Abstract
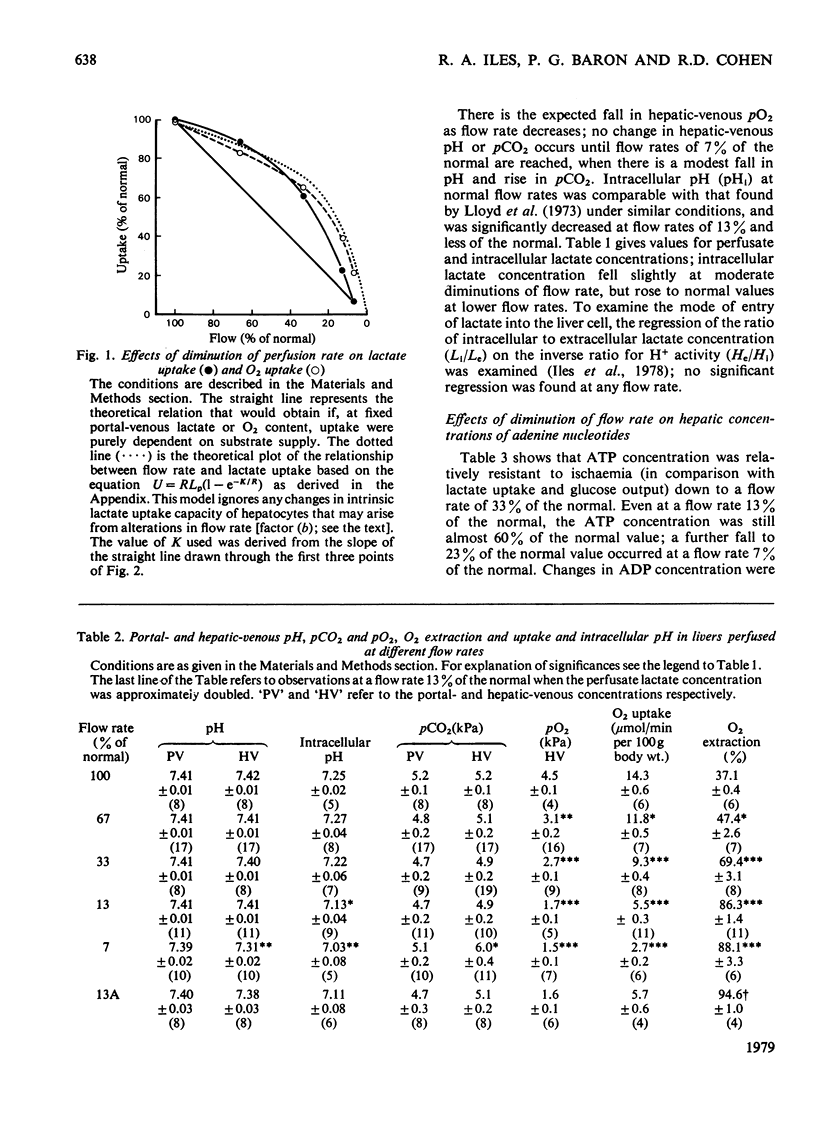
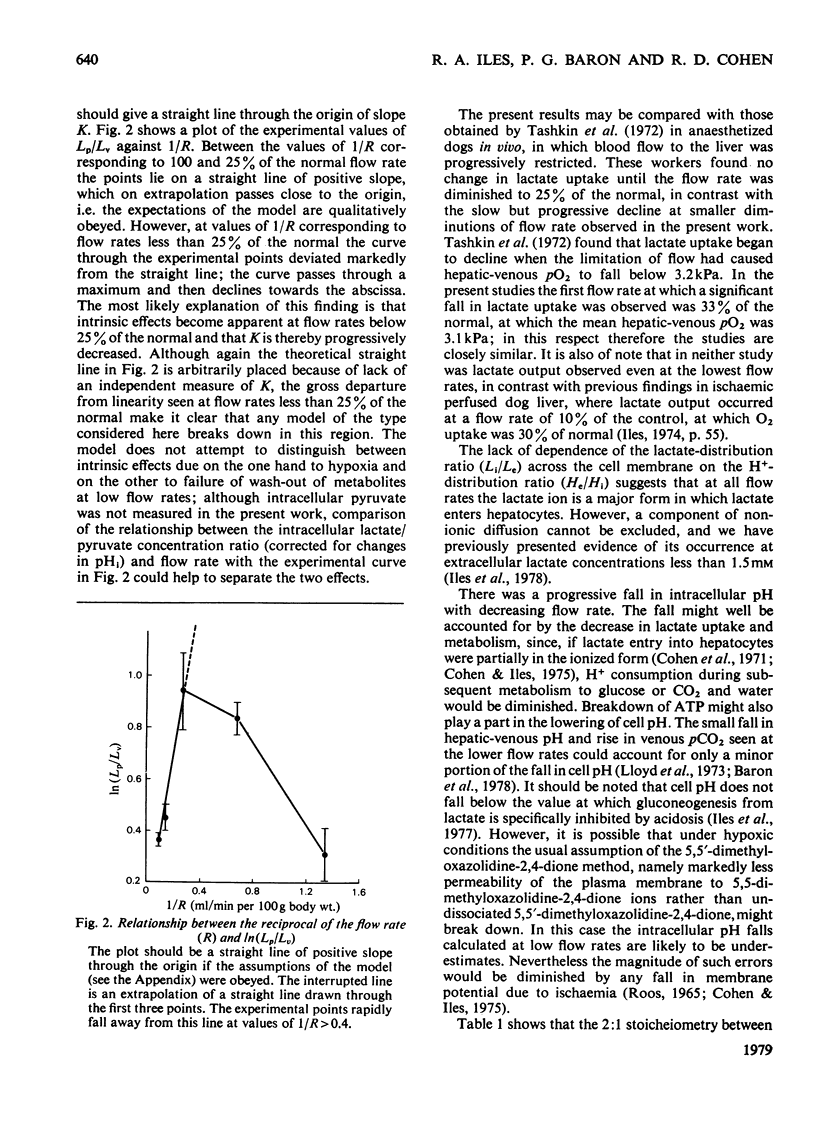
1. Lactate and O2 uptake and glucose output were studied in isolated livers from starved rats at perfusate flow rates varying from 100 to 7% of "normal" (11.25-0.75 ml/min per 100 g body wt.). 2. With moderate diminution of flow rate, lactate and oxygen uptake fell more slowly than would be expected if uptake purely depended on substrate supply. 3. Use of a mathematical model suggests that the intrinsic capacity of the liver for lactate uptake is unaffected until the flow rate falls below 25% of "normal". 4. Some lactate uptake was always observed even at 7% of the "normal" flow rate. 5. At flow rates below 33% of the "normal", lactate was increasingly metabolized by pathways other than gluconeogenesis, which became a progressively less important consumer of available O2. 6. ATP content decreased with diminution of flow rate, but substantially less markedly than did lactate uptake and glucose output. 7. Intracellular pH fell from a mean value of 7.25 at "normal" flow rate to 7.03 at 7% of the "normal" flow rate.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron P. G., Iles R. A., Cohen R. D. Effect of varying PCO2 on intracellular pH and lactate consumption in the isolated perfused rat liver. Clin Sci Mol Med. 1978 Aug;55(2):175–181. doi: 10.1042/cs0550175. [DOI] [PubMed] [Google Scholar]
- Brosnan J. T., Krebs H. A., Williamson D. H. Effects of ischaemia on metabolite concentrations in rat liver. Biochem J. 1970 Mar;117(1):91–96. doi: 10.1042/bj1170091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R. D., Iles R. A., Barnett D., Howell M. E., Strunin J. The effect of changes in lactate uptake on the intracellular pH of the perfused rat liver. Clin Sci. 1971 Aug;41(2):159–170. doi: 10.1042/cs0410159. [DOI] [PubMed] [Google Scholar]
- Cohen R. D., Iles R. A. Intracellular pH: measurement, control, and metabolic interrelationships. CRC Crit Rev Clin Lab Sci. 1975 Sep;6(2):101–143. doi: 10.3109/10408367509151567. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Park C. R. Control of gluconeogenesis in liver. I. General features of gluconeogenesis in the perfused livers of rats. J Biol Chem. 1967 Jun 10;242(11):2622–2636. [PubMed] [Google Scholar]
- Guder W. G., Schmidt U. Liver cell heterogeneity. The distribution of pyruvate kinase and phosphoenolpyruvate carboxykinase (GTP) in the liver lobule of fed and starved rats. Hoppe Seylers Z Physiol Chem. 1976 Dec;357(12):1793–1800. doi: 10.1515/bchm2.1976.357.2.1793. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Brosnan J. T. Effects of ischaemia on content of metabolites in rat liver and kidney in vivo. Biochem J. 1970 Nov;120(1):105–111. doi: 10.1042/bj1200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles R. A., Baron P. G., Cohen R. D. Mechanism of the effect of varying PCO2 on gluconeogenesis from lactate in the perfused rat liver. Clin Sci Mol Med. 1978 Aug;55(2):183–188. doi: 10.1042/cs0550183. [DOI] [PubMed] [Google Scholar]
- Iles R. A., Cohen R. D., Rist A. H., Baron P. G. The mechanism of inhibition by acidosis of gluconeogenesis from lactate in rat liver. Biochem J. 1977 Apr 15;164(1):185–191. doi: 10.1042/bj1640185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd M. H., Iles R. A., Simpson B. R., Strunin J. M., Layton J. M., Cohen R. D. The effect of simulated metabolic acidosis on intracellular pH and lactate metabolism in the isolated perfused rat liver. Clin Sci Mol Med. 1973 Oct;45(4):543–549. doi: 10.1042/cs0450543. [DOI] [PubMed] [Google Scholar]
- NOVIKOFF A. B. Cell heterogeneity within the hepatic lobule of the rat: staining reactions. J Histochem Cytochem. 1959 Jul;7(4):240–244. doi: 10.1177/7.4.240. [DOI] [PubMed] [Google Scholar]
- Roos A. Intracellular pH and intracellular buffering power of the cat brain. Am J Physiol. 1965 Dec;209(6):1233–1246. doi: 10.1152/ajplegacy.1965.209.6.1233. [DOI] [PubMed] [Google Scholar]
- Tashkin D. P., Goldstein P. J., Simmons D. H. Hepatic lactate uptake during decreased liver perfusion and hyposemia. Am J Physiol. 1972 Oct;223(4):968–974. doi: 10.1152/ajplegacy.1972.223.4.968. [DOI] [PubMed] [Google Scholar]
- Yudkin J., Cohen R. D., Slack B. The haemodynamic effects of metabolic acidosis in the rat. Clin Sci Mol Med. 1976 Mar;50(3):177–184. doi: 10.1042/cs0500177. [DOI] [PubMed] [Google Scholar]


