Abstract
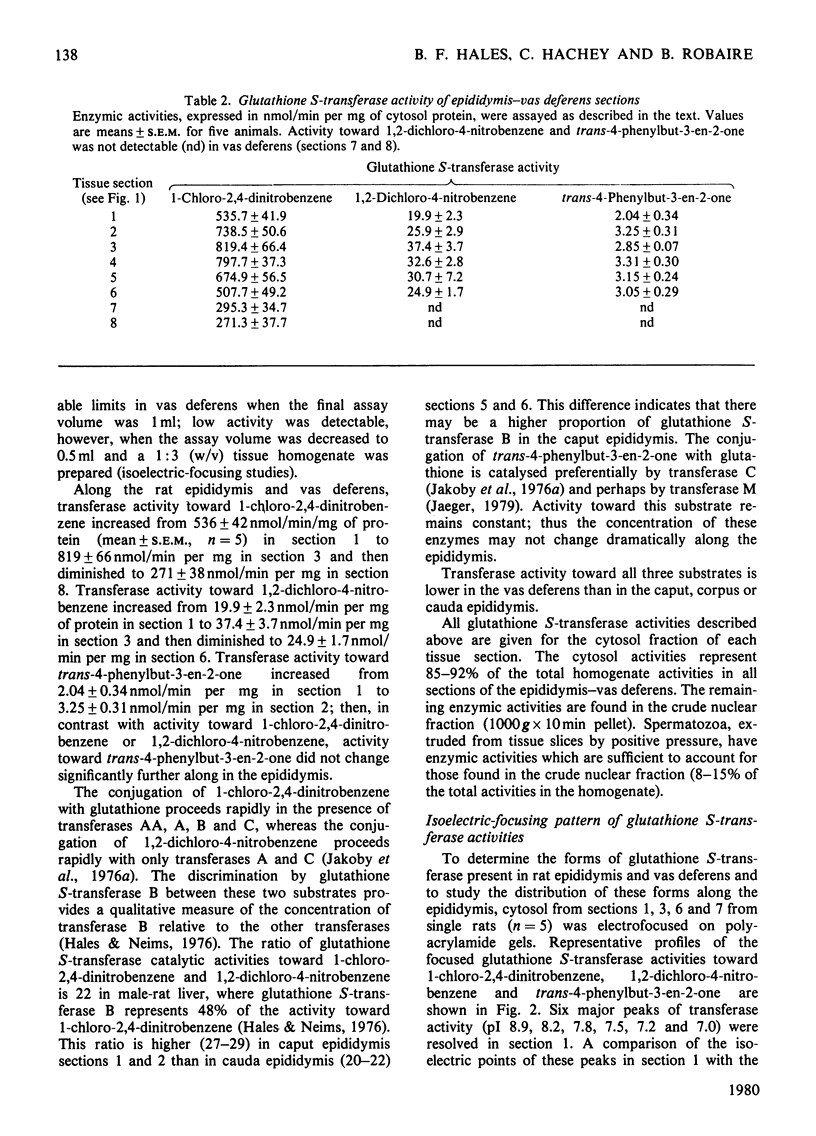
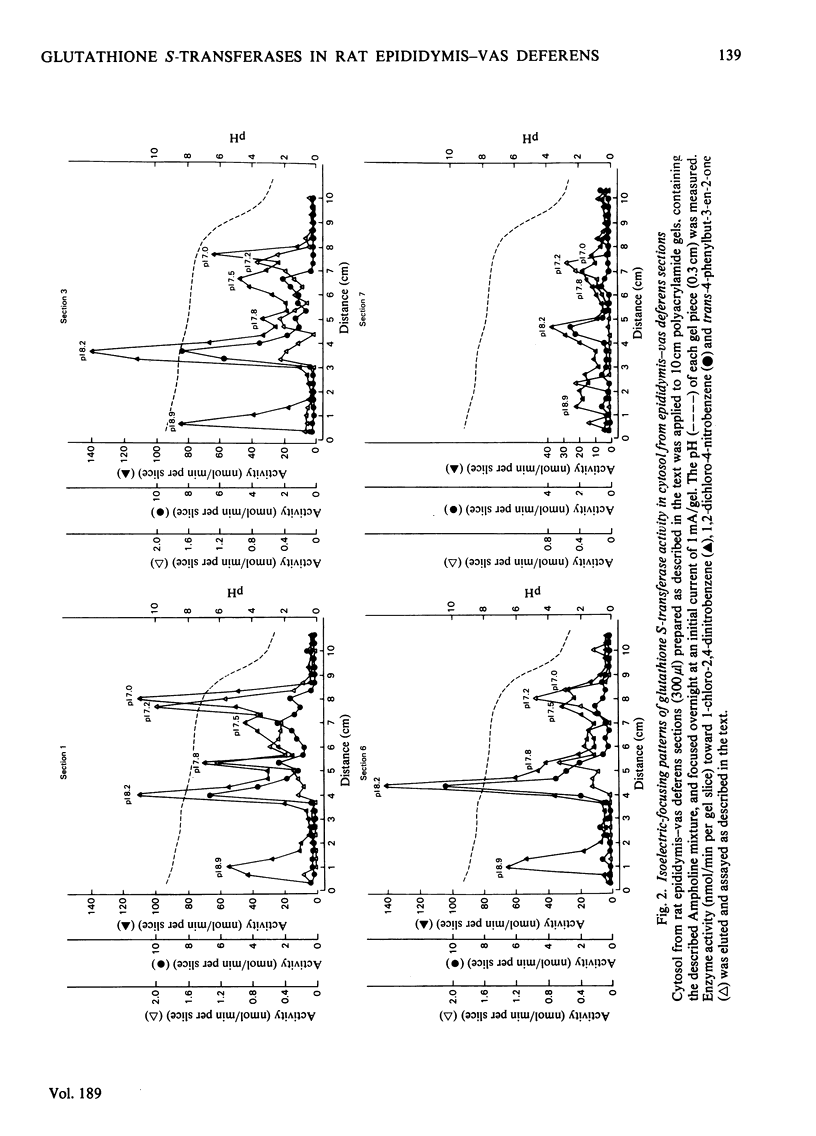
The presence of the glutathione S-transferases, enzymes that catalyse the conjugation of glutathione with a variety of compounds, is reported here, for the first time, in the mammalian epididymis–vas deferens. These glutathione S-transferases, approx. 50% of those from rat liver on a per-mg-of-protein basis, are resolved by isoelectric focusing into six peaks, each with a characteristic isoelectric point and substrate specificity. By these same criteria, the first three peaks (pI 8.9, 8.2 and 7.8) can be identified as transferases B, A and C respectively. The fifth peak (pI7.2) may correspond to transferase M; the fourth (pI7.5) and sixth (pI7.0) peaks do not correspond to previously described transferases. The distribution of transferase activity towards any one substrate studied differs in sequential sections of the epididymis and vas deferens; in addition, the longitudinal-distribution pattern differs for each of the three substrates studied. Isoelectric focusing of the cytosol fractions of the different sections further substantiates these observations. The potential significance of these enzymes and of their distribution in terms of epididymal function, maturation of spermatozoa, is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson A. M., Talalay P., Keen J. H., Jakoby W. B. Relationship between the soluble glutathione-dependent delta 5-3-ketosteroid isomerase and the glutathione S-transferases of the liver. Proc Natl Acad Sci U S A. 1977 Jan;74(1):158–162. doi: 10.1073/pnas.74.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaquier J. A., Cameo M. S., Burgos M. H. The role of androgens in the maturation of epididymal spermatozoa in the guinea pig. Endocrinology. 1972 Mar;90(3):839–842. doi: 10.1210/endo-90-3-839. [DOI] [PubMed] [Google Scholar]
- Calvin H. I., Bedford J. M. Formation of disulphide bonds in the nucleus and accessory structures of mammalian spermatozoa during maturation in the epididymis. J Reprod Fertil Suppl. 1971 May;13(Suppl):65–75. [PubMed] [Google Scholar]
- Chang T. S., Morton B. Epididymal sulfhydryl oxidase: a sperm-protective enzyme from the male reproductive tract. Biochem Biophys Res Commun. 1975 Sep 2;66(1):309–315. doi: 10.1016/s0006-291x(75)80329-9. [DOI] [PubMed] [Google Scholar]
- DeLap L. W., Tate S. S., Meister A. gamma-glutamyl transpeptidase and related enzyme activities inthe reproductive system of the male rat. Life Sci. 1977 Feb 15;20(4):673–679. doi: 10.1016/0024-3205(77)90472-6. [DOI] [PubMed] [Google Scholar]
- Fjellstedt T. A., Allen R. H., Duncan B. K., Jakoby W. B. Enzymatic conjugation of epoxides with glutathione. J Biol Chem. 1973 May 25;248(10):3702–3707. [PubMed] [Google Scholar]
- Gillham B. The mechanism of the reaction between glutathione and 1-menaphthyl sulphate catalysed by a glutathione S-transferase from rat liver. Biochem J. 1973 Dec;135(4):797–804. doi: 10.1042/bj1350797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferase AA from rat liver. Arch Biochem Biophys. 1976 Aug;175(2):710–716. doi: 10.1016/0003-9861(76)90563-4. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Hales B. F., Jaeger V., Neims A. H. Isoelectric focusing of glutathione S-transferases from rat liver and kidney. Biochem J. 1978 Dec 1;175(3):937–943. doi: 10.1042/bj1750937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales B. F., Neims A. H. A sex difference in hepatic glutathione S-transferase B and the effect of hypophysectomy. Biochem J. 1976 Nov 15;160(2):223–229. doi: 10.1042/bj1600223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwack G., Ketterer B., Arias I. M. Ligandin: a hepatic protein which binds steroids, bilirubin, carcinogens and a number of exogenous organic anions. Nature. 1971 Dec 24;234(5330):466–467. doi: 10.1038/234466a0. [DOI] [PubMed] [Google Scholar]
- Mukhtar H., Lee I. P., Bend J. R. Glutathione S-transferase activities in rat and mouse sperm and human semen. Biochem Biophys Res Commun. 1978 Aug 14;83(3):1093–1098. doi: 10.1016/0006-291x(78)91507-3. [DOI] [PubMed] [Google Scholar]
- Mukhtar H., Lee I. P., Foureman G. L., Bend J. R. Epoxide metabolizing enzyme activities in rat testes: postnatal development and relative activity in interstitial and spermatogenic cell compartments. Chem Biol Interact. 1978 Sep;22(2-3):153–165. doi: 10.1016/0009-2797(78)90122-9. [DOI] [PubMed] [Google Scholar]
- Mukhtar H., Philpot R. M., Bend J. R. Epoxide-metabolizing enzyme activities and cytochrome P-450 content of rat ovaries during pregnancy. Biochem Biophys Res Commun. 1978 Mar 15;81(1):89–98. doi: 10.1016/0006-291x(78)91634-0. [DOI] [PubMed] [Google Scholar]
- Orgebin-Crist M. C. Sperm maturation in rabbit epididymis. Nature. 1967 Nov 25;216(5117):816–818. doi: 10.1038/216816a0. [DOI] [PubMed] [Google Scholar]
- Pabst M. J., Habig W. H., Jakoby W. B. Glutathione S-transferase A. A novel kinetic mechanism in which the major reaction pathway depends on substrate concentration. J Biol Chem. 1974 Nov 25;249(22):7140–7147. [PubMed] [Google Scholar]
- Prohaska J. R., Ganther H. E. Glutathione peroxidase activity of glutathione-s-transferases purified from rat liver. Biochem Biophys Res Commun. 1976 May 23;76(2):437–445. doi: 10.1016/0006-291x(77)90744-6. [DOI] [PubMed] [Google Scholar]
- Robaire B. Effects of unilateral orchidectomy on rat epididymal delta 4-5 alpha-reductase and 3 alpha-hydroxysteroid dehydrogenase. Can J Physiol Pharmacol. 1979 Sep;57(9):998–1003. doi: 10.1139/y79-149. [DOI] [PubMed] [Google Scholar]
- Robaire B., Ewing L. L., Zirkin B. R., Irby D. C. Steroid delta4-5alpha-reductase and 3alpha-hydroxysteroid dehydrogenase in the rat epididymis. Endocrinology. 1977 Nov;101(5):1379–1390. doi: 10.1210/endo-101-5-1379. [DOI] [PubMed] [Google Scholar]
- Robb G. W., Amann R. P., Killian G. J. Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J Reprod Fertil. 1978 Sep;54(1):103–107. doi: 10.1530/jrf.0.0540103. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Grau E. M., Meister A. Conversion of glutathione to glutathione disulfide by cell membrane-bound oxidase activity. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2715–2719. doi: 10.1073/pnas.76.6.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate S. S., Orlando J. Conversion of glutathione to glutathione disulfide, a catalytic function of gamma-glutamyl transpeptidase. J Biol Chem. 1979 Jul 10;254(13):5573–5575. [PubMed] [Google Scholar]


