Abstract
BNPS-skatole (3-bromo-3-methyl-2-(2-nitrophenyl)thiol-3H-indole) and N-bromosuccinimide have been used to specifically cleave at peptide bonds after amino acids with available C-gamma=C-delta double bonds, i.e. tryptophan, tyrosine and histidine. The resulting C-terminal lactones conveniently attach to amino-glass supports for sequencing with DABITC (4-NN-dimethylaminoazobenzene 4'-isothiocyanate). Also, peptides having such C-termini, i.e. from a chymotryptic digest, can be readily made to react with these reagents and thus be easily attached and sequenced by solid-phase methods.
Full text
PDF

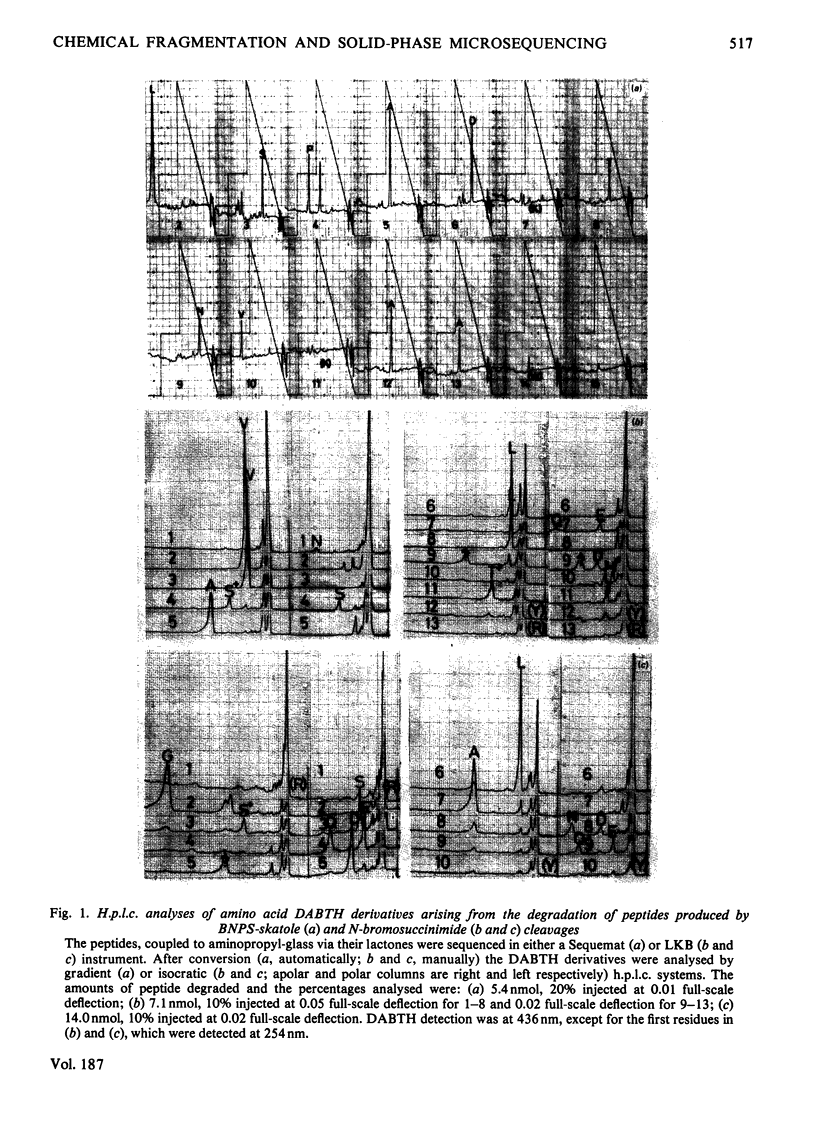


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Horn M. J., Laursen R. A. Solid-phase edman degradation: attachment of carboxyl-terminal homoserine peptides to an insoluble resin. FEBS Lett. 1973 Nov 1;36(3):285–288. doi: 10.1016/0014-5793(73)80392-8. [DOI] [PubMed] [Google Scholar]
- Hughes G. J., Winterhalter K. H., Lutz H., Wilson K. J. Microsequence analysis: III. Automatic solid-phage sequencing using DABITC. FEBS Lett. 1979 Dec 1;108(1):92–97. doi: 10.1016/0014-5793(79)81185-0. [DOI] [PubMed] [Google Scholar]
- Hughes G. J., Winterhalter K. H., Wilson K. J. Microsequence analysis: I. Peptide isolation using high-performance liquid chromatography. FEBS Lett. 1979 Dec 1;108(1):81–86. doi: 10.1016/0014-5793(79)81183-7. [DOI] [PubMed] [Google Scholar]
- Mahoney W. C., Hermodson M. A. High-yield cleavage of tryptophanyl peptide bonds by o-iodosobenzoic acid. Biochemistry. 1979 Aug 21;18(17):3810–3814. doi: 10.1021/bi00584a026. [DOI] [PubMed] [Google Scholar]
- Savige W. E., Fontana A. Cleavage of the tryptophanyl peptide bond by dimethyl sulfoxide-hydrobromic acid. Methods Enzymol. 1977;47:459–469. doi: 10.1016/0076-6879(77)47046-0. [DOI] [PubMed] [Google Scholar]
- Wachter E., Werhahn R. Attachment of tryptophanyl peptides to 3-aminopropyl-glass suited for subsequent solid-phase Edman degradation. Anal Biochem. 1979 Aug;97(1):56–64. doi: 10.1016/0003-2697(79)90327-0. [DOI] [PubMed] [Google Scholar]
- Wilson K. J., Hunziker P., Hughes G. J. Microsequence analysis: IV. Automatic liquid-phase sequencing using DABITC. FEBS Lett. 1979 Dec 1;108(1):98–102. doi: 10.1016/0014-5793(79)81186-2. [DOI] [PubMed] [Google Scholar]
- Wilson K. J., Rodger K., Hughes G. J. Microsequence analyses: II. DABTH-amino acid identification by high-performance liquid and thin-layer chromatography. FEBS Lett. 1979 Dec 1;108(1):87–91. doi: 10.1016/0014-5793(79)81184-9. [DOI] [PubMed] [Google Scholar]



