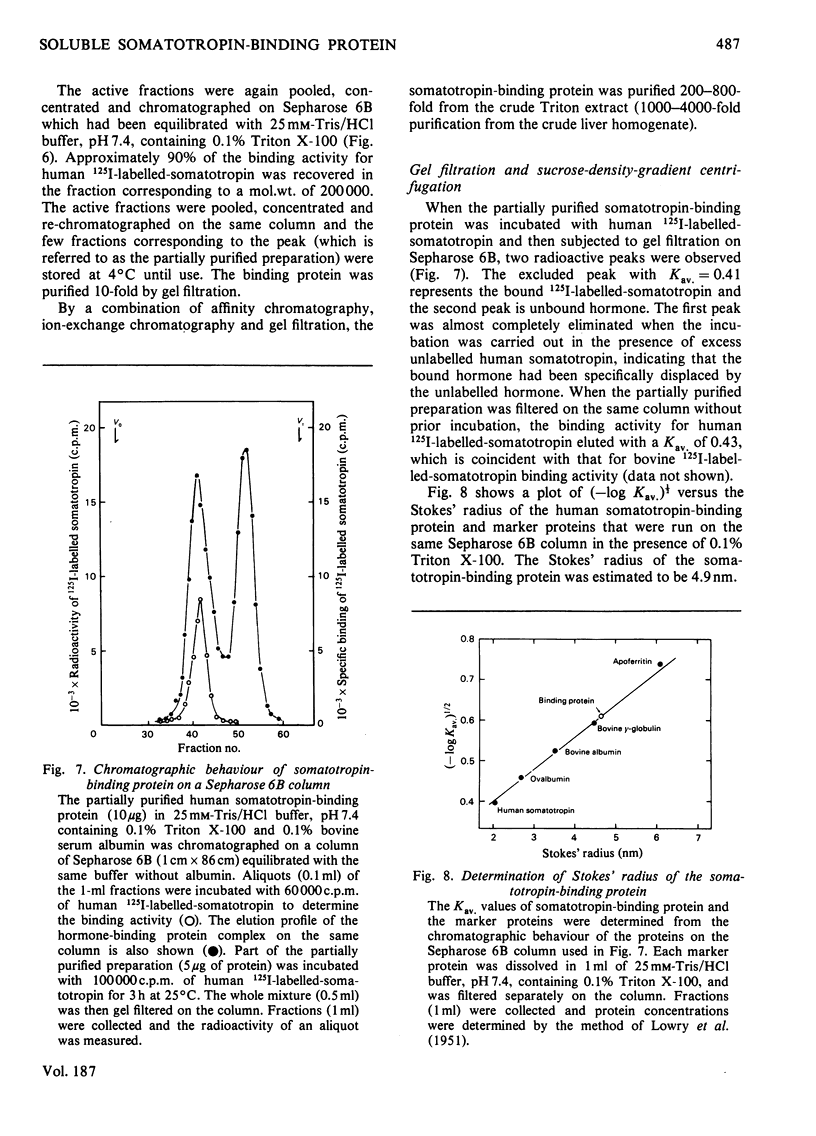
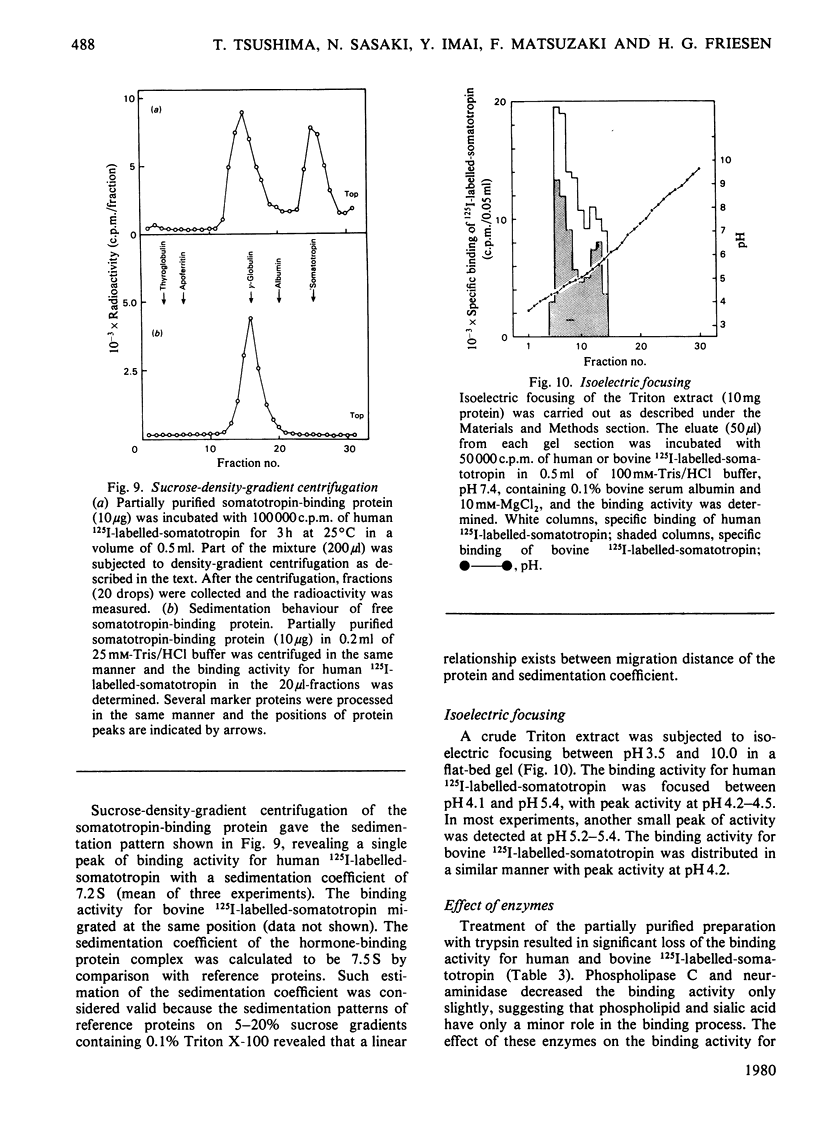
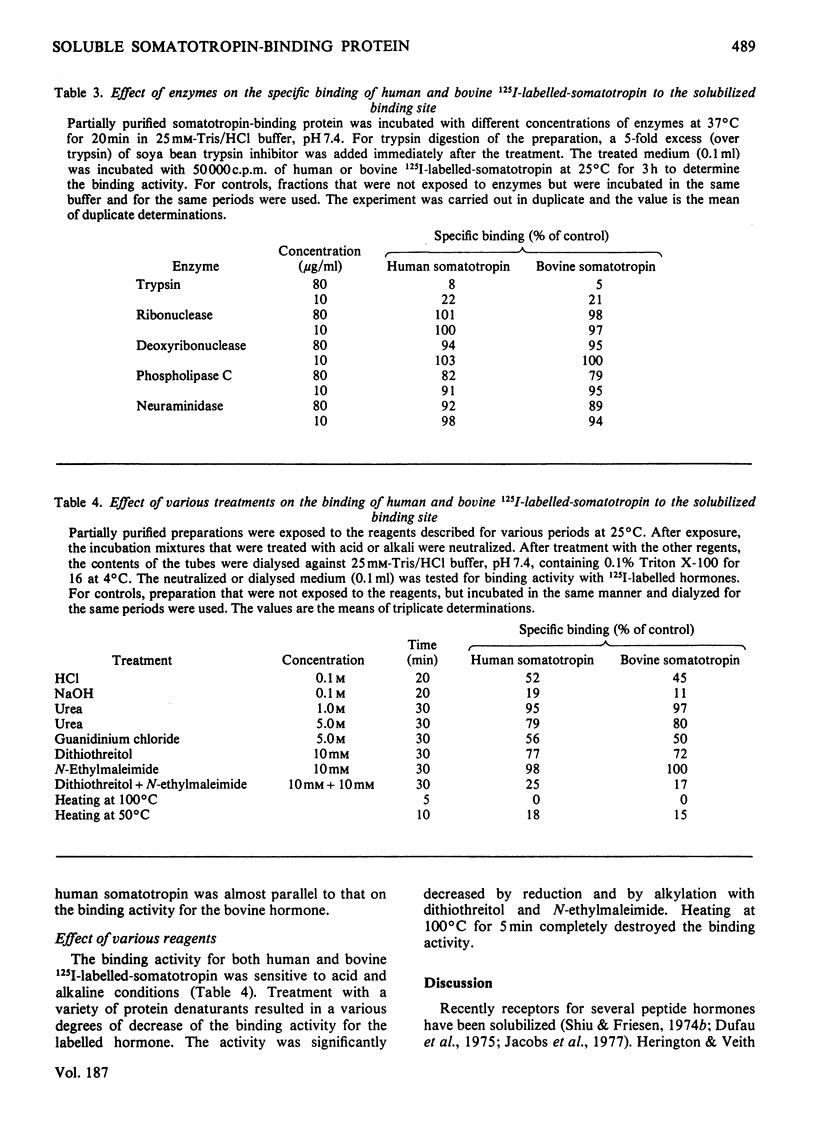
A specific binding site for somatotropin was solubilized by 1% (v/v) Triton X-100 from a crude particulate membrane fraction of pregnant rabbit liver, partially purified and characterized. The solubilized binding site retained many of the characteristics observed in the original particulate fraction, indicating that extraction of the binding site with Triton X-100 does not cause any major changes in its properties. The binding of human 125I-labelled-somatotropin to the solubilized binding site is a saturable and reversible process, depending on temperature, incubation time, pH and ionic environment. Analysis of the kinetic data revealed a finite number of binding sites with an affinity constant of 0.32 x 10(10)M-1. The binding activity for human 125I-labelled-somatotropin was adsorbed to a concanavalin-A-Sepharose column and was dissociated from the column with alpha-methyl-D-glucoside, suggesting that the binding protein may be a glycoprotein. Using affinity chromatography on concanavalin-A-Sepharose, ion-exchange chromatography on DEAE-cellulose and gel filtration on Sepharose 6B, the binding protein was purified 1000-4000-fold from the original liver homogenate. When the partially purified preparation was chromatographed on Sepharose 6B, the binding protein eluted as a molecule with an apparent molecular weight of 200000, with a Stokes' radius of 4.9 nm. Sucrose-density-gradient centrifugation of the preparation showed that the sedimentation coefficient of the binding protein was 7.2S. Isoelectric focusing experiments revealed that a major part of the protein has an acidic pI (4.2-4.5). Exposure of the protein to trypsin decreased the binding activity for human 125I-labelled-somatotropin or bovine 125I-labelled-somatotropin, whereas ribonuclease, deoxyribonuclease, phospholipase C or neuraminidase had little or no effect.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carr D., Friesen H. G. Growth hormone and insulin binding to human liver. J Clin Endocrinol Metab. 1976 Mar;42(3):484–493. doi: 10.1210/jcem-42-3-484. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Isolation of the insulin receptor of liver and fat-cell membranes (detergent-solubilized-( 125 I)insulin-polyethylene glycol precipitation-sephadex). Proc Natl Acad Sci U S A. 1972 Feb;69(2):318–322. doi: 10.1073/pnas.69.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P., Tell G. P. Insulin-like activity of concanavalin A and wheat germ agglutinin--direct interactions with insulin receptors. Proc Natl Acad Sci U S A. 1973 Feb;70(2):485–489. doi: 10.1073/pnas.70.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufau M. L., Charreau E. H., Catt K. J. Characteristics of a soluble gonadotropin receptor from the rat testis. J Biol Chem. 1973 Oct 25;248(20):6973–6982. [PubMed] [Google Scholar]
- Dufau M. L., Ryan D. W., Baukal A. J., Catt K. J. Gonadotropin receptors. Solubilization and purification by affinity chromatography. J Biol Chem. 1975 Jun 25;250(12):4822–4824. [PubMed] [Google Scholar]
- Dufau M. L., Ryan D., Catt K. J. Disulphide groups of gonadotropin receptors are essential for specific binding of human chorionic gonadotropin. Biochim Biophys Acta. 1974 Apr 22;343(2):417–422. doi: 10.1016/0304-4165(74)90106-8. [DOI] [PubMed] [Google Scholar]
- Herington A. C., Veith N. M. Solubilization of a growth hormone-specific receptor from rabbit liver. Endocrinology. 1977 Sep;101(3):984–987. doi: 10.1210/endo-101-3-984. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Shechter Y., Bissell K., Cuatrecasas P. Purification and properties of insulin receptors from rat liver membranes. Biochem Biophys Res Commun. 1977 Aug 8;77(3):981–988. doi: 10.1016/s0006-291x(77)80074-0. [DOI] [PubMed] [Google Scholar]
- Jänne J., Raina A. On the stimulation of ornithine decarboxylase and RNA polymerase activity in rat liver after treatment with growth hormone. Biochim Biophys Acta. 1969 Feb 18;174(2):769–772. doi: 10.1016/0005-2787(69)90310-4. [DOI] [PubMed] [Google Scholar]
- Kelly P. A., Posner B. I., Tsushima T., Friesen H. G. Studies of insulin, growth hormone and prolactin binding: ontogenesis, effects of sex and pregnancy. Endocrinology. 1974 Aug;95(2):532–539. doi: 10.1210/endo-95-2-532. [DOI] [PubMed] [Google Scholar]
- Lesniak M. A., Gorden P., Roth J., Gavin J. R., 3rd Binding of 125I-human growth hormone to specific receptors in human cultured lymphocytes. Characterization of the interaction and a sensitive radioreceptor assay. J Biol Chem. 1974 Mar 25;249(6):1661–1667. [PubMed] [Google Scholar]
- Lesniak M. A., Roth J., Gorden P., Gavin J. R., 3rd Human growth hormone radioreceptor assay using cultured human lymphocytes. Nat New Biol. 1973 Jan 3;241(105):20–22. doi: 10.1038/newbio241020a0. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- McConaghey P., Sledge C. B. Production of "sulphation factor" by the perfused liver. Nature. 1970 Mar 28;225(5239):1249–1250. doi: 10.1038/2251249b0. [DOI] [PubMed] [Google Scholar]
- Phillips L. S., Herington A. C., Karl I. E., Daughaday W. H. Comparison of somatomedin activity in perfusates of normal and hypophysectomized rat livers with and without added growth hormone. Endocrinology. 1976 Mar;98(3):606–614. doi: 10.1210/endo-98-3-606. [DOI] [PubMed] [Google Scholar]
- Posner B. I. Characterization and modulation of growth hormone and prolactin binding in mouse liver. Endocrinology. 1976 Mar;98(3):645–654. doi: 10.1210/endo-98-3-645. [DOI] [PubMed] [Google Scholar]
- Posner B. I., Kelly P. A., Shiu R. P., Friesen H. G. Studies of insulin, growth hormone and prolactin binding: tissue distribution, species variation and characterization. Endocrinology. 1974 Aug;95(2):521–531. doi: 10.1210/endo-95-2-521. [DOI] [PubMed] [Google Scholar]
- Shiu R. P., Friesen H. G. Blockade of prolactin action by an antiserum to its receptors. Science. 1976 Apr 16;192(4236):259–261. doi: 10.1126/science.176727. [DOI] [PubMed] [Google Scholar]
- Shiu R. P., Friesen H. G. Properties of a prolactin receptor from the rabbit mammary gland. Biochem J. 1974 May;140(2):301–311. doi: 10.1042/bj1400301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu R. P., Friesen H. G. Solubilization and purification of a prolactin receptor from the rabbit mammary gland. J Biol Chem. 1974 Dec 25;249(24):7902–7911. [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Tsushima T., Friesen H. G. Radioreceptor assay for growth hormone. J Clin Endocrinol Metab. 1973 Aug;37(2):334–337. doi: 10.1210/jcem-37-2-334. [DOI] [PubMed] [Google Scholar]


