Abstract
The presence of glomerular diseases in pregnancy presents challenges to both patients and nephrologists. The preconception planning in patients with kidney disease involves comprehensive stratification, treatment optimization, and comorbidity assessment, requiring nephrologists to engage in well-informed decision-making processes alongside their patients. There is a necessity for a multidisciplinary approach to meet their complex healthcare needs. Effective control of blood pressure, proteinuria, and disease activity are pivotal in mitigating adverse pregnancy events. This comprehensive review intends to equip nephrologists with the requisite knowledge and understanding to navigate the intricate landscape of glomerular diseases in pregnancy. It delves into the challenges associated with the diagnosis of glomerular diseases, the significance of preconception counseling, and the nuances of antenatal and postnatal care. Additionally, the article provides insights into the management and prognosis of glomerular diseases, shedding light on the judicious use of immunosuppression as a therapeutic tool.
Keywords: Antenatal care, Glomerular diseases, Pregnancy, Pre-eclampsia, Steroids
Introduction
The convergence of glomerular diseases and pregnancy presents a complex and intricate subject matter that poses challenges for both patients and nephrologists. From the patient’s standpoint, the pursuit of motherhood embodies a profound societal aspiration, marking a natural phase in the trajectory of life. For the nephrologist, this juncture signals the looming specter of potentially poor outcomes, evoking the cautionary words of an often-quoted editorial: “Children of women with renal disease used to be born dangerously or not at all—not at all, if their doctors had their way.”1 It is imperative to recognize that the coexistence of glomerular diseases and pregnancy is not a prohibitive conundrum, but rather a complex clinical scenario demanding nuanced consideration. This review serves as a resounding call to move beyond dogmatic prohibition and toward a informed, evidence-based approach to the management of pregnancy in women with glomerular diseases.
Physiological alterations in kidneys during pregnancy
Women’s kidneys undergo significant structural, functional, and hemodynamic alterations during pregnancy.2 An increase in maternal cardiac output begins as early as week five of pregnancy, resulting in decreased systemic vascular resistance, increased heart rate, and decreased systolic and diastolic blood pressure (BP).3 Glomerular filtration rate (GFR) increases by 20% at four weeks gestation and by 45% at nine weeks.4 Renal plasma flow (RPF) increases after conception, ultimately achieving rates that are 50–85% higher.2 The filtration fraction is slightly reduced in early pregnancy because RPF exceeds GFR. Between weeks 12 and the end of pregnancy, the filtration fraction rises because the RPF returns to prepregnancy levels, but the GFR remains elevated.4 These aforementioned alterations are attributed to the hormonal and hemodynamic changes during pregnancy. In a study investigating the impact of glomerular disease during pregnancy in rats with experimentally induced glomerulonephritis (GN), micropuncture revealed elevated glomerular capillary BP and a reduced glomerular capillary ultrafiltration coefficient, Kf, proteinuria and moderate glomerular changes. Pregnancy during the midterm stage did not worsen proteinuria or glomerular morphology, and it did not further affect BP or Kf, indicating the maternal kidney’s ability to enhance RPF in the presence of underlying glomerular diseases.5
Figure 1 shows the comparison between kidney physiological adaptations in pregnancy and the pathophysiology of preeclampsia.
Figure 1:
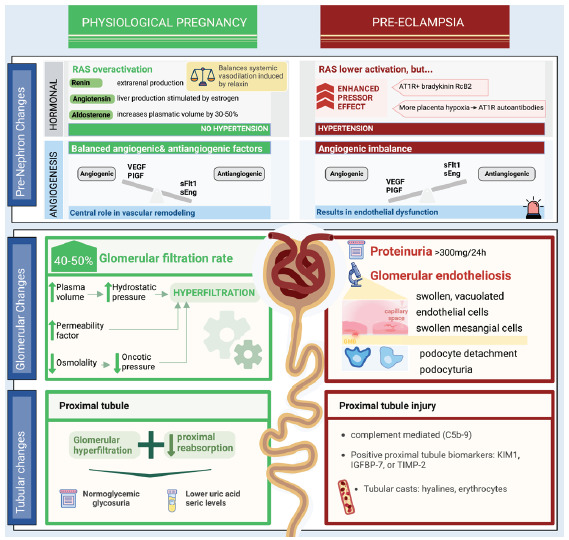
Comparison between kidney physiological adaptations in pregnancy and pathophysiology of preeclampsia. AT1R: Angiotensin II receptor type 1, VEGF: Vascular endothelial growth factor, PlGF: Placental growth factor, Soluble fms-like Tyrosine Kinase-1, sEng: Soluble endoglin, IGFBP: Insulin-like growth factor-binding protein, TIMP2: Tissue inhibitor of metalloproteinases, KIM: Kidney injury molecule, RcB2: Bradykinin B2 receptor, RAS: renin-angiotensin- system.
Epidemiology of glomerular disease in pregnancy
The exact data on the prevalence and type of glomerular diseases in pregnancy is limited due to inconsistencies in the reporting and the diversity of study groups and types of studies. The most frequently reported glomerular diseases diagnosed through kidney biopsies in pregnancy or postpartum period is focal segmental glomerulosclerosis (FSGS), followed by immunoglobulin A nephropathy (IgAN), and lupus nephritis (LN).6,7 In a recently published systematic review on pregnancy and glomerular disease, IgAN was the most commonly reported GN.8 The Australian and New Zealand Dialysis and Transplantation Registry’s analysis revealed that glomerular diseases accounted for 35–56% of end-stage kidney disease (ESKD) cases in women who were started on dialysis before or after pregnancy and those who had received a kidney transplant.9
Interpretation of tests to measure kidney structure and function during pregnancy GFR estimation by conventional equations
GFR estimating equations based on serum creatinine are not reliable in the pregnant state. However, owing to the difficulty of obtaining measured GFR using inulin clearance, timed urinary collections, and risk of radiation as well as fetal exposure in radionuclide imaging-based GFR, GFR estimating equations are still commonly utilized for the diagnosis of reduced GFR in pregnancy.10 All the commonly used estimating equations based on serum creatinine tend to underestimate GFR, and even the 2021 CKD EPI equation did not include pregnant women in the validation cohort.11,12 Cystatin C-based equations are also not reliable in the pregnant state.13 Measuring a 24-hour urinary creatinine clearance has been considered to be the standard of care among the commonly used techniques, even though timed urine collection is difficult in pregnancy due to urinary stasis. Another pitfall is the lack of studies with preconception/”baseline” serum creatinine. Serum creatinine in pregnancy falls to around 86% or less of the nonpregnant upper normal limit. Hence, an elevated serum creatinine level exceeding 0.8 mg/dl in a pregnant woman could suggest underlying kidney disease. Nonetheless, the assessment should be based on the comprehensive clinical context.14
Proteinuria
Increased urinary protein excretion up to 150–200 mg/day is common in normal pregnancies.15 Increased ultrafiltration coefficients increases glomerular basement permeability in late pregnancy, and a decreased creatinine tubular reabsorption can explain isolated gestational proteinuria.16,17 The generally accepted cutoff to define proteinuria during pregnancy is >300 mg/24 hours or spot urine protein to creatinine ratio of 0.3 (dipstick 1+).15 Isolated proteinuria, especially in the moderately elevated range (>300 mg/g creatinine) occurs in about 8–10% of normotensive pregnancies.15 In terms of practical considerations, 24-hour urine collection is time-consuming and prone to collection deficiencies. Recent studies have revealed a positive correlation between urinary albumin-to-creatinine ratio (uACR) and urinary protein-to-creatinine ratio (uPCR), suggesting that these two biomarkers are closely related and exhibit equivalent performance in predicting adverse pregnancy outcomes.15,18,19 A prospective cohort study, including over 11,000 women with low risk for developing preeclampsia (excluded patients with hypertension, diabetes, and a history of preeclampsia), showed single episodes of isolated gestational proteinuria of ≥1+ on the dipstick evaluation in 7.7% of the women.20 Some studies suggest that isolated gestational proteinuria is a milder form of preeclampsia.21,22
Detection of hematuria
Dipstick positive hematuria can be seen in 20% of pregnant women, while microscopic hematuria is relatively uncommon (2–4%).23 Dipstick hematuria does not correlate with any adverse maternal and fetal outcomes. Other than glomerular causes, uterine distension, increased vascularity, venous pressures, and nutcracker syndrome might contribute to hematuria in pregnancy.24 The finding of acanthocytes and casts in urine may act as a marker of glomerular inflammation. Isolated microhematuria alone does not indicate worsening glomerulonephritis in IgAN but does so in LN as microhematuria is part of the definition of lupus flare and indicates active glomerular inflammation.25 Also, women with LN and secondary antiphospholipid syndrome might be on anticoagulant therapy, which might predispose them to further hematuria. For pregnant women with ANCA-associated vasculitis (AAV) microhematuria, especially as part of active urine sediments, portends the risk of relapse of the disease.26
Complement levels in pregnancy
The complement system is intricately involved with maintaining normal placentation, and studies have demonstrated that C3 and C4 increase, especially in the second and third trimesters, while C5b-9 (terminal component) remains largely unchanged.27 Therefore, normal or only mildly reduced C3 and C4 levels might mask a lupus flare, superimposed preeclampsia, or both in the pregnant patient.28
Use of serologic markers in preexisting glomerular diseases
In women with preexisting GN, serial follow-up of serologic markers can facilitate monitoring of the underlying disease, along with periodic BP, urinary findings, and kidney function tests. The use of C-reactive protein and erythrocyte sedimentation rate is not preferable during pregnancy, as these can be elevated in uncomplicated pregnancies.29 In women with systemic lupus erythematosus (SLE), anti-double-stranded deoxyribonucleic acid Antibodies (dsDNA Ab) titers correlate with disease severity and flares during pregnancy and higher titers are associated with adverse fetal and maternal outcomes.30 Antineutrophilic cytoplasmic antibody (ANCA) titers have limited value in pregnant patients with AAV to predict the risk of relapse.26 In the case of membranous nephropathy (MN), the data is sparse regarding the use of anti-M-type phospholipase A2 receptor (PLA2R) autoantibodies in the usual follow-up of these pregnancies. No specific serologic markers can reliably differentiate relapse of minimal change disease (MCD) and FSGS from superimposed preeclampsia.
Differentiating disease flares and superimposed preeclampsia
LN can flare up during pregnancy. Conventionally, the presence of active urine sediments, acanthocyturia, and cellular casts in association with hypocomplementemia can reliably differentiate between pregnancy-associated flare and preeclampsia; sometimes, the laboratory findings may be inconclusive. Studies have elucidated the use of soluble fms-like Tyrosine Kinase-1 (sFlt-1) and placental growth factor (PlGF) ratio, which is elevated in preeclampsia, but not so in other kidney diseases.31,32 This test was approved by the Food and Drugs Administration (FDA) of the United States, based on the findings of the PRAECIS study in 2022.33
Table 1 presents these clinical and laboratory criteria that might help in the monitoring of certain kidney diseases in appropriate clinical context.
Table 1:
Differentiating features between flare of lupus nephritis and preeclampsia
| Lupus nephritis | Preeclampsia | |
|---|---|---|
| History | Any time during pregnancy | Onset after 20 weeks |
| Clinical examination | Presence of SLE activity in other organ systems (skin, joints, hematologic) | Hematologic, hepatic, and cerebral manifestations can occur with complicated preeclampsia |
| Urine examination | Presence of active urinary sediment and RBC and WBC casts favors LN | Urine sediment is generally inactive in preeclampsia |
| Biochemistry | Elevated liver enzymes and hyperuricemia are uncommon in active LN | Elevated liver enzymes and hyperuricemia occur more commonly with preeclampsia |
| Complement levels | Hypocomplementemia (low C3, C4) with increasing anti dsDNA titres | Complement levels are usually normal to high in preeclampsia |
| Growth factor levels | sFlt1/PlGF ratio is low, with higher VEGF level in active LN | sFlt1/PlGF ratio is elevated, low VEGF levels |
SLE: Systemic lupus erythematosus, dsDNA: Anti-double-stranded deoxyribonucleic acid antibodies, VEGF: Vascular endothelial growth factor, PlGF: Placental growth factor, sFlt1: Soluble fms-like tyrosine kinase-1, LN: Lupus nephritis, RBC: Red blood cells, WBC: White blood cells.
De novo glomerular diseases
De novo glomerular diseases are suspected when pregnant women without a known history of glomerular diseases present with proteinuria, hematuria or kidney dysfunction. If proteinuria is detected before 20 weeks, likely, that glomerular diseases were already present before the pregnancy. After 20 weeks, the main differential diagnosis is between isolated proteinuria, preeclampsia, and other glomerular diseases. Preeclampsia can occur in 3–5% of pregnancies, making it more prevalent than de novo glomerular disease.34,35
Dysmorphic red blood cells and pleomorphic casts in the sediment of the urine are telltale signs of active GN. Though the data regarding the incidence of de novo glomerular diseases during pregnancy is limited, there have been documented cases of de novo, MCD, FSGS, anti-glomerular basement membrane (anti-GBM) disease, and vasculitis.26,36–40 Interestingly, there are few case reports of de novo MN, revealing positive anti-PLA2R antibodies (Ab).41,42 Even though PLA2R Abs can cross the placenta, newborns are usually proteinuria-free. PLA2R Antibodies also pass from mother to fetus through breast milk. The levels of these Antibodies in the child decreased when breastfeeding ceased.41 In cases of neonatal MN caused by anti-neutral endopeptidase antibodies, mothers do not develop glomerular diseases as they lack the target antigen. While neonatal MN is typically transient due to the short lifespan of maternal antibodies, severe cases can occur, necessitating plasma exchange to reduce antibody titres.43
Treatment leads to clinical improvement and normalization of laboratory parameters.44,45 In most situations, the diagnosis of MCD/FSGS is presumed without doing a kidney biopsy based on the patient’s presentation of abrupt onset of proteinuria, clinical symptoms, and responsiveness to corticosteroids.
De novo primary glomerular diseases can rarely be diagnosed during pregnancy [Table 2]. Figure 2 shows a diagnosis of de novo proteinuria in pregnancy and a kidney biopsy during pregnancy.
Table 2:
Main characteristics of de novo glomerular diseases reported during pregnancy
| Type of glomerular disease | Cases | Clinic | Gestational age at diagnostics | Diagnostics |
|---|---|---|---|---|
| Preeclampsia | 3–5% pregnancies |
Hypertension Dyspnea Persistent headache Blurry vision |
Debut after 20 weeks |
New-onset hypertension and New-onset end-organ damage, including but not necessarily proteinuria, after 20 weeks of gestation Biopsy: Glomerular endotheliosis Rare: FSGS |
| MCD and FSGS36,37,44,74 | Rare, case reports |
Edema Nephrotic range proteinuria Hypertension atypical |
Any |
Kidney biopsy Assumed when abrupt onset, selective nephrotic-range proteinuria, prompt response to steroids |
| Membranous glomerulonephritis 41,42,43,88 | Rare, case reports |
Edema Nephrotic syndrome |
11–28 weeks of gestation |
Kidney biopsy Anti-M-type phospholipase A2 receptor (PLA2R) antibody |
| IgA nephropathy | Rare, case reports78, 87 |
Nephrotic syndrome Rapidly progressive glomerulonephritis (nephrotic range proteinuria + microscopic hematuria/sterile leukocyturia) |
8–24 weeks of gestation | Kidney biopsy only |
| Lupus nephritis | Rare, more frequent lupus flares25, 63, 64 | Sub-nephrotic proteinuria, microscopic hematuria, sterile leukocyturia, AKI | More frequent in second and third trimesters |
Combination of: Clinical criteria Immunologic criteria Kidney biopsy |
| ANCA associated vasculitis26,38,41,45 | More frequent than other glomerular diseases |
Rapidly progressive glomerulonephritis Sub-nephrotic proteinuria, microscopic hematuria, AKI Extrarenal manifestations |
Median 20 weeks of gestation |
Combination of: Clinical features Antibodies: C-, P-ANCA Biopsies: renal and extrarenal |
| GBM antibody disease39 | Rare |
Proteinuria (low-range, sub-nephrotic), hematuria (all case reports) AKI, anemia |
12–28 weeks of gestation |
Anti-GBM antibodies Kidney biopsy |
AKI: Acute kidney injury, ANCA: Antineutrophilic cytoplasmic antibody, FSGS: Focal segmental glomerulosclerosis, MCD: Minimal change disease, GBM: Glomerular basement membrane.
Figure 2:
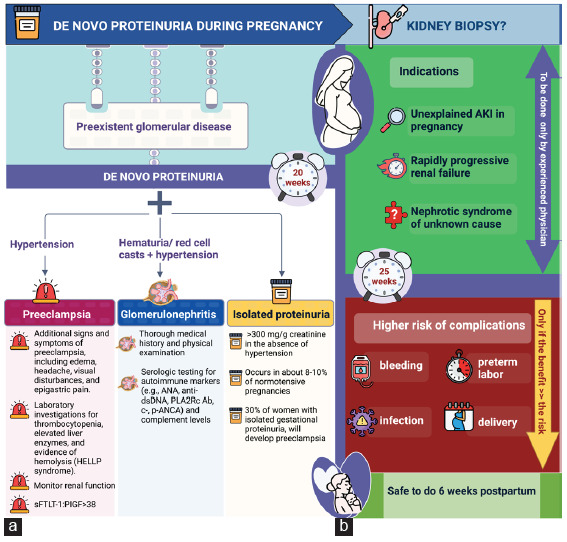
(a) Diagnosis of de novo proteinuria in pregnancy; (b) Kidney biopsy during pregnancy. HELLP: hemolysis, elevated liver enzymes, and low platelet count., sFLT: Soluble fms-like tyrosine kinase-1, PlGF: placenta growth factor, ANA: antinuclear antibody, DSDNA: Anti-double-stranded deoxyribonucleic acid, PLA2R: phospholipase A2 receptor antibody, c-ANCA: Cytoplasmic antineutrophil cytoplasmic antibodies, p-ANCA: Perinuclear anti-neutrophil cytoplasmic antibodies, AKI: Acute kidney injury.
Kidney biopsy
Histopathologic examination of renal tissue is required for accurate diagnosis and provides important prognostic and therapeutic information to facilitate appropriate management. Kidney biopsy in pregnant women should only be considered when the diagnosis obtained from the procedure is going to contribute to facilitating treatment decisions. Common indications for renal biopsy in pregnancy include unexplained renal dysfunction, rapidly progressive renal failure, nephrotic syndrome, when noninvasive methods of diagnosis have failed to elucidate the disease etiology, differentiation between LN flare and preeclampsia or for evaluation of renal allograft dysfunction.
In a case series of 11 pregnant women with SLE who underwent a renal biopsy to evaluate a presumptive flare of LN, proliferative LN was found in 91% of the biopsies. Kidney biopsy guided management in all except one. No maternal and fetal complications related to biopsy were reported.6 In general, pregnancy is regarded as a relative contraindication for a kidney biopsy because of the underlying risk of complications, such as bleeding, infection, or preterm labor and deliveries. Renal biopsy is typically not possible during the later stages of pregnancy, as alternate positioning (i.e., lateral decubitus or seated) may cause the procedure to become more technically difficult. In a series of 15 pregnant women who underwent kidney biopsies before 30 weeks, only one (6.7%) had post-biopsy gross hematuria.46 Out of 243 pregnancy biopsies evaluated in a systematic review, only four had significant bleeding complications at 23–26 weeks. Major complications were identified in 7% of women during pregnancy and 1% after birth. In 66% of cases, performing a kidney biopsy for the diagnosis of glomerulonephritis or preeclampsia resulted in treatment alteration.47 The major complications occurred at a median of 25 weeks of gestation (range 23–26 weeks). Therefore, the benefits of kidney biopsy for clinical diagnosis and management during pregnancy must be carefully weighed against the potential risks. Typically, a kidney biopsy is performed during the gestational period of up to 25 weeks. It is important to consider whether renal biopsy might alter the management of a patient in the later stages of pregnancy. It is suggested to wait for six weeks after childbirth to reduce the likelihood of complications and the resolution of preeclampsia changes. When contemplating the possibility of a renal biopsy in a pregnant patient, two critical questions come to the fore: (i) Is it prudent to proceed with a renal biopsy, considering the patient’s specific clinical condition, gestational stage, and the risk-benefit ratio for a definitive diagnosis? (ii) Could the insights gleaned from the renal biopsy influence the course of action for both the mother and the pregnancy?
Pregnancy planning in women with glomerular diseases
Important antenatal care for women with glomerular diseases should be established before pregnancy. Once the expectations have been identified, planning should address fertility and future pregnancy timing. Key factors to be taken into account in prepregnancy counseling are outlined in Box 1.48 Avoidance of alkylating drugs such as cyclophosphamide (CYC) is ideal if the patient desires pregnancy. However, CYC may be used if there is a concern about renal function loss. New evidence indicates that low-dose protocols are associated with decreased rates of amenorrhea and ovarian failure.49,50 Appropriate workup should be done to evaluate the disease: creatinine, proteinuria, and baseline-specific Antibodies level (anti-dsDNA, anti-Ro, anti-La, anti-PLA2R, ANCA). Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend that patients with active LN should also wait for a minimum of six months after LN is inactive before considering pregnancy.51 KDIGO does not provide specific recommendations for other types of glomerular diseases. Fakhouri et al. defined pregnancy risk according to the time since immunosuppression was stopped. Patients with low-added pregnancy risk are in remission for more than 12–18 months, while medium-added risk is when the patient is in remission for less than 12–18 months. The high added risk is attributed to active glomerular diseases, deteriorating kidney function, and remission for less than six months, cases when pregnancy is advised against.52
Box 1:
Key factors to be taken into account during prepregnancy counseling
Objectives:
Planning
Risk stratification
Kidney disease treatment optimization
Comorbidity evaluation
Multidisciplinary team: to meet complex needs.
Optimal time for pregnancy is determined by:
Glomerular disease activity
Proteinuria serum creatinine avoid pregnancy if active disease or undergoing treatment with teratogenic drugs.
BP should be optimized before conception.
Contraception is indicated until pregnancy is safe.
Immunosuppressants considerations.48,50
Stop CYC and MMF before conception (three months before for CYC and six weeks before for MMF) and replace them with nonteratogenic options while assuring that glomerular diseases remains stable.
While transitioning to safer options, use two contraceptive methods (usually one barrier and one hormonal).
CYC: Cyclophosphamide, MMF: Mycophenolate mofetil, BP: Blood pressure.
Regarding blood pressure control, both KDIGO and the European Society of Cardiology and International Hypertension guideline recommend a target preconception BP of 140/90 mmHg (ESC/ISH-class IA).51,53 Renin-angiotensin-aldosterone blockers (RAASBs), although shown to have teratogenic effects in the second and third pregnancy, were shown in an observational prospective cohort study, including in over 700 RAASBs, exposed pregnancies not to have teratogenic effects during the first pregnancy trimester.54 Nonetheless, compared to control, two recent meta-analyses revealed a higher risk of congenital malformations, cardiovascular malformations, and stillbirths for patients under RAAS blockade.55,56 KDIGO suggests BP values should be optimized before conception with alternative antihypertensive medication.51
Recently, sodium-glucose cotransporter-2 inhibitors (SGLT-2i) have been shown to be nephroprotective but do not have any specific preconception recommendations.52 A systematic review conducted by Muller et al. revealed that animal studies have shown that SGLT2 inhibitors are generally safe in the first trimester of pregnancy. However, when exposed during postnatal day 21–90 in juvenile rats, which corresponds to the late second and third trimester of human renal development, dilatation of the renal pelvis and tubules was observed. Human data utilising a pharmaceutical database that documented inadvertent pregnancies occurring with the usage of SGLT2 inhibitors indicated a higher occurrence of miscarriages and congenital abnormalities. SGLT2 inhibitors are excreted in breast milk and had an impact on the growth of newborn animals in research experiments. However, there is no published data on humans.57
Antenatal care
The antenatal care plan for women with known glomerulonephritis should be developed well before pregnancy. In particular, control of BP, proteinuria, and disease activity are critical factors in mitigating pregnancy adverse events. Risk predictors and approaches to optimizing maternal health before a pregnancy with known glomerulonephritides are shown in Figure 3. Figure 4 shows a best practice framework for antenatal care in women with glomerulonephritis in pregnancy. This antenatal care plan should be individualized for each patient and each healthcare setting. The nature and frequency of monitoring and review will depend onglomerulonephritis type, activity, CKD stage, comorbidities, and psychosocial context. The plan should be revised based on maternal and fetal progress. The focus should be on heightened vigilance for glomerulonephritis flares or relapses, treatable maternal comorbidities, fetal conditions, and the emergence of preeclampsia.
Figure 3:
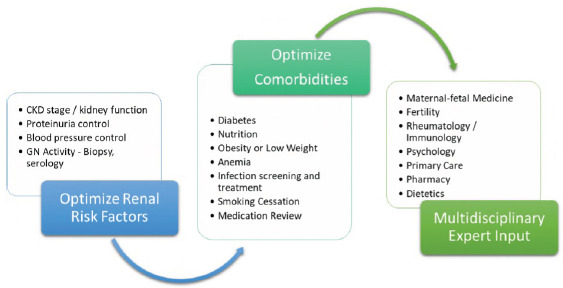
Risk predictors and approaches to optimizing maternal health before a pregnancy with known glomerular disease patients. CKD: Chronic kidney disease, GN: Glomerulonephritis.
Figure 4:
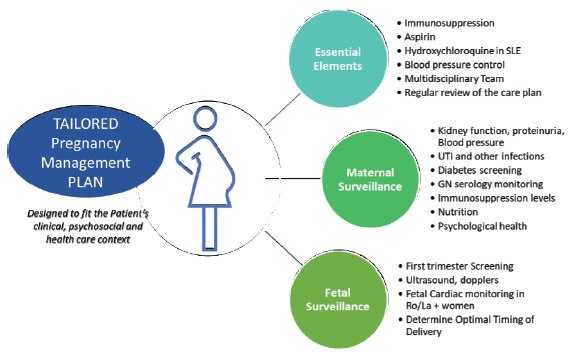
A best practice framework for antenatal care in women with glomerular disease in pregnancy. GN: Glomerulonephritis, SLE: Systemic lupus erythematosus, UTI: Urinary tract infection.
Low-dose aspirin (100–150mg) should be commenced ideally before 12–14 weeks gestation in all women with excess preeclampsia risk, which includes all women with glomerular diseases.19,58 Aspirin should be continued for 35 weeks. The World Health Organization (WHO) has recommended daily calcium supplementation for pregnant women to reduce the incidence of preeclampsia in populations with poor dietary calcium consumption.59 Antihypertensive medication management in pregnancy is beyond the scope of this review and is documented in other guidelines.19,60 Adequate BP control to a target of <140/90 in women with chronic hypertension has been shown to minimize severe hypertension episodes without fetal harm.60,61 Venous thromboembolism is increased women with antiphospholipid antibodies and nephrotic syndrome. In this setting, anticoagulation with low molecular weight heparin should be considered,58 particularly if nephrotic syndrome is severe with hypoalbuminemia (<2 g/dL). Diuretic therapy might be necessary for women with severe nephrotic syndrome and edema. Caution should be exercised to avoid excessive diuresis, as it can adversely affect BP, placental perfusion, and amniotic fluid levels. Nutritional assessment is important to ensure adequate protein and micronutrient intake and correct any hematinic, vitamin, or other nutritional deficiencies.
Special considerations in lupus nephritis
Pregnancy in women with LN is fraught with difficulty due to the high morbidity and mortality rates associated with it; the estimated risk of maternal mortality is around 20 times higher than in healthy women.62 Meta-analysis has shown that active LN in early pregnancy increases the risk of fetal loss, preeclampsia, preterm delivery, and small for gestational age babies. About a quarter of pregnancies experience a maternal flare and 16% experience a renal flare.63 A multicentric prospective cohort analysis of 61 women with LN revealed that each increase in proteinuria by 1 g/d throughout the trimesters increased the odds of preterm delivery by 15%. Even if the disease is quiescent, being lupus anticoagulant-positive, being a non-White or Hispanic person, and using hypertensive medication can result in poor pregnancy outcomes.64 Low levels of C3 and C4, without systemic manifestations, have been linked to an increased risk. Women who have Anti-Ro/Anti-Sjögren’s syndrome-related antigen A (SSA) antibodies should be made aware of the potential risk of fetal heart block. Furthermore, neonatal lupus erythematosus is more likely to occur in women with high-titer anti-La levels.65
Hydroxychloroquine is an important component in mitigating risk in pregnant women with SLE and reducing the risk of congenital heart block and neonatal lupus66 as well as reducing preeclampsia risk.67,68
Management
Pregnancy considerations for immunosuppressive medications
With the advent of effective therapies for GN, a sizeable number of women conceive and carry on pregnancies while on immunosuppressive medications, which have varied effects on feto-maternal outcomes. Table 3 shows the use of immunosuppressive medications, including pregnancy categories, transplacental passage, and associated maternal and fetal complications.69–73
Table 3:
Use of immunosuppressive medications, including pregnancy categories, transplacental passage, and associated maternal and fetal complications
| Immunosuppressive medication | Category | Transplacental passage | Maternal complications | Fetal complications | Evidence for use in pregnant state |
|---|---|---|---|---|---|
|
Glucocorticoids (Prednisone, prednisolone) |
C | 10% | Increases lower urinary tract infections, gestational diabetes, and gestational hypertension | Earlier associated with cleft palate (not proven), transient adrenal insufficiency, thymic hypoplasia |
Safely used in glomerular diseases as first line of agent and kidney transplant settings Boluses of IV steroids (up to 500 mg/day) in rapidly progressive GNs, otherwise maintenance dosing |
|
Calcineurin inhibitors (Cyclosporine, tacrolimus) |
C | 50–70% (based on drug trough levels in fetus in comparison to mother) | Worsening of gestational hypertension and diabetes (in prone patients) | Some case series have indicated a heightened likelihood of low birth weight and preterm birth with the use of tacrolimus. However, these studies have concluded that this correlation is likely attributable to the maternal state rather than the medications.72,73,84 Due to documented cases of hyperkalemia and renal dysfunction in infants who have been exposed, it is recommended to closely monitor renal function and potassium levels in these newborns. Long-term risk of CKD unknown | In SLE flares, quiescent LN, membranous glomerulonephritis |
| Azathioprine | D | Active metabolite, 6-mercaptopurine does not cross placenta | Gastrointestinal side effects, bone marrow suppression, including cytopenias, increased risk of infections | Transient lymphopenia | In quiescent LN, kidney transplant recipients |
| Mycophenolate mofetil (MMF) | D | Gastrointestinal side effects, bone marrow suppression, activation of viral infections | MMF embryopathy—ear, mouth, fingers, ocular organ malformation (EMFO tetrad) (risk of malformations 20%) | Mycophenolate Mofetil (MMF) should be discontinued six weeks prior to planned pregnancies | |
| Cyclophosphamide | D | Congenital malformations, suppressed bone marrow function, neuronal defects reported | Discontinued 12 weeks prior to pregnancies; anecdotally used in second and third trimesters in severe LN, AAV76 | ||
| Hydroxychloroquine | C | Transferred easily (nearly 100%) with similar maternal and cord blood levels | Gastrointestinal side effects, retinopathy | No increase in congenital malformations in fetus noted | Evidence for use in pregnancy is strong (from systematic review and meta-analysis), also decreases the risk of neonatal heart blocks in pregnant females of SLE positive for Ro antibodies |
| Intravenous immunoglobulins (IVIG) | IgG is transferred most easily (90–160%), more so in the third trimester | Systemic inflammatory and allergic reactions, hemolysis, aseptic meningitis | Normal lymphocyte counts observed in neonates born to mothers administered (IVIG) Intravenous immunoglobulin | Case reports mention use as part of combined protocols with dose of IVIG of 200–400 mg/kg/dose described in LN, AAV45,78 | |
| Rituximab | C | As it is chimeric IgG1, it can cross transplacental barrier easily via Fc receptors | Infusion reactions, peripheral B-cell depletion up to six months | Neonatal B cell depletion and nonspecific malformation syndromes (causation unclear) | Case reports in LN, membranous glomerulonephritis.74 AAV exist of rituximab use during and shortly prior to pregnancy with successful outcomes. Rituximab should be discontinued at least 6–12 months before conception, but use in exceptional circumstances is relatively safer in first trimester than others |
| Eculizumab81 | C | It is likely to cross placenta less well than other monoclonal antibodies as IgG2 (which is present in the drug’s Fc portion) transfers poorly across placenta | Safe with respect to maternal outcomes | None | Used in the management of pregnancy-associated HUS. Due to the limited availability of evidence on eculizumab in pregnancy, it is not possible to entirely rule out potential dangers for both the mother and fetus |
AAV: Antineutrophil cytoplasmic antibody-associated vasculitides, LN: Lupus vasculitis, SLE: Systemic lupus erythematosus, GN: Glomerulonephritis, HUS: Hemolytic uremic syndrome
Management strategies in some commonly encountered kidney diseases in pregnancy
Primary nephrotic states
If the pregnant patient with biopsy-proven glomerular diseases relapses with nephrotic proteinuria in the third trimester or close to term, the patient can be managed conservatively with moderate salt restriction, anti-edema measures, and antihypertensives, as deemed necessary. In newly diagnosed cases as well as relapses in the first and second trimesters, pulse of intravenous methylprednisolone followed by doses of 1 mg/kg/day prednisolone have been successfully used in cases of MCD and FSGS. Calcineurin inhibitors, tacrolimus, and cyclosporine have been used in steroid-resistant cases as well as in MN.58 Rituximab is generally avoided for at least a year before planned pregnancy due to its unclear teratogenic potential.74
LN flares
It is advisable to continue hydroxychloroquine throughout pregnancy. Tacrolimus or cyclosporine-based immunosuppression regimens can be continued in pregnancy with quiescent LN.75 Azathioprine is usually substituted in place of mycophenolate mofetil (MMF) during planned conceptions.58 If the flare is refractory to steroids, CYC has been used in exceptional circumstances in the second and third trimesters, though it is contraindicated in pregnancy.76 There are several case reports demonstrating the use of intravenous immunoglobulins, etanercept, and infliximab, safely during pregnancies with SLE with extrarenal manifestations.77,78 Voclosporine, approved recently for proliferative LN, carries a manufacturer’s warning, contraindicating its use in pregnancy.79
ANCA-associated vasculitis (AAV)
AAV is a uncommon among women in the reproductive age group. Steroids alone may not suffice in cases with organ- and life-threatening disease. Reports suggest the use of rituximab in early pregnancy (first and second trimesters) and CYC in later pregnancy (second and third trimesters), and the use of plasma exchange has also been described.26
IgAN
Most studies have focused on the management of hypertension and renal insufficiency.80 Overall, immunosuppressive agents are not recommended in pregnancies with IgAN except for the use of steroids in managing proteinuria and rapidly deteriorating renal function.8
Atypical hemolytic uremic syndrome (HUS)
Specific management strategies for pregnancy-associated and primary thrombotic microangiopathies in pregnancy are beyond the purview of this review. Broadly, in settings where eculizumab is available, atypical HUS is managed with eculizumab on a tapering regimen, ideally over the lifetime of the patient. In women already on maintenance eculizumab for atypical HUS, these biologic agents resulted in successful pregnancies with a low risk of relapse during pregnancy.81 A recent systematic review showed the safety profile of plasma exchange to be comparable among pregnant and nonpregnant patients.82
Delivery and postpartum care
The optimal timing of delivery may be challenging to determine, and there is likely a clinical bias toward early delivery that drives the high rates of preterm birth <37 weeks in women with glomerulonephritis. Once fetal viability is reached, there should be regular discussions about the optimal timing of delivery to balance the gains to the infant from extra gestational age against the risk of materno-fetal deterioration if gestation is prolonged. In this setting, renal parameters, BP, and serological markers will inform maternal health status. Ultrasound assessment of fetal growth, amniotic fluid volume, and Doppler assessments plus cardiotocography will determine fetal well-being.
Postpartum management is important for women with glomerulonephritis. Women must be monitored carefully for postpartum hypertension, disease flare, or deterioration in kidney function. In proteinuric women, reinstating RAAS blockade is important. ACE I is preferable, with most published data supporting the use of enalapril; among ARB, losartan is considered safe due to extensive first-pass metabolism.83 Breastfeeding is rarely contraindicated in most women. Since the greatest drug exposures occur in utero, all antenatal drug regimens can be safely continued in the post-partum state. Tacrolimus levels are similar in breastfed and bottle-fed babies.84
Outcomes
In women with underlying glomerular diseases, both the effect of pregnancy on glomerular diseases and glomerular diseases on pregnancy may be deleterious. There are implications for fetal well-being too. There is robust data on LN and IgAN; however, the data for other glomerular diseases are limited. Further, data on long-term follow-up after pregnancy are sparse.85
In a study reporting pregnancy outcomes in 48 patients with glomerular diseases, 33% were complicated by preeclampsia, 39% showed a doubling of urinary protein, and 27% exhibited a ≥50% increase in serum creatinine. In addition, 13% of pregnancies resulted in perinatal death, and 48% of babies were born prematurely. Outcome differences across glomerular disease subtypes were not different, although the decline in kidney function appeared most frequently in FSGS.86 A study of 413 women of childbearing age with IgAN who were monitored for at one year showed that 25% experienced an increase in proteinuria during pregnancy, with 17% reaching the nephrotic range.87 After delivery, 6% did not return to baseline proteinuria levels. Additionally, 29% developed hypertension and 13% remained hypertensive after childbirth. After adjusting for age, eGFR, mean arterial pressure, proteinuria, and renal histopathology, a faster decline in eGFR (−7.44 vs. −3.90 mL/min/1.73 m2 per year; P = 0.007) and a higher risk of kidney progression events (HR, 5.14; 95% CI, 1.16–22.74) were observed in those with CKD stages 3–4 who became pregnant compared to those who did not. Pregnancy also increased the risk of CKD progression, with an 1.3- to 5-fold increased risk of kidney failure. A retrospective study of 27 pregnancies in MN showed increased risk of adverse maternal-fetal events, including fetal loss (11%), preterm delivery (26%), and severe preeclampsia (15%). Notably, all patients maintained normal kidney function. Heavy proteinuria, hypoalbuminemia, anti-PLA2R positivity, and lack of remission were associated with worse outcomes.88
Recent data from the Cure Glomerulonephropathy study (CureGN), show that complicated pregnancy was associated with greater eGFR decline in the years following glomerulonephritis diagnosis. The analysis also suggested that among those with a pregnancy history, those with MN were the least likely to report a complicated pregnancy (22/134, 16%) and those with FSGS reported the most complications.89 Figure 5 shows approach to glomerular diseases during pregnancy.
Figure 5:
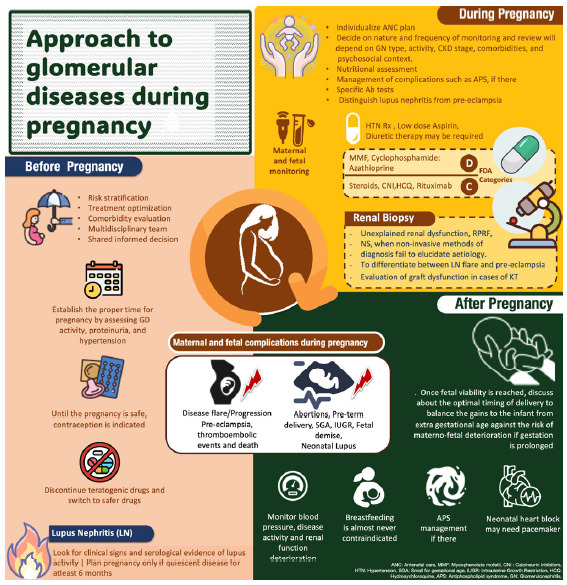
Key points in managing pregnancy in glomerular disease patients.
The review provides a comprehensive analysis of the management strategies utilized for glomerular diseases during pregnancy, with a specific focus on various therapeutic options. Special attention is given to postpartum care, highlighting the importance of diligently monitoring hypertension and disease exacerbations.
Footnotes
How to cite this article: Meena P, Jesudason S, Popa CA, Rao NS, Priyamvada PS. Approach to Glomerular Disease in Pregnancy. Indian J Nephrol. 2024;34:561-72. doi: 10.25259/IJN_26_2024
Conflicts of interest
There are no conflicts of interest.
References
- 1.Pregnancy and renal disease. The Lancet. 1975;306:801–2. [PubMed] [Google Scholar]
- 2.Beers K, Patel N. Kidney physiology in pregnancy. Adv Chronic Kidney Dis. 2020;27:449–54. doi: 10.1053/j.ackd.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Shen M, Tan H, Zhou S, Smith GN, Walker MC, et al. Trajectory of blood pressure change during pregnancy and the role of pre-gravid blood pressure: A functional data analysis approach. Sci Rep. 2017;7:6227. doi: 10.1038/s41598-017-06606-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunlop W. Serial changes in renal haemodynamics during normal human pregnancy. BJOG. 1981;88:1–9. doi: 10.1111/j.1471-0528.1981.tb00929.x. [DOI] [PubMed] [Google Scholar]
- 5.Baylis C, Reese K, Wilson CB. Glomerular effects of pregnancy in a model of glomerulonephritis in the rat. Am J Kidney Dis. 1989;14:452–60. [PubMed] [Google Scholar]
- 6.Day C, Hewins P, Hildebrand S, Sheikh L, Taylor G, et al. The role of renal biopsy in women with kidney disease identified in pregnancy. Nephrol Dial Transplant. 2007;23:201–6. doi: 10.1093/ndt/gfm572. [DOI] [PubMed] [Google Scholar]
- 7.Siligato R, Gembillo G, Cernaro V, Torre F, Salvo A, et al. Maternal and fetal outcomes of pregnancy in nephrotic syndrome due to primary glomerulonephritis. Front Med. 2020;7:563094. doi: 10.3389/fmed.2020.563094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blom K, Odutayo A, Bramham K, Hladunewich MA. Pregnancy and glomerular disease: A systematic review of the literature with management guidelines. CJASN. 2017;12:1862–72. doi: 10.2215/CJN.00130117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wyld ML, Clayton PA, Kennedy SE, Alexander SI, Chadban SJ. Pregnancy outcomes for kidney transplant recipients with transplantation as a child. JAMA Pediatr. 2015;169:e143626. doi: 10.1001/jamapediatrics.2014.3626. [DOI] [PubMed] [Google Scholar]
- 10.Jesudason S, Mohammadi F, Fitzpatrick A. Managing pregnancy in chronic kidney disease: Improving outcomes for mother and baby. IJWH. 2016;8:273–85. doi: 10.2147/IJWH.S76819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, et al. New creatinine- and cystatin c-based equations to estimate gfr without race. N Engl J Med. 2021;385:1737–49. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao M, Vilayur E, Ferreira D, Nanra R, Hawkins J. Estimating the glomerular filtration rate in pregnancy: The evaluation of the Nanra and CKD-EPI serum creatinine-based equations. Obstet Med. 2021;14:31–4. doi: 10.1177/1753495X20904177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saxena AR, Karumanchi SA, Fan SL, Horowitz GL, Hollenberg NK, et al. Correlation of cystatin-c with glomerular filtration rate by inulin clearance in pregnancy. Hypertens in Pregnancy. 2012;31:22–30. doi: 10.3109/10641955.2010.507845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopes Van Balen VA, Van Gansewinkel TAG, De Haas S, Spaan JJ, Ghossein-Doha C, et al. Maternal kidney function during pregnancy: Systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2019;54:297–307. doi: 10.1002/uog.20137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kattah A, Milic N, White W, Garovic V. Spot urine protein measurements in normotensive pregnancies, pregnancies with isolated proteinuria and preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2017;313:R418–24. doi: 10.1152/ajpregu.00508.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts M, Lindheimer MD, Davison JM. Altered glomerular permselectivity to neutral dextrans and heteroporous membrane modeling in human pregnancy. Am J Physiol. 1996;270:F338–43. doi: 10.1152/ajprenal.1996.270.2.F338. [DOI] [PubMed] [Google Scholar]
- 17.Milne JEC, Lindheimer MD, Davison JM. Glomerular heteroporous membrane modeling in third trimester and postpartum before and during amino acid infusion. Am J Physiol. 2002;282:F170–5. doi: 10.1152/ajprenal.00195.2000. [DOI] [PubMed] [Google Scholar]
- 18.Cade TJ, De Crespigny PC, Nguyen T, Cade JR, Umstad MP. Should the spot albumin-to-creatinine ratio replace the spot protein-to-creatinine ratio as the primary screening tool for proteinuria in pregnancy? Pregnancy Hypertens. 2015;5:298–302. doi: 10.1016/j.preghy.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72:24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803. [DOI] [PubMed] [Google Scholar]
- 20.Macdonald-Wallis C, Lawlor DA, Heron J, Fraser A, Nelson SM, et al. Relationships of risk factors for pre-eclampsia with patterns of occurrence of isolated gestational proteinuria during normal term pregnancy. Belizan JM, editor. PLoS ONE. 2011;6:e22115. doi: 10.1371/journal.pone.0022115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holston AM, Qian C, Yu KF, Epstein FH, Karumanchi SA, et al. Circulating angiogenic factors in gestational proteinuria without hypertension. Am J Obstet Gynecol. 2009;200:392.e1–392.e10. doi: 10.1016/j.ajog.2008.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fishel Bartal M, Lindheimer MD, Sibai BM. Proteinuria during pregnancy: Definition, pathophysiology, methodology, and clinical significance. Am J Obstet Gynecol. 2022;226:S819–34. doi: 10.1016/j.ajog.2020.08.108. [DOI] [PubMed] [Google Scholar]
- 23.Brown MA, Holt JL, Mangos GJ, Murray N, Curtis J, et al. Microscopic hematuria in pregnancy: Relevance to pregnancy outcome. Am J Kidney Dis. 2005;45:667–73. doi: 10.1053/j.ajkd.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 24.Sandhu KS, LaCombe JA, Fleischmann N, Greston WM, Lazarou G, et al. Gross and microscopic hematuria: Guidelines for obstetricians and gynecologists. Obstet Gynecol Surv. 2009;64:39–49. doi: 10.1097/OGX.0b013e3181932841. [DOI] [PubMed] [Google Scholar]
- 25.Buyon JP, Kim MY, Guerra MM, Lu S, Reeves E, et al. Kidney outcomes and risk factors for nephritis (Flare/De Novo) in a multiethnic cohort of pregnant patients with lupus. CJASN. 2017;12:940–6. doi: 10.2215/CJN.11431116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh P, Dhooria A, Rathi M, Agarwal R, Sharma K, et al. Successful treatment outcomes in pregnant patients with ANCA-associated vasculitides: A systematic review of literature. Int J of Rheum Dis. 2018;21:1734–40. doi: 10.1111/1756-185X.13342. [DOI] [PubMed] [Google Scholar]
- 27.Richani K, Soto E, Romero R, Espinoza J, Chaiworapongsa T, et al. Normal pregnancy is characterized by systemic activation of the complement system. J Matern Fetal Neonatal Med. 2005;17:239–45. doi: 10.1080/14767050500072722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He Y, Xu B, Song D, Wang Y, Yu F, et al. Normal range of complement components during pregnancy: A prospective study. American J Rep Immunol. 2020;83:e13202. doi: 10.1111/aji.13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Den Broek NR, Letsky EA. Pregnancy and the erythrocyte sedimentation rate. BJOG. 2001;108:1164–7. [PubMed] [Google Scholar]
- 30.Tomer Y, Viegas OA, Swissa M, Koh SC, Shoenfeld Y. Levels of lupus autoantibodies in pregnant SLE patients: Correlations with disease activity and pregnancy outcome. Clin Exp Rheumatol. 1996;14:275–80. [PubMed] [Google Scholar]
- 31.De Jesús GR, Lacerda MI, Rodrigues BC, Dos Santos FC, Do Nascimento AP, et al. Soluble Flt-1, placental growth factor, and vascular endothelial growth factor serum levels to differentiate between active lupus nephritis during pregnancy and preeclampsia. Arthritis Care Res (Hoboken) 2021;73:717–21. doi: 10.1002/acr.24360. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen TH, Bui TC, Vo TM, Tran QM, Luu LTT, et al. Predictive value of the sFlt-1 and PlGF in women at risk for preeclampsia in the south of Vietnam. Pregnancy Hypertension. 2018;14:37–42. doi: 10.1016/j.preghy.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Thadhani R, Lemoine E, Rana S, Costantine MM, Calsavara VF, et al. Circulating angiogenic factor levels in hypertensive disorders of pregnancy. NEJM Evidence. 2022 Nov 22; doi: 10.1056/EVIDoa2200161. Available from: https://evidence.nejm.org/doi/10.1056/EVIDoa2200161 [Last accessed on 2023 Nov 7] [DOI] [PubMed] [Google Scholar]
- 34.Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980–2010: Age-period-cohort analysis. BMJ. 2013;347:f6564. doi: 10.1136/bmj.f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:391–403. doi: 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Horigome M, Kobayashi R, Hanaoka M, Kinguchi S, Kanaoka T, et al. A case of minimal change nephrotic syndrome with pregnancy. CEN Case Rep. 2021;10:315–9. doi: 10.1007/s13730-020-00568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smyth A, Wall CA. Nephrotic syndrome due to focal segmental glomerulosclerosis occurring in early pregnancy. Obstet Med. 2011;4:80–2. doi: 10.1258/om.2011.110010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veltri NL, Hladunewich M, Bhasin A, Garland J, Thomson B. De novo antineutrophil cytoplasmic antibody-associated vasculitis in pregnancy: A systematic review on maternal, pregnancy and fetal outcomes. Clin Kidney J. 2018;11:659–66. doi: 10.1093/ckj/sfy011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomson B, Joseph G, Clark WF, Hladunewich M, Patel A, et al. Maternal, pregnancy and fetal outcomes in de novo anti-glomerular basement membrane antibody disease in pregnancy: A systematic review. Clin Kidney J. 2014;7:450–6. doi: 10.1093/ckj/sfu086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montersino B, Menato G, Colla L, Masturzo B, Piccoli GB, et al. A young woman with proteinuria and hypertension in pregnancy: Is what looks and smells like preeclampsia always preeclampsia? J Nephrol. 2021;34:1677–9. doi: 10.1007/s40620-021-01080-4. [DOI] [PubMed] [Google Scholar]
- 41.Sachdeva M, Beck LH, Miller I, Bijol V, Fishbane S. Phospholipase A2 receptor antibody – positive pregnancy: A case report. Am J Kidney Dis. 2020;76:586–9. doi: 10.1053/j.ajkd.2019.11.011. [DOI] [PubMed] [Google Scholar]
- 42.Uchino E, Takada D, Mogami H, Matsubara T, Tsukamoto T, et al. Membranous nephropathy associated with pregnancy: An anti-phospholipase A2 receptor antibody-positive case report. CEN Case Rep. 2018;7:101–6. doi: 10.1007/s13730-018-0304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Debiec H, Guigonis V, Mougenot B, Decobert F, Haymann JP, et al. Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N Engl J Med. 2002;346:2053–60. doi: 10.1056/NEJMoa012895. [DOI] [PubMed] [Google Scholar]
- 44.Gleeson S, Cardoso F, Lightstone L, Webster P, Munro K, et al. A new approach to de novo minimal change disease in pregnancy. Nephrology. 2021;26:692–3. doi: 10.1111/nep.13871. [DOI] [PubMed] [Google Scholar]
- 45.Alves P, Navarro D, Góis M, Mendes M, Nolasco F. A Pregnant woman with gross hematuria and acute kidney injury. Am J Kidney Dis. 2021;78:A11–2. doi: 10.1053/j.ajkd.2021.05.017. [DOI] [PubMed] [Google Scholar]
- 46.Chen TK, Gelber AC, Witter FR, Petri M, Fine DM. Renal biopsy in the management of lupus nephritis during pregnancy. Lupus. 2015;24:147–54. doi: 10.1177/0961203314551812. [DOI] [PubMed] [Google Scholar]
- 47.Piccoli G, Daidola G, Attini R, Parisi S, Fassio F, et al. Kidney biopsy in pregnancy: Evidence for counselling? A systematic narrative review. BJOG. 2013;120:412–27. doi: 10.1111/1471-0528.12111. [DOI] [PubMed] [Google Scholar]
- 48.Wiles K, Chappell L, Clark K, Elman L, Hall M, et al. Clinical practice guideline on pregnancy and renal disease. BMC Nephrol. 2019;20:401. doi: 10.1186/s12882-019-1560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma SK, Jain S, Bahl P, Potturi P, Rathi M, et al. Ovarian dysfunction with moderate-dose intravenous cyclophosphamide (modified NIH regimen) and mycophenolate mofetil in young adults with severe lupus: A prospective cohort study. Arthritis Res Ther. 2020;22:189. doi: 10.1186/s13075-020-02292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tamirou F, Husson SN, Gruson D, Debiève F, Lauwerys BR, et al. Brief report: The euro-lupus low-dose intravenous cyclophosphamide regimen does not impact the ovarian reserve, as measured by serum levels of anti-müllerian hormone. Arthritis Rheumatol. 2017;69:1267–71. doi: 10.1002/art.40079. [DOI] [PubMed] [Google Scholar]
- 51.Rovin BH, Adler SG, Barratt J, Bridoux F, Burdge KA, et al. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100:S1–276. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 52.Fakhouri F, Schwotzer N, Cabiddu G, Barratt J, Legardeur H, et al. Glomerular diseases in pregnancy: Pragmatic recommendations for clinical management. Kidney Int. 2023;103:264–81. doi: 10.1016/j.kint.2022.10.029. [DOI] [PubMed] [Google Scholar]
- 53.Mancia G, Kreutz R, Brunström M, Burnier M, Grassi G, et al. 2023 ESH guidelines for the management of arterial hypertension the task force for the management of arterial hypertension of the European society of hypertension: Endorsed by the international society of hypertension (ISH) and the European renal association (ERA) J Hypertens. 2023;41:1874–2071. doi: 10.1097/HJH.0000000000003480. [DOI] [PubMed] [Google Scholar]
- 54.Diav-Citrin O, Shechtman S, Halberstadt Y, Finkel-Pekarsky V, Wajnberg R, et al. Pregnancy outcome after in utero exposure to angiotensin converting enzyme inhibitors or angiotensin receptor blockers. Reprod Toxicol. 2011;31:540–5. doi: 10.1016/j.reprotox.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 55.Buawangpong N, Teekachunhatean S, Koonrungsesomboon N. Adverse pregnancy outcomes associated with first trimester exposure to angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers: A systematic review and meta-analysis. Pharmacol Res Perspect. 2020;8:e00644. doi: 10.1002/prp2.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fu J, Tomlinson G, Feig DS. Increased risk of major congenital malformations in early pregnancy use of angiotensin-converting-enzyme inhibitors and angiotensin-receptor-blockers: A meta-analysis. Diabetes Metab Res Rev. 2021;37:e3453. doi: 10.1002/dmrr.3453. [DOI] [PubMed] [Google Scholar]
- 57.Muller DRP, Stenvers DJ, Malekzadeh A, Holleman F, Painter RC, et al. Effects of GLP-1 agonists and SGLT2 inhibitors during pregnancy and lactation on offspring outcomes: A systematic review of the evidence. Front Endocrinol. 2023;14:1215356. doi: 10.3389/fendo.2023.1215356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sammaritano LR, Bermas BL, Chakravarty EE, Chambers C, Clowse MEB, et al. 2020 American college of rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Rheumatol. 2020;72:529–56. doi: 10.1002/art.41191. [DOI] [PubMed] [Google Scholar]
- 59.Gomes F, Ashorn P, Askari S, Belizan JM, Boy E, et al. Calcium supplementation for the prevention of hypertensive disorders of pregnancy: Current evidence and programmatic considerations. Ann N Y Acad Sci. 2022;1510:52–67. doi: 10.1111/nyas.14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tita AT, Szychowski JM, Boggess K, Dugoff L, Sibai B, et al. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022;386:1781–92. doi: 10.1056/NEJMoa2201295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Magee LA, Von Dadelszen P, Rey E, Ross S, Asztalos E, et al. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015;372:407–17. doi: 10.1056/NEJMoa1404595. [DOI] [PubMed] [Google Scholar]
- 62.Clowse MEB, Jamison M, Myers E, James AH. A national study of the complications of lupus in pregnancy. Am J Obstet Gynecol. 2008;199:127.e1–127.e6. doi: 10.1016/j.ajog.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smyth A, Oliveira GHM, Lahr BD, Bailey KR, Norby SM, et al. A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin J Am Soc Nephrol. 2010;5:2060–8. doi: 10.2215/CJN.00240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buyon JP, Kim MY, Guerra MM, Laskin CA, Petri M, et al. Predictors of pregnancy outcomes in patients with lupus: A cohort study. Ann Intern Med. 2015;163:153–63. doi: 10.7326/M14-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jaeggi E, Laskin C, Hamilton R, Kingdom J, Silverman E. The importance of the level of maternal anti-Ro/SSA antibodies as a prognostic marker of the development of cardiac neonatal lupus erythematosus. J Am Coll Cardiol. 2010;55:2778–84. doi: 10.1016/j.jacc.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 66.Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78:736–45. doi: 10.1136/annrheumdis-2019-215089. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y, Wei Y, Zhang Y, Yang H. Hydroxychloroquine significantly decreases the risk of preeclampsia in pregnant women with autoimmune disorders: A systematic review and meta-analysis. Clin Rheumatol. 2023;42:1223–35. doi: 10.1007/s10067-022-06496-2. [DOI] [PubMed] [Google Scholar]
- 68.Clowse MEB, Eudy AM, Balevic S, Sanders-Schmidler G, Kosinski A, et al. Hydroxychloroquine in the pregnancies of women with lupus: A meta-analysis of individual participant data. Lupus Sci Med. 2022;9:e000651. doi: 10.1136/lupus-2021-000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Colla L, Diena D, Rossetti M, Manzione AM, Marozio L, et al. Immunosuppression in pregnant women with renal disease: Review of the latest evidence in the biologicsera. J Nephrol. 2018;31:361–83. doi: 10.1007/s40620-018-0477-3. [DOI] [PubMed] [Google Scholar]
- 70.Boulay H, Mazaud-Guittot S, Supervielle J, Chemouny JM, Dardier V, et al. Maternal, foetal and child consequences of immunosuppressive drugs during pregnancy in women with organ transplant: A review. Clin Kidney J. 2021;14:1871–8. doi: 10.1093/ckj/sfab049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ponticelli C, Moroni G. Fetal toxicity of immunosuppressive drugs in pregnancy. JCM. 2018;7:552. doi: 10.3390/jcm7120552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kainz A, Harabacz I, Cowlrick IS, Gadgil SD, Hagiwara D. Review of the course and outcome of 100 pregnancies in 84 women treated with tacrolimus. Transplantation. 2000;70:1718–21. doi: 10.1097/00007890-200012270-00010. [DOI] [PubMed] [Google Scholar]
- 73.Nevers W, Pupco A, Koren G, Bozzo P. Safety of tacrolimus in pregnancy. Can Fam Physician. 2014;60:905–6. [PMC free article] [PubMed] [Google Scholar]
- 74.Holden F, Bramham K, Clark K. Rituximab for the maintenance of minimal change nephropathy – A report of two pregnancies. Obstet Med. 2020;13:145–7. doi: 10.1177/1753495X18813739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Webster P, Wardle A, Bramham K, Webster L, Nelson-Piercy C, et al. Tacrolimus is an effective treatment for lupus nephritis in pregnancy. Lupus. 2014;23:1192–6. doi: 10.1177/0961203314540353. [DOI] [PubMed] [Google Scholar]
- 76.Clowse ME, Magder L, Petri M. Cyclophosphamide for lupus during pregnancy. Lupus. 2005;14:593–7. doi: 10.1191/0961203305lu2169oa. [DOI] [PubMed] [Google Scholar]
- 77.Mitchell K, Kaul M, Clowse M. The management of rheumatic diseases in pregnancy. Scand J Rheumatol. 2010;39:99–108. doi: 10.3109/03009740903449313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wenderfer SE, Thacker T. Intravenous immunoglobulin in the management of lupus nephritis. Autoimmune Diseases. 2012;2012:1–10. doi: 10.1155/2012/589359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dao KH, Bermas BL. Systemic lupus erythematosus management in pregnancy. IJWH. 2022;14:199–211. doi: 10.2147/IJWH.S282604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang F, Lu JD, Zhu Y, Wang TT, Xue J. Renal outcomes of pregnant patients with immunoglobulin a nephropathy: A systematic review and meta-analysis. Am J Nephrol. 2019;49:214–24. doi: 10.1159/000496410. [DOI] [PubMed] [Google Scholar]
- 81.Sarno L, Tufano A, Maruotti GM, Martinelli P, Balletta MM, et al. Eculizumab in pregnancy: A narrative overview. J Nephrol. 2019;32:17–25. doi: 10.1007/s40620-018-0517-z. [DOI] [PubMed] [Google Scholar]
- 82.Wind M, Gaasbeek AGA, Oosten LEM, Rabelink TJ, Van Lith JMM, et al. Therapeutic plasma exchange in pregnancy: A literature review. Eur J Obstet Gynecol Reprod Biol. 2021;260:29–36. doi: 10.1016/j.ejogrb.2021.02.027. [DOI] [PubMed] [Google Scholar]
- 83.Kearney L, Wright P, Fhadil S, Thomas M. Postpartum cardiomyopathy and considerations for breastfeeding. Card Fail Rev. 2018;4:112. doi: 10.15420/cfr.2018.21.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bramham K, Chusney G, Lee J, Lightstone L, Nelson-Piercy C. Breastfeeding and tacrolimus: Serial monitoring in breast-fed and bottle-fed infants. Clin J Am Soc Nephrol. 2013;8:563–7. doi: 10.2215/CJN.06400612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marinaki S, Tsiakas S, Skalioti C, Kapsia E, Lionaki S, et al. Pregnancy in women with preexisting glomerular diseases: A single-center experience. Front Med. 2022;9:801144. doi: 10.3389/fmed.2022.801144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O’Shaughnessy MM, Jobson MA, Sims K, Liberty AL, Nachman PH, et al. Pregnancy outcomes in patients with glomerular disease attending a single academic center in north carolina. Am J Nephrol. 2017;45:442–51. doi: 10.1159/000471894. [DOI] [PubMed] [Google Scholar]
- 87.Su X, Lv J, Liu Y, Wang J, Ma X, et al. Pregnancy and kidney outcomes in patients with IgA nephropathy: A cohort study. Am J Kidney Dis. 2017;70:262–9. doi: 10.1053/j.ajkd.2017.01.043. [DOI] [PubMed] [Google Scholar]
- 88.Liu ZN, Cui Z, He YD, Zhang YM, Wang F, et al. Membranous nephropathy in pregnancy. Am J Nephrol. 2020;51:304–17. doi: 10.1159/000505175. [DOI] [PubMed] [Google Scholar]
- 89.Reynolds ML, Oliverio AL, Zee J, Hendren EM, O’Shaughnessy MM, et al. Pregnancy history and kidney disease progression among women enrolled in cure glomerulonephropathy. Kidney Int Rep. 2023;8:805–17. doi: 10.1016/j.ekir.2023.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]


