Abstract
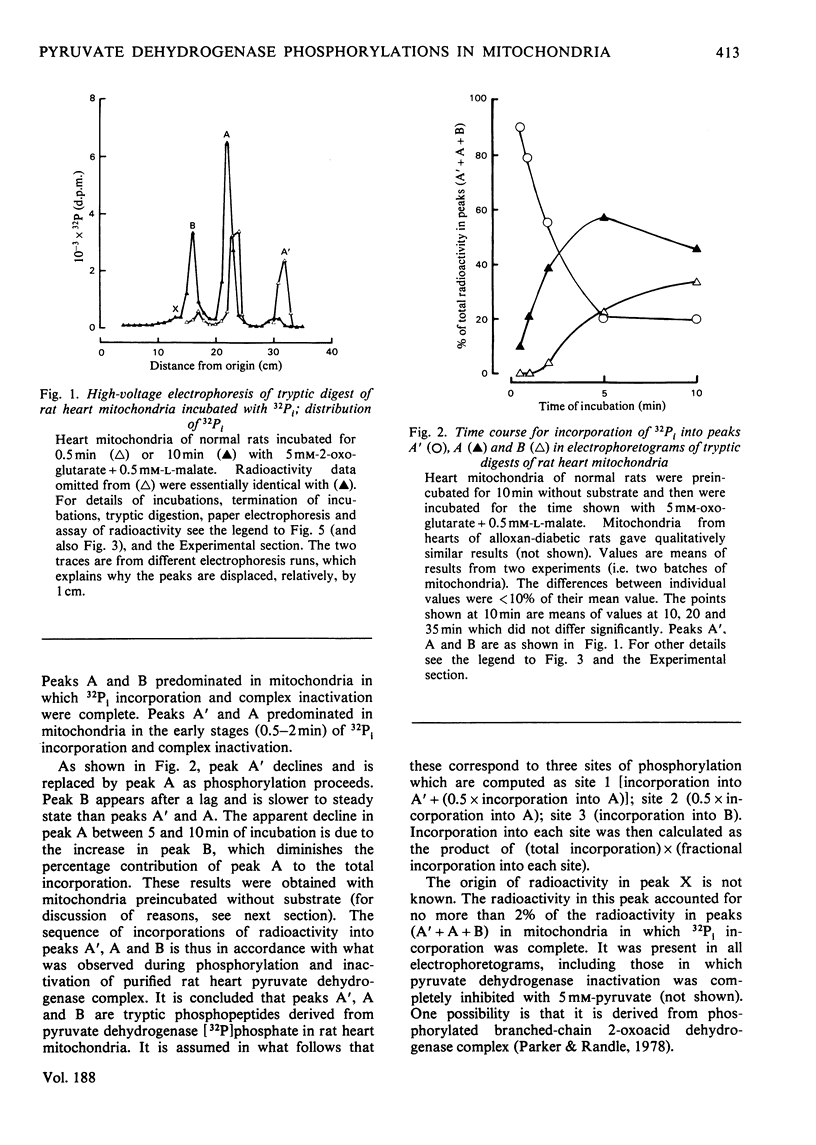
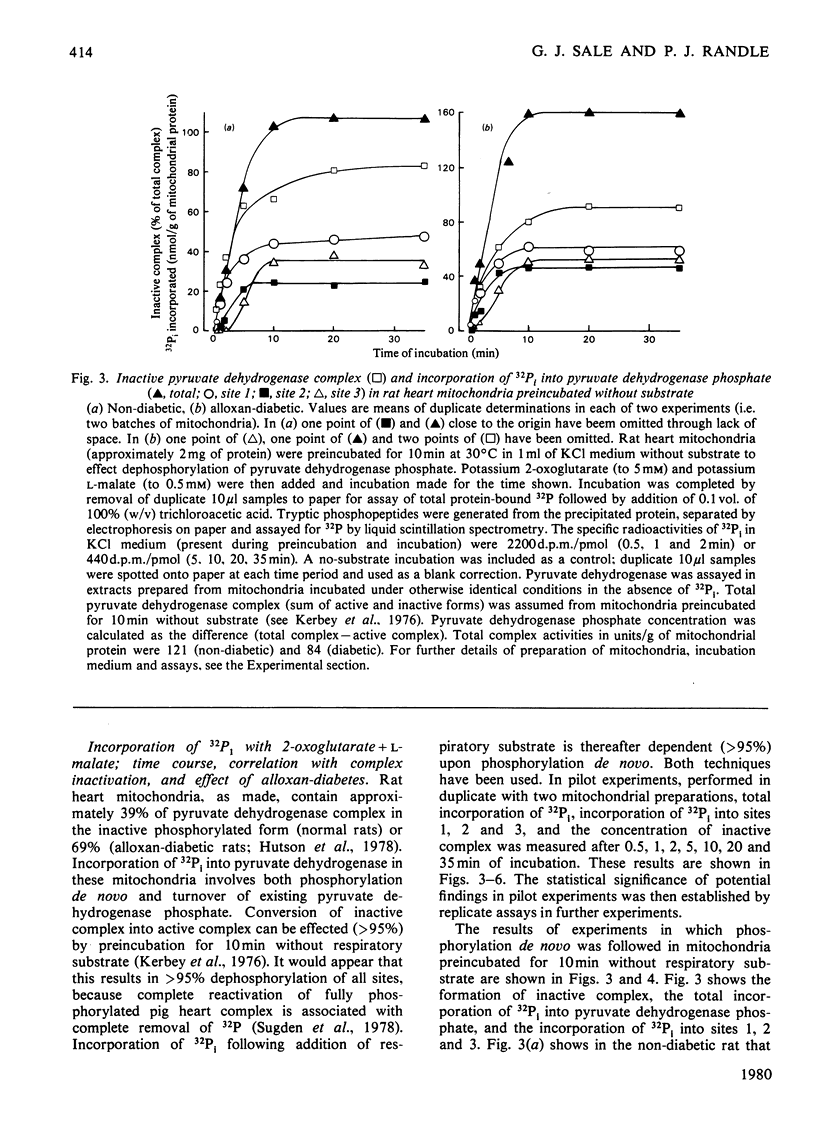
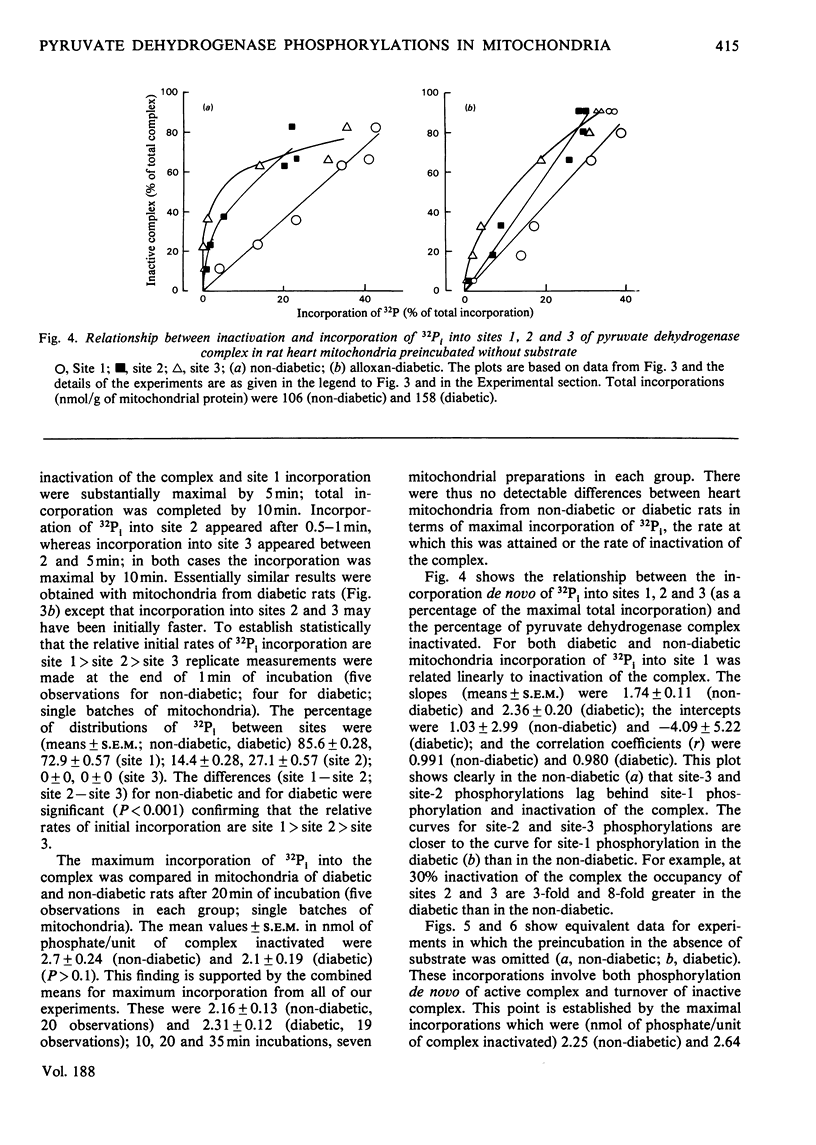
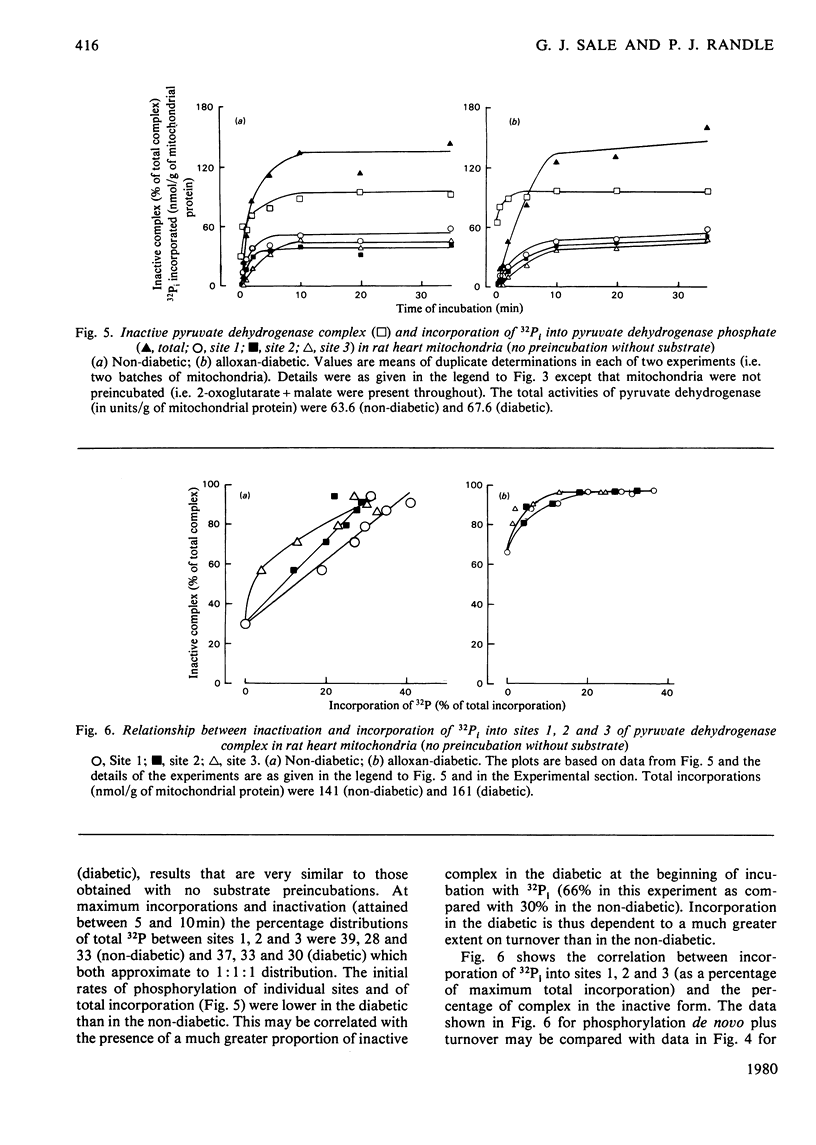
1. Evidence is given for three sites of phosphorylation in the alpha-chains of the decarboxylase component of purified rat heart pyruvate dehydrogenase complex, analogous to those established for procine and bovine complexes. Inactivation of rat heart complex was correlated with phosphorylation of site 1. Relative initial rates of phosphorylation were site 1 greater than site 2 greater than site 3. 2. Methods are described for measurement of incorporation of 32Pi into the complex in rat heart mitochondria oxidizing 2-oxoglutarate + L-malate (total, sites 1, 2 and 3). Inactivation of the complex was related linearly to phosphorylation of site 1 in mitochondria of normal or diabetic rats. The relative initial rates of phosphorylation were site 1 greater than site 2 greater than site 3. Rates of site-2 and site-3 phosphorylation may have been closer to that of site 1 in mitochondria of diabetic rats than in mitochondria of normal rats. 3. The concentration of inactive (phosphorylated) complex was varied in mitochondria from normal rats by inhibiting the kinase reaction with pyruvate at concentrations ranging from 0.15 to 0.4 mM. The results showed that the concentration of inactive complex is related linearly to incorporation of 32Pi into site 1. Inhibition of 32Pi incorporations with pyruvate at all concentrations over this range was site 3 greater than site 2 greater than site 1. 4. With mitochondria from diabetic rats, pyruvate (0.15-0.4 mM) inhibited incorporation of 32Pi into site 3, but it had no effect on the concentration of inactive complex or on incorporations of 32Pi into site 1 or site 2. It is concluded that site-3 phosphorylation is not required for inactivation of the complex in rat heart mitochondria. 5. Evidence is given that phosphorylation of sites 2 and 3 may inhibit reactivation of the complex by dephosphorylation in rat heart mitochondria.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper R. H., Randle P. J., Denton R. M. Regulation of heart muscle pyruvate dehydrogenase kinase. Biochem J. 1974 Dec;143(3):625–641. doi: 10.1042/bj1430625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. H., Randle P. J., Denton R. M. Stimulation of phosphorylation and inactivation of pyruvate dehydrogenase by physiological inhibitors of the pyruvate dehydrogenase reaction. Nature. 1975 Oct 30;257(5529):808–809. doi: 10.1038/257808a0. [DOI] [PubMed] [Google Scholar]
- Coore H. G., Denton R. M., Martin B. R., Randle P. J. Regulation of adipose tissue pyruvate dehydrogenase by insulin and other hormones. Biochem J. 1971 Nov;125(1):115–127. doi: 10.1042/bj1250115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin J. D., Reimann E. M. Assay of cyclic AMP-dependent protein kinases. Methods Enzymol. 1974;38:287–290. doi: 10.1016/0076-6879(74)38044-5. [DOI] [PubMed] [Google Scholar]
- Hansford R. G. Studies on the effects of coenzyme A-SH: acetyl coenzyme A, nicotinamide adenine dinucleotide: reduced nicotinamide adenine dinucleotide, and adenosine diphosphate: adenosine triphosphate ratios on the interconversion of active and inactive pyruvate dehydrogenase in isolated rat heart mitochondria. J Biol Chem. 1976 Sep 25;251(18):5483–5489. [PubMed] [Google Scholar]
- Hughes W. A., Denton R. M. Evidence for multi-site phosphorylation of pyruvate dehydrogenase within intact mitochondria [proceedings]. Biochem Soc Trans. 1978;6(6):1228–1230. doi: 10.1042/bst0061228. [DOI] [PubMed] [Google Scholar]
- Hughes W. A., Denton R. M. Incorporation of 32Pi into pyruvate dehydrogenase phosphate in mitochondria from control and insulin-treated adipose tissue. Nature. 1976 Dec 2;264(5585):471–473. doi: 10.1038/264471a0. [DOI] [PubMed] [Google Scholar]
- Hutson N. J., Kerbey A. L., Randle P. J., Sugden P. H. Conversion of inactive (phosphorylated) pyruvate dehydrogenase complex into active complex by the phosphate reaction in heart mitochondria is inhibited by alloxan-diabetes or starvation in the rat. Biochem J. 1978 Aug 1;173(2):669–680. doi: 10.1042/bj1730669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson N. J., Randle P. J. Enhanced activity of pyruvate dehydrogenase kinase in rat heart mitochondria in alloxan-diabetes or starvation. FEBS Lett. 1978 Aug 1;92(1):73–76. doi: 10.1016/0014-5793(78)80724-8. [DOI] [PubMed] [Google Scholar]
- Kerbey A. L., Radcliffe P. M., Randle P. J. Diabetes and the control of pyruvate dehydrogenase in rat heart mitochondria by concentration ratios of adenosine triphosphate/adenosine diphosphate, of reduced/oxidized nicotinamide-adenine dinucleotide and of acetyl-coenzyme A/coenzyme A. Biochem J. 1977 Jun 15;164(3):509–519. doi: 10.1042/bj1640509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbey A. L., Radcliffe P. M., Randle P. J., Sugden P. H. Regulation of kinase reactions in pig heart pyruvate dehydrogenase complex. Biochem J. 1979 Aug 1;181(2):427–433. doi: 10.1042/bj1810427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbey A. L., Randle P. J., Cooper R. H., Whitehouse S., Pask H. T., Denton R. M. Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. Biochem J. 1976 Feb 15;154(2):327–348. doi: 10.1042/bj1540327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Hucho F., Reed L. J. Alpha-keto acid dehydrogenase complexes. XI. Comparative studies of regulatory properties of the pyruvate dehydrogenase complexes from kidney, heart, and liver mitochondria. Proc Natl Acad Sci U S A. 1969 Sep;64(1):227–234. doi: 10.1073/pnas.64.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker P. J., Randle P. J. Inactivation of rat heart branched-chain 2-oxoacid dehydrogenase complex by adenosine triphosphate. FEBS Lett. 1978 Nov 1;95(1):153–156. doi: 10.1016/0014-5793(78)80072-6. [DOI] [PubMed] [Google Scholar]
- Pettit F. H., Pelley J. W., Reed L. J. Regulation of pyruvate dehydrogenase kinase and phosphatase by acetyl-CoA/CoA and NADH/NAD ratios. Biochem Biophys Res Commun. 1975 Jul 22;65(2):575–582. doi: 10.1016/s0006-291x(75)80185-9. [DOI] [PubMed] [Google Scholar]
- Severson D. L., Denton R. M., Pask H. T., Randle P. J. Calcium and magnesium ions as effectors of adipose-tissue pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1974 May;140(2):225–237. doi: 10.1042/bj1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Hutson N. J., Kerbey A. L., Randle P. J. Phosphorylation of additional sites on pyruvate dehydrogenase inhibits its re-activation by pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1978 Feb 1;169(2):433–435. doi: 10.1042/bj1690433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Kerbey A. L., Randle P. J., Waller C. A., Reid K. B. Amino acid sequences around the sites of phosphorylation in the pig heart pyruvate dehydrogenase complex. Biochem J. 1979 Aug 1;181(2):419–426. doi: 10.1042/bj1810419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Randle P. J. Regulation of pig heart pyruvate dehydrogenase by phosphorylation. Studies on the subunit and phosphorylation stoicheiometries. Biochem J. 1978 Aug 1;173(2):659–668. doi: 10.1042/bj1730659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teague W. M., Pettit F. H., Yeaman S. J., Reed L. J. Function of phosphorylation sites on pyruvate dehydrogenase. Biochem Biophys Res Commun. 1979 Mar 15;87(1):244–252. doi: 10.1016/0006-291x(79)91672-3. [DOI] [PubMed] [Google Scholar]
- Yeaman S. J., Hutcheson E. T., Roche T. E., Pettit F. H., Brown J. R., Reed L. J., Watson D. C., Dixon G. H. Sites of phosphorylation on pyruvate dehydrogenase from bovine kidney and heart. Biochemistry. 1978 Jun 13;17(12):2364–2370. doi: 10.1021/bi00605a017. [DOI] [PubMed] [Google Scholar]


