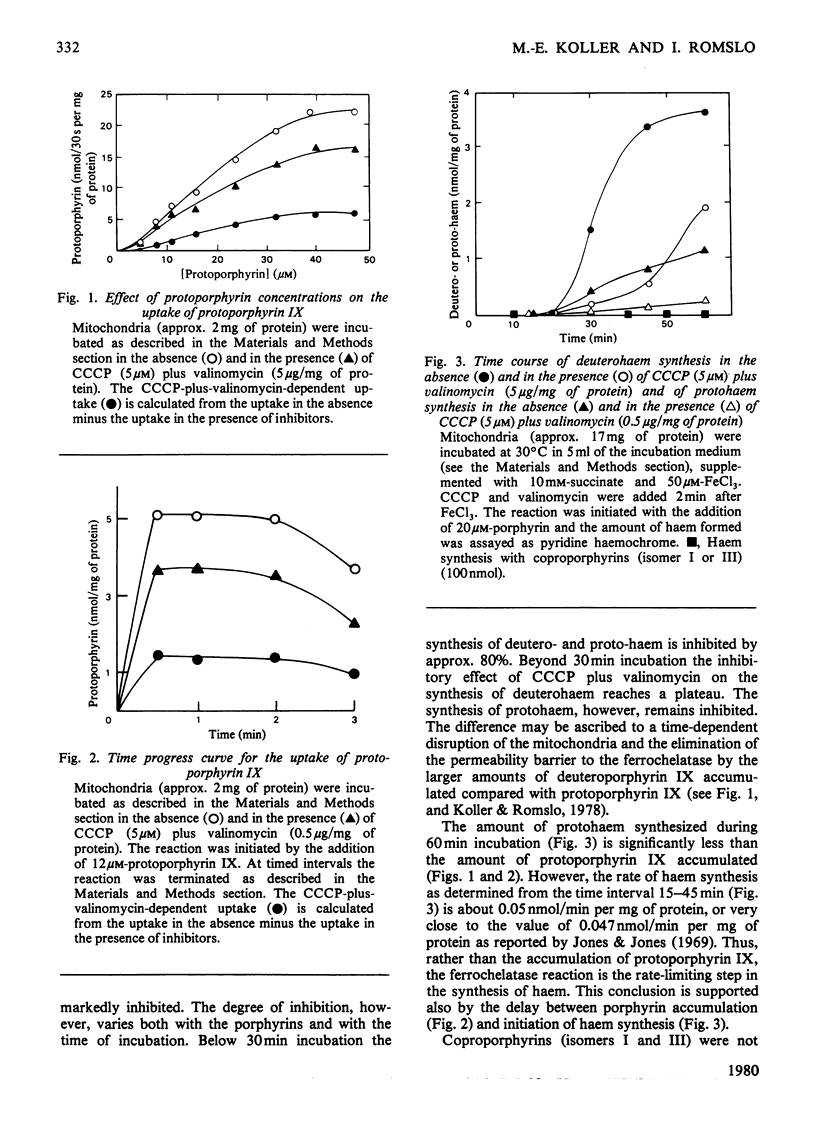
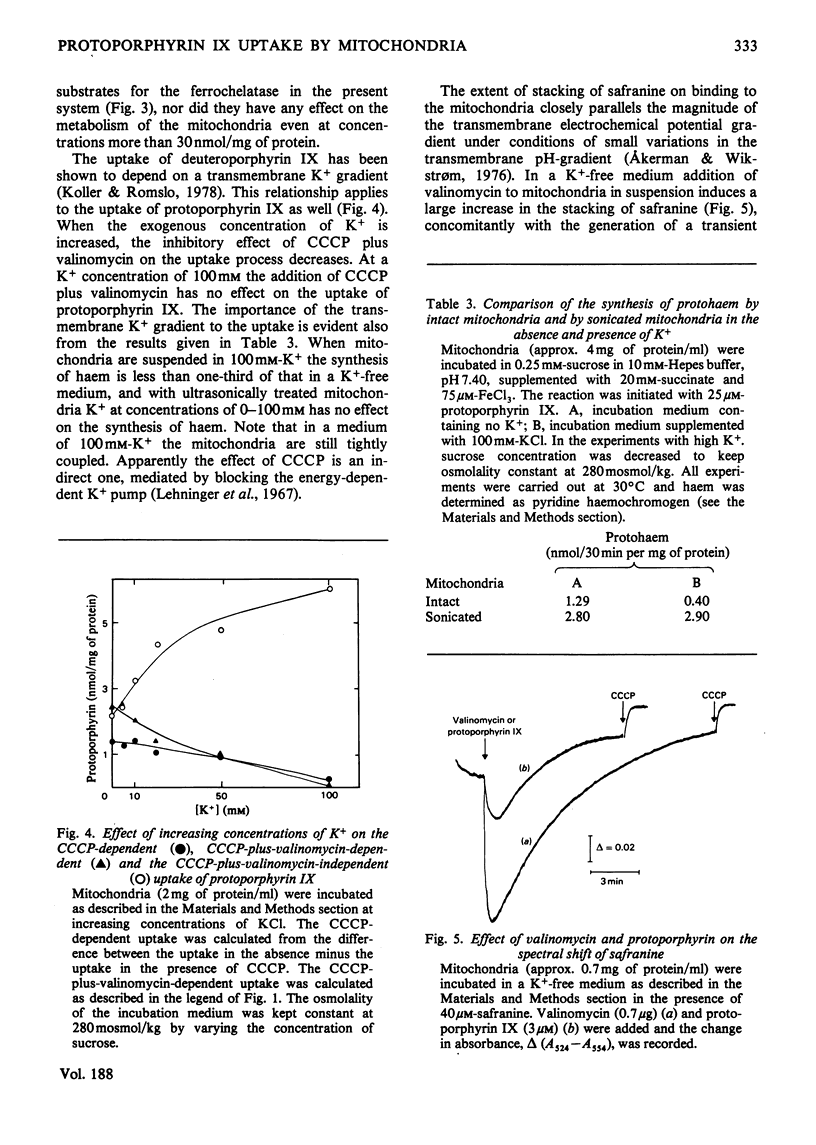
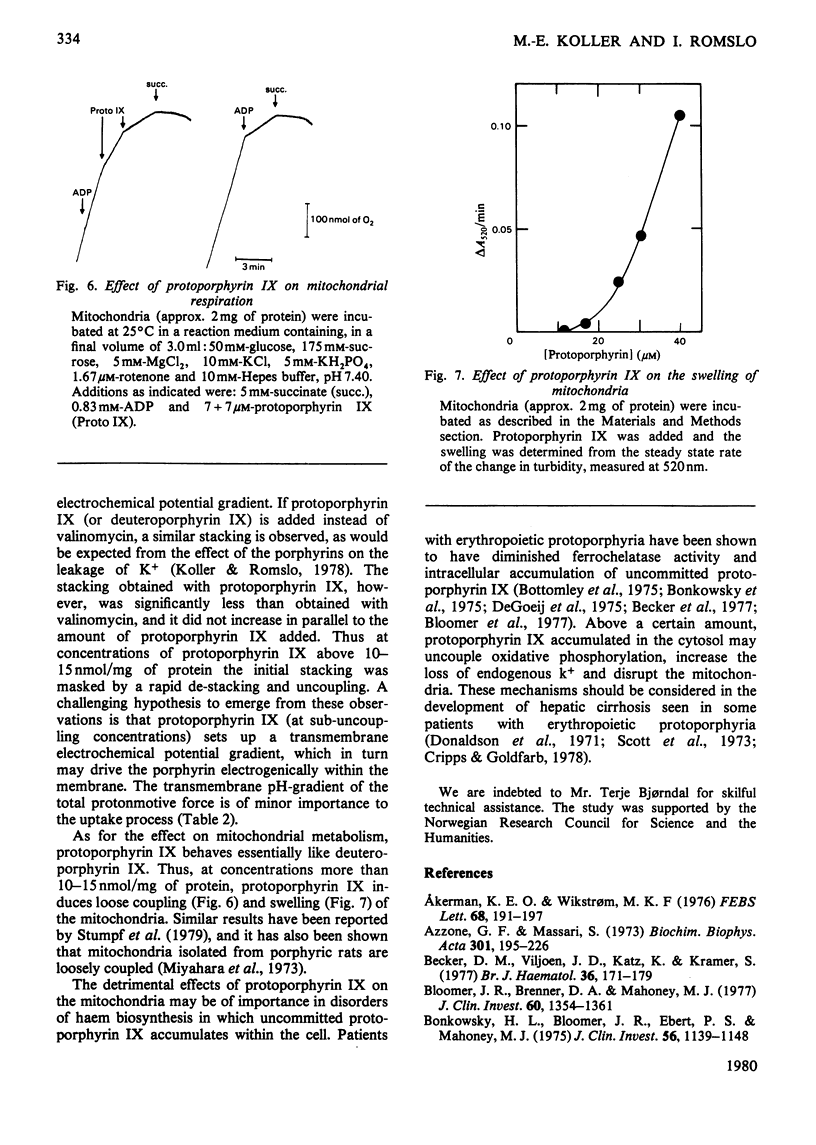
Abstract
Rat liver mitochondria accumulate protoporphyrin IX from the suspending medium into the inner membrane in parallel with the magnitude of the transmembrane K+ gradient (K+in/K+out). Only protoporphyrin IX taken up in parallel with the transmembrane K+ gradient is available for haem synthesis. Coproporphyrins (isomers I and III) are not taken up by the mitochondria. The results support the suggestion by Elder & Evans [(1978) Biochem. J. 172, 345-347] that the prophyrin to be taken up by the inner mitochondrial membrane belongs to the protoporphyrin(ogen) IX series. Protoporphyrin IX at concentrations above 15 nmol/mg of protein has detrimental effects on the structural and functional integrity of the mitochondria. The relevance of these effects to the hepatic lesion in erythropoietic protoporphyria is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerman K. E., Wikström M. K. Safranine as a probe of the mitochondrial membrane potential. FEBS Lett. 1976 Oct 1;68(2):191–197. doi: 10.1016/0014-5793(76)80434-6. [DOI] [PubMed] [Google Scholar]
- Azzone G. F., Massari S. Active transport and binding in mitochondria. Biochim Biophys Acta. 1973 Dec 31;301(3):195–226. doi: 10.1016/0304-4173(73)90004-9. [DOI] [PubMed] [Google Scholar]
- Becker D. M., Viljoen J. D., Katz J., Kramer S. Reduced ferrochelatase activity: a defect common to porphyria variegata and protoporphyria. Br J Haematol. 1977 Jun;36(2):171–179. doi: 10.1111/j.1365-2141.1977.tb00637.x. [DOI] [PubMed] [Google Scholar]
- Bloomer J. R., Brenner D. A., Mahoney M. J. Study of factors causing excess protoporphyrin accumulation in cultured skin fibroblasts from patients with protoporphyria. J Clin Invest. 1977 Dec;60(6):1354–1361. doi: 10.1172/JCI108895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowsky H. L., Bloomer J. R., Ebert P. S., Mahoney M. J. Heme synthetase deficiency in human protoporphyria. Demonstration of the defect in liver and cultured skin fibroblasts. J Clin Invest. 1975 Nov;56(5):1139–1148. doi: 10.1172/JCI108189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley S. S., Tanaka M., Everett M. A. Diminished erythroid ferrochelatase activity in protoporphyria. J Lab Clin Med. 1975 Jul;86(1):126–131. [PubMed] [Google Scholar]
- Cripps D. J., Goldfarb S. S. Erythropoietic protoporphyria: hepatic cirrhosis. Br J Dermatol. 1978 Mar;98(3):349–354. doi: 10.1111/j.1365-2133.1978.tb06163.x. [DOI] [PubMed] [Google Scholar]
- Donaldson E. M., McCall A. J., Magnus I. A., Simpson J. R., Caldwell R. A., Hargreaves T. Erythropoietic protoporphyria: two deaths from hepatic cirrhosis. Br J Dermatol. 1971 Jan;84(1):14–24. doi: 10.1111/j.1365-2133.1971.tb14191.x. [DOI] [PubMed] [Google Scholar]
- Drabkin D. L. The environment of function of liver and red blood cells. Ann N Y Acad Sci. 1975 Apr 15;244:603–623. doi: 10.1111/j.1749-6632.1975.tb41557.x. [DOI] [PubMed] [Google Scholar]
- Elder G. H., Evans J. O. Evidence that the coproporphyrinogen oxidase activity of rat liver is situated in the intermembrane space of mitochondria. Biochem J. 1978 May 15;172(2):345–347. doi: 10.1042/bj1720345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatmark T., Terland O., Helle K. B. Electron carriers of the bovine adrenal chromaffin granules. Biochim Biophys Acta. 1971 Jan 12;226(1):9–19. doi: 10.1016/0005-2728(71)90173-3. [DOI] [PubMed] [Google Scholar]
- Grandchamp B., Phung N., Nordmann Y. The mitochondrial localization of coproporphyrinogen III oxidase. Biochem J. 1978 Oct 15;176(1):97–102. doi: 10.1042/bj1760097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. S., Jones O. T. Permeability properties of mitochondrial membranes and the regulation of haem biosynthesis. Biochem Biophys Res Commun. 1970 Nov 25;41(4):1072–1079. doi: 10.1016/0006-291x(70)90195-6. [DOI] [PubMed] [Google Scholar]
- Jones M. S., Jones O. T. The structural organization of haem synthesis in rat liver mitochondria. Biochem J. 1969 Jul;113(3):507–514. doi: 10.1042/bj1130507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller M. E., Romslo I. Studies on the uptake of porphyrin by isolated rat liver mitochondria. Biochim Biophys Acta. 1978 Aug 8;503(2):238–250. doi: 10.1016/0005-2728(78)90185-8. [DOI] [PubMed] [Google Scholar]
- LABBE R. F., HUBBARD N., CAUGHEY W. S. Porphyrin specificity of ferro:protoporphyrin chelatase from rat liver. Biochemistry. 1963 Mar-Apr;2:372–374. doi: 10.1021/bi00902a033. [DOI] [PubMed] [Google Scholar]
- Miyahara M., Hirata K., Tada H., Seno S. Mitochondrial metabolism in porphyric rat liver. Biochim Biophys Acta. 1973 Oct 19;325(1):47–53. doi: 10.1016/0005-2728(73)90149-7. [DOI] [PubMed] [Google Scholar]
- NISHIDA G., LABBE R. F. Heme biosynthesis; on the incorporation of iron into protoporphyrin. Biochim Biophys Acta. 1959 Feb;31(2):519–524. doi: 10.1016/0006-3002(59)90028-9. [DOI] [PubMed] [Google Scholar]
- PORRA R. J., JONES O. T. Studies on ferrochelatase. 1. Assay and properties of ferrochelatase from a pig-liver mitochondrial extract. Biochem J. 1963 Apr;87:181–185. doi: 10.1042/bj0870181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulson R. The enzymic conversion of protoporphyrinogen IX to protoporphyrin IX in mammalian mitochondria. J Biol Chem. 1976 Jun 25;251(12):3730–3733. [PubMed] [Google Scholar]
- Romslo I., Flatmark T. Energy-dependent accumulation of iron by isolated rat liver mitochondria. II. Relationship to the active transport of Ca2+. Biochim Biophys Acta. 1973 Oct 19;325(1):38–46. doi: 10.1016/0005-2728(73)90148-5. [DOI] [PubMed] [Google Scholar]
- SANO S., GRANICK S. Mitochondrial coproporphyrinogen oxidase and protoporphyrin formation. J Biol Chem. 1961 Apr;236:1173–1180. [PubMed] [Google Scholar]
- Scott A. J., Ansford A. J., Webster B. H., Stringer H. C. Erythropoietic protoporphyria with features of a sideroblastic anaemia terminating in liver failure. Am J Med. 1973 Feb;54(2):251–259. doi: 10.1016/0002-9343(73)90230-1. [DOI] [PubMed] [Google Scholar]
- Stumpf D. A., McCabe E. R., Parks J. K., Bullen W. W., Schiff S. Loosely coupled mitochondrial oxidative phosphorylation induced by protoporphyrin. Biochem Med. 1979 Apr;21(2):182–189. doi: 10.1016/0006-2944(79)90070-x. [DOI] [PubMed] [Google Scholar]
- de Goeij A. F., Christianse K., van Steveninck J. Decreased haem synthetase activity in blood cells of patients with erythropoietic protoporphyria. Eur J Clin Invest. 1975 Sep 12;5(5):397–400. doi: 10.1111/j.1365-2362.1975.tb00470.x. [DOI] [PubMed] [Google Scholar]


