ABSTRACT
There has been a growing interest in bacteriophages as therapeutic agents to treat multidrug-resistant bacterial infections. The present work aimed at expanding the microbiological and molecular characterization of lytic phages ZC01 and ZC03 and investigating their efficacy in the control of Pseudomonas aeruginosa infection in an invertebrate animal model. These two phages were previously isolated from composting using P. aeruginosa strain PA14 as the enrichment host and had their genomes sequenced. ZC01 and ZC03 present, respectively, siphovirus and podovirus morphotypes. ZC01 was recently classified into the genus Abidjanvirus, while ZC03 belongs to Zicotriavirus genus of the Schitoviridae N4-like viruses. Through proteomics analysis, we identified virion structural proteins of ZC01 and ZC03, including a large virion-associated RNA polymerase that is characteristic of N4-like viruses, some hypothetical proteins whose annotation should be changed to virion structural proteins and a putative peptidoglycan hydrolase. Phages ZC01 and ZC03 exhibit a limited yet distinct host range, with moderate to high efficiency of plating (EOP) values observed for a few P. aeruginosa clinical isolates. Phage susceptibility assays in PA14 mutant strains point to the type-IV pilus (T4P) as the primary receptor for phages ZC01 and ZC03, and the major pilin (PilAPA14) is the T4P component recognized by these phages. Moreover, both phages significantly increase survival of Galleria mellonella larvae infected with PA14 strain. Taken together, these results underpin the therapeutic potential of these phages to treat infections by P. aeruginosa and lay the groundwork for a more detailed investigation of phage-bacteria-specific recognition mechanisms.
IMPORTANCE
Phage therapy is gaining increasing interest in cases of difficult-to-treat bacterial human infections, such as carbapenem-resistant Pseudomonas aeruginosa. In this work, we investigated the molecular mechanism underlying the interaction of the lytic phages ZC01 and ZC03 with the highly virulent P. aeruginosa PA14 strain and their efficacy to treat PA14 infection in Galleria mellonella larvae, a commonly used invertebrate model for phage therapy. We depicted the protein composition of ZC01 and ZC03 viral particles and identified pilin A, the major component of type-4 pilus, as the receptor recognized by these phages. Our findings indicate that phages ZC01 and ZC03 may be further used for developing therapies to treat multidrug-resistant P. aeruginosa infections.
KEYWORDS: Pseudomonas aeruginosa, PA14, phage therapy, phage receptor, type-4 pilus
INTRODUCTION
Bacteriophages (phages) are viruses that rely on a bacterial host for propagation. Phages are mainly classified according to their life cycle into lysogenic and lytic, in the latter of which the host cells are lysed and mature phage particles (virions) are released (1). Phages generally display high specificity toward bacterial species or strains, and this is determined by the mechanisms of phage adsorption to host cells (2) and by bacterial antiphage defense systems (3).
The receptors known to be involved in phage adsorption to Gram-negative bacteria are lipopolysaccharides (LPS), capsular polysaccharides, pili, flagella, and outer membrane proteins (2, 4, 5). Phages, on the other hand, have receptor-binding proteins (RBPs) responsible for the specific recognition and interaction with the receptor displayed on the surface of bacterial cells, thus initiating the infection process (2). As the first point of contact with bacterial cells, RBPs are the primary determinants of the phage ability to infect one or more bacterial strains, an attribute referred to as phage host range. RBPs are typically located on tail fibers or tailspikes which may have enzymatic activity that binds and degrades carbohydrate moieties on the bacterial surface (6–8).
Similarly to other bacterial species, the major receptors that have been implicated in phage adsorption to Pseudomonas aeruginosa are type IV pili (T4P) (9–12) and LPS (13–17). Spontaneous mutations in genes responsible for T4P or LPS synthesis can result in phage-resistant mutants, particularly under laboratory conditions (17–20). On the other hand, virulence reduction of phage-resistant bacteria may occur if the phage targets a virulence factor (5, 15, 21, 22).
P. aeruginosa belongs to the ESKAPEE group of pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, P. aeruginosa, Enterobacter spp., and Escherichia coli) which became a serious concern due to the worldwide antimicrobial resistance (AMR) increase in nosocomial and community-acquired infections (23–27). Multidrug-resistant P. aeruginosa causes acute or chronic infection in immunocompromised individuals with chronic obstructive pulmonary disease, cystic fibrosis, cancer, traumas, burns, sepsis, and ventilator-associated pneumonia (28). In addition to the development of new antimicrobials to combat AMR (29), phage therapy is gaining increasing interest in cases of difficult-to-treat ESKAPEE human infections (30–33), such as carbapenem-resistant P. aeruginosa (34–36).
PAO1 and PA14 (or UCBPP-PA14) are commonly used as laboratory reference P. aeruginosa strains to study this bacterial species (37–40). While our PAO1 is a derivative of the original PAO1 isolate which was obtained from a wound at Melbourne, PA14 was one of the strains isolated from burn wound patients at a hospital in Pennsylvania (37, 38, 41). PA14 has been shown to be highly virulent in both animals and plants (42, 43), and its use in research is gradually matching that of P. aeruginosa PAO1. Until now, thousands of phages that infect P. aeruginosa PAO1, PA14, and/or clinical strains have been characterized, and some of them exhibit a broad spectrum of activity against P. aeruginosa clinical isolates (34, 44–49). Nevertheless, new P. aeruginosa phages are continuously being discovered and characterized through phage isolation studies and predictions retrieved from metagenomics data set (50, 51).
The Pseudomonas phages ZC01 and ZC03 were isolated from composting samples using PA14 strain as the enrichment host and previously classified as Siphoviridae and Podoviridae, respectively, based on their double-strand DNA (dsDNA) genomes and morphotypes of tailed phages (46). Phage ZC01 was recently classified into the genus Abidjanvirus, while ZC03 is a single species of the new Zicotriavirus genus (52, 53). These two phages had their genomes analyzed, but their biological properties were not fully explored yet. In this work, we extended the molecular characterization of phages ZC01 and ZC03 and investigated the mechanism underlying their interaction with P. aeruginosa. Both phages were evaluated regarding their phage therapy potential against PA14 infection in Galleria mellonella larvae. Our findings indicate that these phages may be further used for developing therapies to treat multidrug-resistant P. aeruginosa infections.
RESULTS
Update of ZC01 and ZC03 phage characteristics
The phages ZC01 and ZC03 were isolated from composting samples using P. aeruginosa strain PA14 as enrichment host and exhibit, respectively, siphovirus and podovirus morphotypes (46) (Fig. 1). Based on their complete genome sequences (sequence accessions NC_052965 and NC_048638, these phages are currently classified as Caudoviricetes; Mesyanzhinovviridae; Bradleyvirinae; Abidjanvirus; Abidjanvirus ZC01 and Caudoviricetes; Schitoviridae; Zicotriavirus; Zicotriavirus ZC03 according to the new taxonomy of bacterial viruses that abolished the morphology-based families Myoviridae, Siphoviridae, and Podoviridae (52). ZC01 genome is highly similar (93% coverage and 96% identity) to the genome of phage Ab18 (54). While Ab18 infects P. aeruginosa strain PAO1 but not PA14 (54), ZC01 infects PA14 but not PAO1 (46). ZC03 and phage ZC08 (46) are the only members of the genus Zicotriavirus of the Schitoviridae N4-like viruses that encode three RNA polymerase genes, including a large (~3,500 aa) virion-associated RNA polymerase that is characteristic of this family (55).
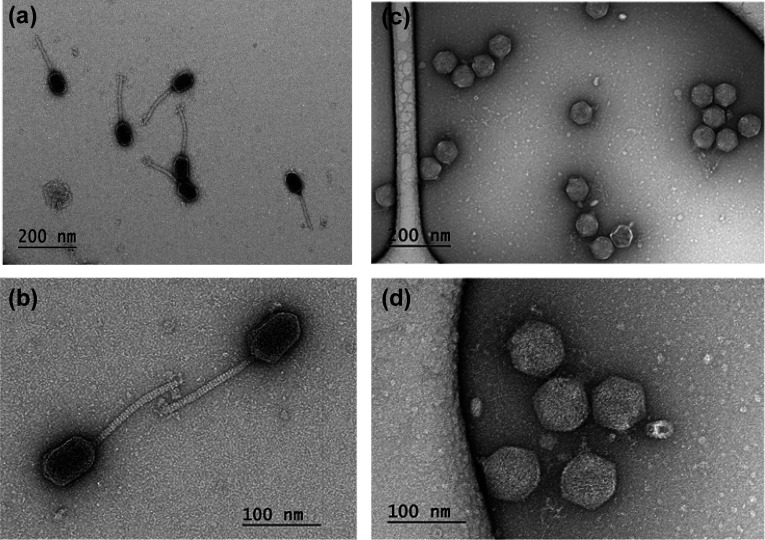
Fig 1.
Transmission electron micrographs of ZC01 and ZC03 purified phage particles, negatively stained with uranyl acetate. Different areas on a grid show intact ZC01 (a and b) and ZC03 (c and d) phage particles with siphovirus (ZC01) and podovirus (ZC03) morphotypes as previously described (46).
Both phages form clear lysis plaques, which is typical for lytic phages. ZC03 presents a latent period of ~50 min and a calculated burst size of 10 phage particles per infected cell (46). On the of other hand, ZC01 presents a latent period of ~100 min and a calculated burst size of 87 phage particles per infected cell (Fig. S1). The bacteriolytic effect of phages ZC01 and ZC03 on PA14 strain was investigated through time-killing curves at different multiplicities of infection (MOIs). A better lytic effect was observed at MOIs higher than 1 for both phages (Fig. S2). It is worth noting that until 12 h, no secondary bacterial growth was detected upon ZC01 and ZC03 infection, indicating a prolonged control of bacterial growth under these conditions, with no apparent phage resistance emergence.
Phage ZC01 was stable at 25°C and 37°C, but its viability decreased upon incubation at 16°C, 42°C, and 60°C. In contrast, phage ZC03 was moderately stable at 16°C and 42°C, but at 60°C, its titer was drastically reduced. Both phages were fully inactivated by incubation at 80°C or when exposed to UV light for 20 min. ZC01 and ZC03 were reasonably stable in chloroform 10% and in pH values ranging from 4 to 12. Both phages became unviable at pH 2. These results are summarized in Table 1.
TABLE 1.
Viability of phages ZC01 and ZC03 under different conditions
| Phage | Temperature | |||||
|---|---|---|---|---|---|---|
| 16°C | 25°C | 37°C | 42°C | 60°C | 80°C | |
| ZC01 | 24.4 ± 15 | 103.3 ± 1 | 116.0 ± 22 | 33.9 ± 3 | 30.4 ± 13 | 0.2 ± 1 |
| ZC03 | 89.8 ± 20 | 96.0 ± 2.5 | 89.9 ± 19 | 76.5 ± 26 | 3.9 ± 3.7 | 0.0 |
Incubations of phage suspension at different temperatures, pHs, and CHCl3 were performed for 60 min. Phage % viability (mean ± SD from three independent assays) was relative to the initial phage titer (109 PFU/mL) in control condition (25°C in Saline-Magnesium buffer pH 7.5) before the test incubations.
Proteomics of ZC01 and ZC03 phage particles
Mass spectrometry-based proteomics of highly pure ZC01 and ZC03 phage particles (Fig. 1) identified 51 proteins out of 78 predicted open reading frames (ORFs) in the ZC01 genome (Table S1) and 65 proteins out of 85 predicted ORFs in the ZC03 genome (Table S2). For both phage particles, the in-gel trypsin digestion outperformed the in-solution trypsin digestion (shotgun proteomics) in terms of the identification of higher number of polypeptides. Table 2 lists 18 proteins of ZC01 virions which had a coverage higher than 20% and a minimum of three peptides (Table S1). The major head protein (ZC01_055) and the tail length tape measure protein (ZC01_065) had the highest coverage and number of peptides. Other typical structural proteins, such as portal protein (ZC01_047), head morphogenesis protein (ZC01_048), tail fiber assembly protein (ZC01_068 and ZC01_069), and tail protein (ZC01_072), are among the proteins that have been reliably identified in ZC01 virions.
TABLE 2.
Proteins of ZC01 and ZC03 virions identified by proteomic analysis which had a coverage higher than 20% and presented a minimum of three peptidesa
| ZC01 ORF ID | Predicted function | ZC03 ORF ID | Predicted function |
|---|---|---|---|
| ZC01_047 | Portal protein | ZC03_001 | Hypothetical protein |
| ZC01_048 | Head morphogenesis protein | ZC03_002 | Tail assembly protein |
| ZC01_054 | Head scaffolding protein | ZC03_003 | Hypothetical protein |
| ZC01_055 | Major head protein | ZC03_004 | Hypothetical protein |
| ZC01_056 | Hypothetical protein | ZC03_005 | Tail fiber protein |
| ZC01_057 | Virion structural protein | ZC03_006 | Tail fiber protein |
| ZC01_058 | Head-tail adaptor Ad1 | ZC03_008 | Hypothetical protein |
| ZC01_060 | Tail completion or Neck1 protein | ZC03_012 | Single-stranded DNA-binding protein |
| ZC01_061 | Tail terminator protein | ZC03_014 | Hypothetical protein |
| ZC01_062 | Minor tail protein | ZC03_015 | Virion RNA polymerase |
| ZC01_065 | Tail length tape measure protein | ZC03_016 | Peptidoglycan hydrolase |
| ZC01_066 | Structural protein | ZC03_017 | Hypothetical protein |
| ZC01_067 | Structural protein | ZC03_018 | Hypothetical protein |
| ZC01_068 | Tail assembly protein | ZC03_019 | Virion structural protein |
| ZC01_069 | Tail assembly protein | ZC03_021 | Major head protein |
| ZC01_072 | Tail protein | ZC03_022 | Tail length tape measure protein |
| ZC01_073 | Hypothetical protein | ZC03_024 | Portal protein |
| ZC01_075 | Baseplate hub subunit and tail lysozyme | ZC03_028 | Hypothetical protein |
| ZC03_029 | Hypothetical protein | ||
| ZC03_030 | Virion structural protein | ||
| ZC03_044 | Hypothetical protein | ||
| ZC03_087 | Thymidylate synthase | ||
| ZC03_089 | RIIB lysis inhibitor | ||
| ZC03_094 | Hypothetical protein |
The complete lists of proteins identified with the respective number of peptides and coverage from in-gel and in-solution proteomics are presented on Tables S1 and S2. ORF amino acid sequences and functional prediction can be found along with ZC01 (accession NC_052965) and ZC03 (accession NC_048638) NCBI Reference Sequence genome annotation.
For ZC03, 24 proteins (Table 2) had a coverage higher than 20% and a minimum of three peptides (Table S2). Among the predominant proteins identified, in terms of coverage and number of peptides, are the large virion-associated RNA polymerase (ZC03_015), typical of the N4-Like viruses (family Schitoviridae), which carry this 399.4 kDa enzyme inside their capsids. Other dominant proteins identified are a putative peptidoglycan hydrolase (ZC03_016), the major head protein (ZC03_021), and the portal protein (ZC03_024). Proteomics of ZC03 virion also identified two ORFs predicted as tail fiber proteins (ZC03_005 and ZC03_006).
ZC01 and ZC03 host-range evaluation
Previously reported drop test assays revealed that phages ZC01 and ZC03 present a narrow host range producing clear lysis plaques in just 3 out of 18 P. aeruginosa isolates. Moreover, both phages infect the reference strain PA14 but not strain PAO1 (46). Additional drop test assays were performed using 66 P. aeruginosa clinical and environmental isolates (Table S3) some of them with multidrug-resistance phenotypes. While seven isolates were susceptible to both phages, two isolates were susceptible to ZC03 only, and one isolate was susceptible just to ZC01. Altogether, and including the three susceptible isolates previously identified, 11.5% (8/69) of the isolates were susceptible to ZC01 and ZC03, respectively, whereas 16% (11/69) and 14% (10/69) of the P. aeruginosa isolates were susceptible to ZC01 or ZC03. However, the efficiency of plating (EOP) of none of these 13 susceptible clinical isolates has surpassed that of the strain PA14 (Table 3). While for most of these 13 isolates EOP values of ZC01 were moderate (>0.1%), lower EOP values (<0.1%) were calculated for ZC03 (Table 3). In contrast, higher EOP values (~50%) were calculated for the lung isolates Fc79a M and Fc79a PAB NM infected by ZC03.
TABLE 3.
EOP of phages ZC01 and ZC03 for selected clinical isolatesa
| P. aeruginosa strain/isolate | Drop test assay | EOP | Source | ||
|---|---|---|---|---|---|
| ZC01 | ZC03 | ZC01 | ZC03 | ||
| PA14b | + | + | 100% | 100% | Reference strain |
| 3845 GSP-3 producer | + | + | 3.4% | 0.02% | HIV patient feces |
| ALERTA 226 (GES-5 carbapenemase-producer) | − | + | nd | 0.06% | Hospital |
| ALERTA 275 (VIM-7 carbapenemase-producer) | − | + | nd | 0.05% | Hospital |
| ALERTA 395 (IMP-18 metallo-beta-lactamase producer) | + | + | 5% | 9% | Hospital |
| Fc79a M | + | + | 2% | 49% | Lung secretion |
| Fc79a PAB NM | + | + | 8% | 51% | Lung secretion |
| Fc7f NM | + | − | 2% | nd | Lung secretion |
| MT222 | + | + | 14% | 0.01% | Tracheal aspirate |
| P13.612 | + | + | 20% | 0.03% | Hospital |
| SC-61 | + | + | 8% | 2% | Nasal secretion |
| H6044c | + | − | 0.6% | nd | Blood |
| H6086c | + | − | 14% | nd | Blood |
| 5757c | + | + | 9.8% | 0.045% | Blood and urine |
Clear lysis plaque (+) and no lysis/turbid plaque (−). The EOP value of 100% was considered for the host strain. nd: not determined.
Host strain.
Isolates analyzed in previous work (46). The full list of the 69 strains evaluated is shown on Table S3.
Identification of type 4 pilus as the receptor for ZC01 and ZC03
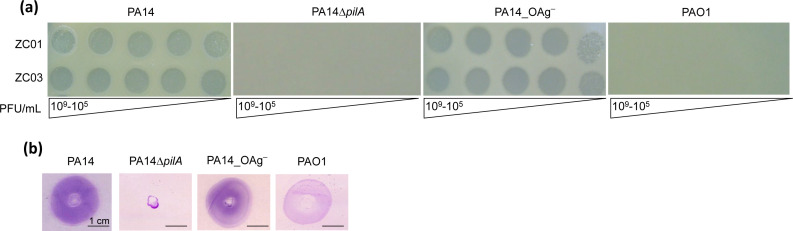
Commonly identified receptors for P. aeruginosa phages are LPS and T4P (4). To determine whether T4P or LPS would function as receptors for ZC01 and ZC03, mutants PA14ΔpilA, lacking pilin A, and PA14_OAg–, lacking the O antigen, were tested on phage infection assays. As shown in Fig. 2a, both phages form clear lysis plaques in strain PA14 and in PA14_OAg–, but not in PA14ΔpilA strain. As previously observed (46), PAO1 strain is resistant to phages ZC01 or ZC03 (Table S3). Twitching motility, which is dependent on functional T4P, was fully abolished in the PA14ΔpilA but was not altered in the other strains, including the PA14_OAg– (Fig. 2b). These findings strongly suggest that T4P is the primary receptor for ZC01 and ZC03 adsorption to PA14 cells.
Fig 2.
Infection of P. aeruginosa PA14 depends on T4P but not on LPS. (a) ZC01 and ZC03 phage lysates at the indicated titers (109–105 PFU/mL) were spotted on wild-type strains PAO1 and PA14 and on T4P (PA14ΔpilA) and LPS (PA14_OAg–) null mutants. Photographs were taken after overnight incubation at 37°C. (b) Twitching motility assays of wild-type and mutant strains were analyzed by staining the plates with 0.1% crystal violet after 48 h. Images show a representative experiment of at least three independent biological replicates.
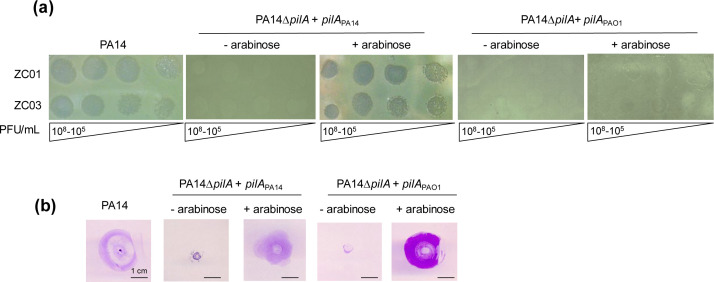
To verify that pilin A, a major component of T4P in P. aeruginosa, is indeed the primary determinant for ZC01 and ZC03 adsorption to PA14 cells, pilA of PA14 (pilAPA14) or pilA of PAO1 (pilAPAO1) was used to complement the PA14ΔpilA mutant strain. As shown in Fig. 3a, PA14ΔpilA complemented with pilAPA14, but not pilAPAO1, can be infected by the two phages. This result suggests that pilin A sequence variation may be the primary host barrier for ZC01 and ZC03 susceptibility. PA14ΔpilA, when complemented with either pilAPA14 or pilAPAO1, exhibits its twitching motility restored, suggesting that T4P is functional upon complementation (Fig. 3b).
Fig 3.
Expression of PilAPA14 restores phage-susceptibility of the PA14ΔpilA mutant. (a) ZC01 and ZC03 phage lysates at the indicated titers (108–105 PFU/mL) were spotted on PA14 and on PA14ΔpilA expressing pilAPA14 or pilAPAO1 upon induction with arabinose. Photographs were taken after overnight incubation at 37°C. (b) Twitching motility assays of wild-type and complemented strains induced or not with arabinose were analyzed by staining the plates with 0.1% crystal violet after 48 h. Images show a representative experiment of at least three independent biological replicates.
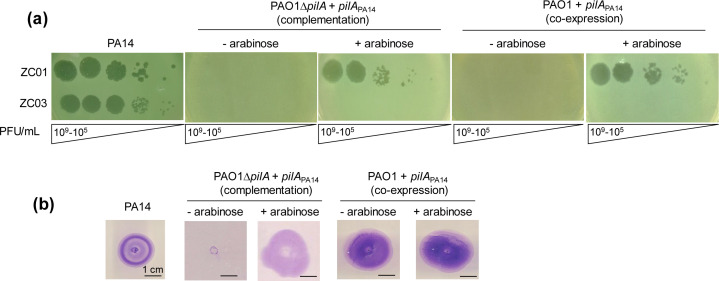
To further evaluate the dependence of PA14 pilin A as the receptor for ZC01 and ZC03, pilAPA14 was introduced in PAO1 (co-expression) and in a PAO1ΔpilA mutant (complementation). PilAPA14 turns these PAO1 strains susceptible to infection by phage ZC01 but not to ZC03 (Fig. 4a). Twitching motility was restored in the PAO1ΔpilA upon complementation, and it was not altered by co-expression of PilAPA14 in wild-type PAO1 (Fig. 4b).
Fig 4.
Infection of P. aeruginosa PAO1 and PAO1 ΔpilA expressing PilAPA14. (a) ZC01 and ZC03 phage lysates at the indicated titers (109–105 PFU/mL) were spotted on PAO1 or PAO1ΔpilA expressing PilAPA14 upon induction using arabinose. Photographs were taken after overnight incubation at 37°C. (b) Twitching motility assays of wild-type and complemented strains induced or not with arabinose were analyzed by staining the plates with 0.1% crystal violet after 48 h. Images show a representative experiment of at least three independent biological replicates.
Evaluation of therapeutic potential of phages ZC01 and ZC03 in G. mellonella
The therapeutic potential of phages ZC01 and ZC03 against P. aeruginosa infection was evaluated in the animal model G. mellonella. As shown in Fig. 5, G. mellonella larvae infected with PA14 strain showed 75% and 100% mortality, respectively, after 18 and 20 h of inoculation. Treatment of PA14-infected larvae with ZC01 phage resulted in survival rates at 20 h of 53% and 68% at MOI of 20 and 100, respectively (Fig. 5a). After 24 h, ZC01 treatment at both MOIs resulted in an increase of 15%–21% (P < 0.0001) of the survival rate. Treatment with ZC03 resulted in survival rates of ~40% (P < 0.0001) at MOIs of 20 and 100, after 20–24 h post infection (Fig. 5b), although a dose-dependent effect was not observed as verified for ZC01. It should be mentioned that uninfected larvae inoculated with the phages or buffer alone presented 100% survival up to 24 h of the assay.
Fig 5.
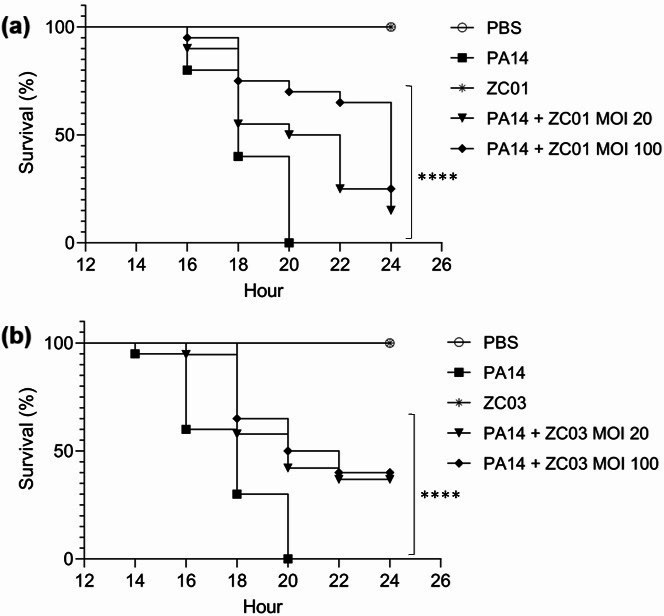
In vivo efficacy of phages ZC01 and ZC03 against P. aeruginosa PA14 strain in G. mellonella infection model. Survival curves of G. mellonella larvae treated with ZC01 (a) or ZC03 (b). G. mellonella larvae were injected with buffer-only [phosphate-buffered saline (PBS)], PA14-only (5 × 103 CFU/mL), phage-only (105 PFU/mL), and phage at MOI of 20 (105 PFU/mL) or 100 (5 × 105 PFU/mL) 1 h post-infection with PA14 (5 × 103 CFU/mL). The larvae were monitored at 2 h intervals, for 24 h. The data represent three independent experiments with 20 animals per treatment. Log rank (Mantel-Cox) test (****, P < 0.0001).
DISCUSSION
In this work, we report an extended characterization of the phages ZC01 and ZC03 which were previously isolated from a thermophilic composting operation at the São Paulo Zoo Park (Brazil) using P. aeruginosa PA14 as the enrichment host (46). ZC01 is a siphovirus currently classified within the Abidjanvirus genus, and ZC03 is a podovirus that belongs to the Zicotriavirus genus of the Schitoviridae N4-like viruses. These phages are devoid of any known lysogenic, virulence, or toxin genes that would preclude their use in phage therapy. Nevertheless, both genomes encode several ORFs of unknown function which require further characterization to verify that they are not harmful as therapeutic phages. Interestingly, although phages ZC01 and ZC03 were isolated from a thermophilic compost, they are not thermostable but maintain viability at 37°C and pH 7.5, which is considered satisfactory for therapeutic phages (56).
Besides the typical structural proteins of Caudoviricetes, proteins of unknown function (hypothetical proteins) were reliably identified by proteomics as components of ZC01 and ZC03 viral particles. It is worth noting that ZC01_056 and ZC01_073 amino acid sequences are conserved (>93% coverage and >57% identity) in other phages from the Abidjanvirus genus. On the other hand, some of the hypothetical proteins identified in the ZC03 proteome (ZC03_001, ZC03_003, ZC03_004, ZC03_008, ZC03 _014, ZC03_029, and ZC03_044) have similar counterparts only in Pseudomonas phage ZC08 (46) which together with ZC03 are the unique members of Zicotriavirus genus. Further analysis of the ZC03 proteome revealed hypothetical proteins ZC03_017 and ZC03_018, which exhibit similarity to counterparts in other phages. Conversely, proteins ZC03_028 and ZC03_095 appear to be unique to phage ZC03. These observations support a change in the current annotation of these hypothetical proteins to virion structural proteins. We highlight ORFs ZC03_005 and ZC03_006 predicted as tail fiber proteins in ZC03 proteome which do not share amino acid sequence similarity. It has yet to be investigated whether ZC03_005 and ZC03_006 make up distinct tail fibers that could function as distinct RBPs, or whether both together are components of the ZC03 tail fibers.
Among the proteins identified in the ZC03 virion proteome, there is an 831 amino acids-protein (ZC03_016) which has an almost identical ortholog (100% coverage and 96% identity) only in Pseudomonas phage ZC08 (NCBI Reference Sequence accession NC_048639). ZC03_016 contains a lytic transglycosylase domain that belongs to the lysozyme-like domain superfamily predicted as peptidoglycan hydrolase according to InterPro classification (57). Virion-associated peptidoglycan hydrolase (VAPGH) proteins are generally attached to the viral particle contacting the bacterial surface in the first step of the infection process to locally degrade the bacterial cell wall peptidoglycan (58, 59). These enzymes have been proposed as antimicrobial and biotechnological tools to fight against numerous pathogens (56, 59–61). Thus, the predicted VAPGH domain in ZC03_016 can be further explored as an antimicrobial against P. aeruginosa and other Gram-negative pathogens.
By using a new set of 66 P. aeruginosa clinical isolates, we have confirmed the narrow and relatively distinct host range of phages ZC01 and ZC03. Despite this restricted host range, for a few clinical isolates, moderate-to-high EOP values were observed. According to predictions based on their genome sequences, these isolates present distinct sequence types as well as distinct serotypes from the PA14 or PAO1 strains (data not shown). Further genomic analyses may help to explain the moderate susceptibility of these strains to phages ZC01 and ZC03. While the host range of phages has traditionally been linked to receptor-associated properties (2), recent research has highlighted the significance of defense system quantity in determining P. aeruginosa strains susceptibility to phages (62).
As reported for other phages infecting P. aeruginosa (9–11, 18, 63), T4P is the primary receptor for phages ZC01 and ZC03 because they did not lyse a T4P-less host mutant (PA14ΔpilA). Phage adsorption along the pilus length occurs probably by their binding to PilA (the major component of T4P) (64). While T4P is a well-established receptor for phage adsorption, the exact mode of phage binding has not been clarified. Alterations in PilA sequence or its glycosylation can interfere with phage infection (18). Here, we provide additional evidence that PilA is indeed the T4P component recognized by ZC01 and ZC03 and that variations in pilin A sequence influence host recognition. Complementation of the T4P-less host mutant (PA14ΔpilA) with PilAPAO1 cannot restore susceptibility to infections of phages ZC01 and ZC03. Nevertheless, PilAPA14 expressed in PAO1ΔpilA turns this strain susceptible to infection by phage ZC01, but not to ZC03. From these observations, we can conclude that PAO1 does not carry anti-phage defense systems to abolish ZC01 infection as this phage can be replicated in PAO1 expressing PilAPA14.
The opposite situation was observed for phage ZC03, which did not infect PAO1 expressing PilAPA14, even though its T4P is functional. Thus, although pilin A sequence variation may be the primary host barrier for phage susceptibility, it is not sufficient to overcome PAO1 resistance to ZC03 infection. We can foresee some possible explanations for this result, such as that ZC03 adsorption is impaired by modifications of PA14 pilin A such as O-glycosylation (18) when it is expressed in PAO1. Another possible explanation could be that a surface structure, such as LPS, prevents ZC03 interaction with PAO1 by masking the host receptor. For instance, PAO1 LPS (O5 serotype) has an O-antigen composition quite distinct of PA14 LPS (serotype O19) (13, 65). Alternatively, ZC03 adsorption may depend on a second receptor recognized only in PA14 cells. Moreover, we cannot exclude that PAO1 carries anti-phage defense systems that impair ZC03 replication.
Phages ZC01 and ZC03 showed promising efficacy to treat PA14 infection in G. mellonella larvae as single-dose treatments with phages ZC01 or ZC03 significantly increase the survival of larvae infected with PA14 strain. It is worth mentioning that these phages exhibit a narrow host range and target a receptor (PilA) that is highly variable among clinical isolates (66, 67), which can be seen as drawbacks of using these phages in therapeutic applications (68). Nevertheless, our results warrant further studies to explore other dose regimens and/or the treatment with these phages together in a cocktail with other phages of different host ranges and/or targeting different receptors in larvae infected with PA14 or other susceptible clinical strains. Our work also lays the groundwork for a more detailed investigation of phage-bacteria-specific recognition mechanisms, especially considering ZC03, which is so far the only representative of the Zicotriavirus genus.
MATERIALS AND METHODS
P. aeruginosa strains and culture conditions
P. aeruginosa reference strains PA14 (69) and PAO1 (70), as well as clinical and environmental P. aeruginosa isolates (Table S3), were grown overnight at 37°C in TSB (tryptic soy broth) containing or not 1.5% agar (TSB-agar). Bacterial stocks were maintained in TSB supplemented with 10% glycerol at −80°C or in liquid nitrogen storage tank.
Propagation and purification of phages ZC01 and ZC03
Previously isolated phages ZC01 and ZC03 (46) were propagated in P. aeruginosa PA14 using the double-layer agar technique (71) and purified following formerly described protocols (72, 73). Briefly, 0.5 mL of log-phase bacterial culture was mixed with the phage suspension (~50 µL) at an MOI of 0.01 and incubated for 10 min at 37°C. The phage-bacteria suspension was then mixed with 13 mL of molten TSB containing 0.7% agar (TSB-top-agar 0.7%) and then poured on a TSB-agar Petri dish. After overnight incubation at 37°C, 15 mL of Saline-Magnesium (SM) buffer (3% NaCl, 10 mM MgSO4, 10 mM CaCl2, 30 mM Tris-HCl, and pH 7.5) was added, and after gently shaking for 1 h at room temperature, the Petri dishes were incubated at 4°C overnight and for additional 1 h at room temperature without shaking. The lysates obtained from each plate were pooled, transferred to 50 mL conical tubes, and centrifuged at 6,000 × g for 20 min at 4°C. The supernatants were transferred to clean tubes, and chloroform was added to the final concentration of 10% and immediately centrifuged at 6,000 × g for 20 min at 4°C. The supernatant was filtered through a 0.22 µm membrane and titrated to calculate phages forming units per mL (PFU/mL) . A solution of 2.5 M NaCl in 20% polyethylene glycol 8000 was added to the filtered phage suspension in a 4:1 vol ratio. After brief mixing, the mixture was kept at 4°C for 24 h, and phage particles were pelleted by centrifugation (6,000 × g, 20 min, 4°C). The supernatant was then discarded, a new round of centrifugation was done, and the pellet with no traces of the supernatant was resuspended in SM buffer overnight at 4°C. The phages were further purified by cesium chloride (CsCl) density gradient ultracentrifugation (40,000 × g, 4 h, 4°C). Phages concentrated in a single band in the CsCl gradient were collected with a needle and syringe and subjected to dialysis by centrifugation through Amicon Ultra-15 Centrifugal Filters 100 kDa with two washes of SM buffer (5× the collected volume). Purified phages were titrated using a double-layer agar technique and stored at 4°C in SM buffer. Phages stocks were maintained in SM buffer supplemented with 10% glycerol at −80°C.
Transmission electron microscopy
Around 3 µL of purified phage suspension was gently placed on glow-discharged carbon-coated 300 mesh copper grids. After about 1 min, excess liquid was blotted off, and the grid was stained with 2% uranyl acetate and air-dried. The negatively stained phage particles were visualized with a JEOL JEM 2100 transmission electron microscope (JEOL Ltd, Tokyo, Japan) at operating voltage of 100 kV, and the images were registered digitally according to protocols of the Chemistry Institute Analytical Center (CA-USP, https://ca2.iq.usp.br/).
Spot test and EOP assays
For the spot test assay, 120 µL of log-phase P. aeruginosa culture was mixed with 4 mL of molten TSB top-agar 0.7% and poured onto a TSB-agar Petri dish. After solidification, 4 µL of 10-fold serial dilutions (four dilutions) of the phage suspension was gently dropped over the top agar and examined for the presence of lysis plaques after overnight incubation at 37°C.
For the EOP assay, an overnight culture of phage susceptible P. aeruginosa strain was diluted to OD600 nm = 1.0 (~3 × 109 colony forming units per mL or CFU/mL), incubated for 10 min at 37°C with 10 µL of 10-fold serial dilutions (eight dilutions) prepared from phage stocks at 1013–1016 PFU/mL. The phage-bacteria suspension was mixed with 7 mL of molten TSB-top-agar 0.7%, poured on a 90 mm-TSB-agar Petri dish, and incubated overnight at 37°C. The EOP (74) was calculated by dividing the number of lysis plaques produced in each susceptible strain (for a fixed dose of phages) by the number of plaques produced in the host strain P. aeruginosa strain PA14. EOP values >0.1% and >50% are considered moderate or high, respectively (75).
One-step growth curve
A total of 5 mL of a log-phase P. aeruginosa PA14 culture grown in TSB at 37°C was mixed with 5 mL of a phage suspension to reach an MOI < 0.1. After incubation for 10 min at 37°C, the mixture was centrifuged at 6,000 × g for 15 min, and the supernatant was collected and titrated to determine the amount of phage that did not adsorb onto the bacteria. The pellet was resuspended in 20 mL of TSB and incubated at 37°C without shaking. Every 10 min up to 190 min of incubation, 1 mL was collected and immediately titrated by the double-layer agar method (71) by plating 10-fold serial dilutions with P. aeruginosa PA14 and determining the number of lysis plaques produced (PFU/mL).
Time-killing curve
To investigate the antibacterial effect of phages ZC01 and ZC03, overnight P. aeruginosa PA14 cultures were diluted to 106 CFU/mL, which is equivalent to OD600nm of 0.2, and infected with phage at different MOIs (100, 10, 1, 0.1, and 0.01). The control sample had no phages. One hundred fifty microliter of each mixture was transferred to six wells of a 96-well plate and incubated at 37°C for 12 h. OD600nm changes were measured every 15 min of incubation using the SpectraMax Paradigm microplate reader (Molecular Devices, CA, USA).
Phage stability evaluation
To evaluate the effect of pH on phage stability, 100 µL of the phage suspension (1 × 109 PFU/mL) prepared in SM buffer (pH 7.5) was mixed with 900 µL of universal buffer solution (150 mM KCl, 10 mM KH2PO4, 10 mM sodium citrate, and 10 mM H3BO3) at pHs 2, 4, 7.5, 9, and 12, followed by incubation for 60 min at 37°C. As a control, the phages were mixed with SM buffer (pH 7.5). The effect of temperature on phage stability was evaluated by incubation of 50 µL of the phage suspension (1 × 108 PFU/mL) at 16°C, 25°C, 37°C, 42°C, 60°C, and 80°C in a thermal cycler for 60 min. To evaluate the effect of chloroform, the phage suspension (1 × 109 PFU/mL) was mixed with chloroform to a final concentration of 10% (vol/vol) and incubated for 60 min at room temperature. To determine the phage tolerance to UV light, 100 µL of a phage suspension was exposed to UV light (254 nm) of a germicidal lamp in a biosafety cabinet for 20 min. After the treatments, the samples were immediately titrated by the double-layer agar method (71) by plating 10-fold serial dilutions with P. aeruginosa PA14 and determining PFU/mL.
Mass spectrometry-based proteomics
The identification of peptides of pure preparations of ZC01 and ZC03 phage particles was performed using two methods of mass spectrometry-based proteomics (SDS-PAGE followed by in-gel digestion and in-solution digestion). In the first approach, the phage particle samples (~100 µg of total protein) were resuspended in 50 mM Tris pH 6.8, 25 mM DTT, 10% glycerol, 1% SDS, 0.025% bromophenol blue, and subjected to SDS-PAGE (76). The gel was stained with Coomassie blue G250, the bands were cut out (Fig. S3), washed with 50 mM NH4HCO3, 40% acetonitrile (ACN), and subjected to sequential incubations with 10 mM dithiothreitol (DTT), 100 mM iodoacetoamide, and ACN prior to trypsin digestion using 100 ng of sequencing grade trypsin and 10 mM NH4HCO3 for 16 h at 37°C. Digestion was stopped with 10% formic acid (FA), and the peptides were extracted with 40% ACN, 0.1% FA, concentrated by vacuum centrifugation, and desalted with Zip-Tip C18 Cartridge column. For in-solution digestion, the phage particles suspension (~100 µg of total protein) was dried by vacuum centrifugation and suspended in 8 M urea, 10 mM DTT, and protease inhibitors cocktail and was incubated for 30 min at 30°C. The samples were diluted 10 times to 0.8 M urea, and sequencing grade trypsin was added (1:50 enzyme:total protein) followed by incubation at room temperature for 16 h at 37°C. The digestion was stopped by adding 1% trifluoroacetic acid, and the samples were desalted with Zip-Tip C18 Cartridge column. Samples were then subjected to nanoflow liquid chromatography coupled to mass spectrometry at the BIOMASS Core Facility at the Center for Research Facilities (CEFAP-USP, https://cefap.icb.usp.br/) using an Easy-nLC system coupled to LTQ-Orbitrap Velos mass spectrometer (Thermo Fisher Scientific Inc., MA, USA). Samples were resuspended in 0.1% FA and loaded onto a C18 PicoFrit column [C18 PepMap, 75 µm id × 10 cm, 3.5 µm particle size, and 100 Å pore size (New Objective, Ringoes, NJ, USA)] and separated with a gradient from 100% mobile phase A (0.1% FA) to 34% phase B (0.1% FA, 95% ACN) during 60 min, at a flow rate of 300 nL/min. Samples were analyzed in duplicate. The LTQ-Orbitrap Velos was operated in positive polarity with data-dependent acquisition. The full scan was obtained in the Orbitrap at a resolution of 60,000 FWHM in the 350–1,500 m/z mass range. The 20 most abundant peptide ions obtained in the MS full scan were selected for MS/MS, fragmented using CID at 35 normalized collision energy, and dynamic excluded for 15 s. All raw data were assessed in the Xcalibur software (Thermo Fisher Scientific Inc., MA, USA). Tandem mass spectra were processed and searched against an in-house database composed of annotated ORFs in phages ZC01 and ZC03 genomes (NCBI Reference Sequence accessions NC_052965.1 and NC_048638.1) using Proteome Discovery v. 1.4 (Thermo Fisher Scientific Inc., MA, USA) and SEQUEST (77), with the following parameters: precursor mass tolerance of 10 ppm; MS/MS mass tolerance 0.6 Da (CID data). Trypsin was selected as enzyme, carbamidomethyl cysteine as fixed modification and oxidation of methionine as variable modification. The False Discovery Rates (FDRs) were calculated using the algorithm Percolator with equal or less than 0.01. Protein FDR was calculated in the Proteome Discoverer software and kept below 1%. The mass spectrometry proteomics data have been deposited to the ProteomeXchange via Consortium the PRIDE (78) partner repository with the data set identifier PXD055478 (https://www.ebi.ac.uk/pride/archive/).
P. aeruginosa mutants and complementation of PA14ΔpilA and PAO1ΔpilA
The deletion mutants ∆pilA were constructed by allelic replacement and do not twitch (79). The spontaneous mutant PA14_OAg– is part of our mutant collection and lacks the O-antigen structures of LPS (79). For complementation of the ∆pilA mutants, the pilA genes from PA14 and PAO1 strains were amplified by PCR using the following primer pairs: PilA_PA14_fwd_ccgtttttttgggctagcgTATCAATGGAGAGATACATGAAAGCTC; PilA_PA14_rev_gcggccgctctagaactagtTTAGCGGCATTCGCTCGG and PilA_PAO1_fwd_cccgtttttttgggctagcgATGAAAGCTCAAAAAGGC; PilA_PAO1_rev_gcggccgctctagaactagtTTAGTTATCACAACCTTTCG. The resulting PCR amplicons were cloned into pJN105 plasmid (80) using the one-step sequence and ligation-independent cloning (SLIC) protocol (81). The vector was linearized using inverse PCR with the following primer pair: pJN105_rev CGCTAGCCCAAAAAAACG; pJN105_fwd ACTAGTTCTAGAGCGGCC. Resulting SLIC constructs were transformed into E. coli DH5α BL21(DE3), and recombinant clones were selected with ampicillin (50 µg/mL). The authenticity of the cloned genes was verified by Sanger sequencing of the inserts. Constructs were introduced into the target P. aeruginosa strains by electroporation. Transformants were selected with gentamicin (50 µg/mL), and the expression of cloned pilA was induced with 0.2% L-arabinose.
Twitching motility assays
Macroscopic twitching motility assays were performed by stabbing a single colony through a 3-mm-thick TSB-1% agar plate. After incubation at 37°C for 24 h in a humidified chamber, the agar was removed, and the twitching zone was stained for 15 min with 1% crystal violet. The stained area is proportional to the cells ability to twitch, which is dependent on T4P.
Phage treatment of G. mellonella larvae infected with P. aeruginosa
The assays of G. mellonella phage treatment were based on previously described protocols (44, 82). Briefly, G. mellonella larvae with size ranging from 2.0 to 2.5 cm in length and body weight from 150 to 200 mg were surface sterilized with 70% ethanol, separated into groups of 20 larvae, and placed in polystyrene Petri dishes (140 mm of diameter). A 10 µL inoculum of P. aeruginosa PA14 (5 × 103 CFU/mL) prepared in phosphate-buffered saline (PBS) was injected into the larva hemolymph behind the last proleg using a 10 µL Hamilton syringe. After 60 min, 10 µL of a phage suspension (1 × 105 or 5 × 105 PFU/mL) or PBS (positive control group) was delivered behind the last proleg on the opposite site to the bacterial injection site. Negative control groups (one group injected with PBS only to assess the impact of any negative effect from the injection process, and one group injected with phage suspension only to assess toxicity of the phage suspension) were also included. The larvae were kept at 37°C fed with pollen and beeswax. After 13 h post-infection, the larvae survival on each group was monitored every 2 h, for 12 h. The larvae were recorded as dead when they did not move in response to touch. Kaplan–Meier survival curves and log-rank (Mantel–Cox) statistical test were performed using GraphPad Prism 10.2.2 (GraphPadSoftware LLC.).
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to the members of the Biology of Bacteria and Bacteriophages Research Center (CEPID B3) for discussions and to Drs. Ana Cristina Gales, Ana Lúcia da Costa Darini, Cintya de Oliveira Souza, Karla Lima, and Nilton Erbet Lincopan Huenuman for providing various P. aeruginosa isolates for host range assays.
Funding for this work was provided by the São Paulo Research Foundation (FAPESP; grant numbers 2021/10577–0, 2018/18257–1, 2018/15549–1, 2020/04923–0, and 2022/11334–6) and the National Council for Scientific and Technological Development (CNPq; grant number 314701/2020–6). A.P.S.J. is supported by a PhD fellowship from the Coordination for the Improvement of Higher Education Personnel (CAPES). A.M.D.S., G.P., and K.I. are supported in part by Research Fellowship Awards from CNPq. The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
L.F.M. and A.M.D.S. designed the research. L.F.M. and A.P.S.J. conducted the experiments. R.B.Q. and G.G.S. contributed to the large-scale phage purification and transmission electron microscopy. C.B.A. and G.P. supplied laboratory infrastructure and performed proteomic analysis. G.G.N. and R.L.B. generated P. aeruginosa PA14 deletion mutants. G.S. and K.I. supplied the laboratory infrastructure and assistance for G. mellonella assays. L.F.M., A.P.S.J., F.P.N.R., G.G.S., R.L.B., and A.M.D.S. participated in the interpretation of the experiments and analysis of the data. L.F.M. and A.M.D.S. wrote the manuscript. All authors read, provided critical review, and approved the final manuscript.
Contributor Information
Layla Farage Martins, Email: layla@iq.usp.br.
Ethel Bayer-Santos, The University of Texas at Austin, Austin, Texas, USA.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.01527-24.
One-step growth curve for phage ZC01.
Time-killing curves of P. aeruginosa PA14 exposed to phages ZC01 and ZC03.
ZC01 and ZC03 phage particles subjected to SDS-PAGE.
Tables S1 and S2.
Host range of phages ZC01 and ZC03 on 69 clinical or environmental P. aeruginosa isolates.
An accounting of the reviewer comments and feedback.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Correa AMS, Howard-Varona C, Coy SR, Buchan A, Sullivan MB, Weitz JS. 2021. Revisiting the rules of life for viruses of microorganisms. Nat Rev Microbiol 19:501–513. doi: 10.1038/s41579-021-00530-x [DOI] [PubMed] [Google Scholar]
- 2. Nobrega FL, Vlot M, de Jonge PA, Dreesens LL, Beaumont HJE, Lavigne R, Dutilh BE, Brouns SJJ. 2018. Targeting mechanisms of tailed bacteriophages. Nat Rev Microbiol 16:760–773. doi: 10.1038/s41579-018-0070-8 [DOI] [PubMed] [Google Scholar]
- 3. Georjon H, Bernheim A. 2023. The highly diverse antiphage defence systems of bacteria. Nat Rev Microbiol 21:686–700. doi: 10.1038/s41579-023-00934-x [DOI] [PubMed] [Google Scholar]
- 4. Bertozzi Silva J, Storms Z, Sauvageau D. 2016. Host receptors for bacteriophage adsorption. FEMS Microbiol Lett 363:fnw002. doi: 10.1093/femsle/fnw002 [DOI] [PubMed] [Google Scholar]
- 5. Shen Y, Loessner MJ. 2021. Beyond antibacterials - exploring bacteriophages as antivirulence agents. Curr Opin Biotechnol 68:166–173. doi: 10.1016/j.copbio.2020.11.004 [DOI] [PubMed] [Google Scholar]
- 6. Klumpp J, Dunne M, Loessner MJ. 2023. A perfect fit: bacteriophage receptor-binding proteins for diagnostic and therapeutic applications. Curr Opin Microbiol 71:102240. doi: 10.1016/j.mib.2022.102240 [DOI] [PubMed] [Google Scholar]
- 7. Knecht LE, Veljkovic M, Fieseler L. 2019. Diversity and function of phage encoded depolymerases. Front Microbiol 10:2949. doi: 10.3389/fmicb.2019.02949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Le S, He X, Tan Y, Huang G, Zhang L, Lux R, Shi W, Hu F. 2013. Mapping the tail fiber as the receptor binding protein responsible for differential host specificity of Pseudomonas aeruginosa bacteriophages PaP1 and JG004. PLoS One 8:e68562. doi: 10.1371/journal.pone.0068562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim ES, Bae HW, Cho YH. 2018. A pilin region affecting host range of the Pseudomonas aeruginosa RNA phage, PP7. Front Microbiol 9:247. doi: 10.3389/fmicb.2018.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang L, Zhang TT, Li LL, Zheng C, Tan DM, Wu NN, Wang MY, Zhu TY. 2022. Characterization of Pseudomonas aeruginosa bacteriophage L5 which requires type IV pili for infection. Front Microbiol 13:9. doi: 10.3389/fmicb.2022.907958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bradley DE, Pitt TL. 1974. Pilus-dependence of four Pseudomonas aeruginosa bacteriophages with non-contractile tails. J Gen Virol 24:1–15. doi: 10.1099/0022-1317-24-1-1 [DOI] [PubMed] [Google Scholar]
- 12. Budzik JM, Rosche WA, Rietsch A, O’Toole GA. 2004. Isolation and characterization of a generalized transducing phage for Pseudomonas aeruginosa strains PAO1 and PA14. J Bacteriol 186:3270–3273. doi: 10.1128/JB.186.10.3270-3273.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huszczynski SM, Lam JS, Khursigara CM. 2020. The role of Pseudomonas aeruginosa lipopolysaccharide in bacterial pathogenesis and physiology. Pathogens 9:6. doi: 10.3390/pathogens9010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pan X, Cui X, Zhang F, He Y, Li L, Yang H. 2016. Genetic evidence for O-specific antigen as receptor of Pseudomonas aeruginosa phage K8 and its genomic analysis. Front Microbiol 7:252. doi: 10.3389/fmicb.2016.00252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Olszak T, Shneider MM, Latka A, Maciejewska B, Browning C, Sycheva LV, Cornelissen A, Danis-Wlodarczyk K, Senchenkova SN, Shashkov AS, Gula G, Arabski M, Wasik S, Miroshnikov KA, Lavigne R, Leiman PG, Knirel YA, Drulis-Kawa Z. 2017. The O-specific polysaccharide lyase from the phage LKA1 tailspike reduces Pseudomonas virulence. Sci Rep 7:16302. doi: 10.1038/s41598-017-16411-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li LY, Pan XW, Cui XL, Sun QH, Yang XJ, Yang HJ. 2016. Characterization of Pseudomonas aeruginosa phage K5 genome and identification of its receptor related genes. J Basic Microbiol 56:1344–1353. doi: 10.1002/jobm.201600116 [DOI] [PubMed] [Google Scholar]
- 17. Pourcel C, Midoux C, Vergnaud G, Latino L. 2020. The basis for natural multiresistance to phage in Pseudomonas aeruginosa. Antibiotics (Basel) 9:339. doi: 10.3390/antibiotics9060339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harvey H, Bondy-Denomy J, Marquis H, Sztanko KM, Davidson AR, Burrows LL. 2018. Pseudomonas aeruginosa defends against phages through type IV pilus glycosylation. Nat Microbiol 3:47–52. doi: 10.1038/s41564-017-0061-y [DOI] [PubMed] [Google Scholar]
- 19. Shen M, Zhang H, Shen W, Zou Z, Lu S, Li G, He X, Agnello M, Shi W, Hu F, Le S. 2018. Pseudomonas aeruginosa MutL promotes large chromosomal deletions through non-homologous end joining to prevent bacteriophage predation. Nucleic Acids Res 46:4505–4514. doi: 10.1093/nar/gky160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Labrie SJ, Samson JE, Moineau S. 2010. Bacteriophage resistance mechanisms. Nat Rev Microbiol 8:317–327. doi: 10.1038/nrmicro2315 [DOI] [PubMed] [Google Scholar]
- 21. León M, Bastías R. 2015. Virulence reduction in bacteriophage resistant bacteria. Front Microbiol 6:343. doi: 10.3389/fmicb.2015.00343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gurney J, Brown SP, Kaltz O, Hochberg ME. 2020. Steering phages to combat bacterial pathogens. Trends Microbiol 28:85–94. doi: 10.1016/j.tim.2019.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moradali MF, Ghods S, Rehm BHA. 2017. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol 7:39. doi: 10.3389/fcimb.2017.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pendleton JN, Gorman SP, Gilmore BF. 2013. Clinical relevance of the ESKAPE pathogens. Expert Rev Anti Infect Ther 11:297–308. doi: 10.1586/eri.13.12 [DOI] [PubMed] [Google Scholar]
- 25. De Oliveira DMP, Forde BM, Kidd TJ, Harris PNA, Schembri MA, Beatson SA, Paterson DL, Walker MJ. 2020. Antimicrobial resistance in ESKAPE pathogens. Clin Microbiol Rev 33:00181–19. doi: 10.1128/CMR.00181-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pottier M, Gravey F, Castagnet S, Auzou M, Langlois B, Guérin F, Giard J-C, Léon A, Le Hello S. 2023. A 10-year microbiological study of Pseudomonas aeruginosa strains revealed the circulation of populations resistant to both carbapenems and quaternary ammonium compounds. Sci Rep 13:2639. doi: 10.1038/s41598-023-29590-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rajput A, Seif Y, Choudhary KS, Dalldorf C, Poudel S, Monk JM, Palsson BO. 2021. Pangenome analytics reveal two-component systems as conserved targets in ESKAPEE pathogens. mSystems 6:e00981-20. doi: 10.1128/mSystems.00981-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qin SG, Xiao W, Zhou CM, Pu QQ, Deng X, Lan LF, Liang HH, Song XR, Wu M. 2022. Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Sig Transduct Target Ther 7:27. doi: 10.1038/s41392-022-01056-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Butler MS, Henderson IR, Capon RJ, Blaskovich MAT. 2023. Antibiotics in the clinical pipeline as of December 2022. J Antibiot 76:431–473. doi: 10.1038/s41429-023-00629-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kortright KE, Chan BK, Koff JL, Turner PE. 2019. Phage therapy: a renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe 25:219–232. doi: 10.1016/j.chom.2019.01.014 [DOI] [PubMed] [Google Scholar]
- 31. Strathdee SA, Hatfull GF, Mutalik VK, Schooley RT. 2023. Phage therapy: from biological mechanisms to future directions. Cell 186:17–31. doi: 10.1016/j.cell.2022.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pirnay JP. 2020. Phage therapy in the year 2035. Front Microbiol 11:1171. doi: 10.3389/fmicb.2020.01171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pirnay J-P, Djebara S, Steurs G, Griselain J, Cochez C, De Soir S, Glonti T, Spiessens A, Vanden Berghe E, Green S, et al. 2024. Personalized bacteriophage therapy outcomes for 100 consecutive cases: a multicentre, multinational, retrospective observational study. Nat Microbiol 9:1434–1453. doi: 10.1038/s41564-024-01705-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Makky S, Abdelrahman F, Rezk N, Easwaran M, El-Shibiny A. 2023. Phages for treatment Pseudomonas aeruginosa infection. Prog Mol Biol Transl Sci 201:1–19. doi: 10.1016/bs.pmbts.2023.03.014 [DOI] [PubMed] [Google Scholar]
- 35. Chen P, Liu Z, Tan X, Wang H, Liang Y, Kong Y, Sun W, Sun L, Ma Y, Lu H. 2022. Bacteriophage therapy for empyema caused by carbapenem-resistant Pseudomonas aeruginosa. Biosci Trends 16:158–162. doi: 10.5582/bst.2022.01147 [DOI] [PubMed] [Google Scholar]
- 36. Tkhilaishvili T, Winkler T, Müller M, Perka C, Trampuz A. 2019. Bacteriophages as adjuvant to antibiotics for the treatment of periprosthetic joint infection caused by multidrug-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 64:5. doi: 10.1128/AAC.00924-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mathee K. 2018. Forensic investigation into the origin of Pseudomonas aeruginosa PA14 - old but not lost. J Med Microbiol 67:1019–1021. doi: 10.1099/jmm.0.000778 [DOI] [PubMed] [Google Scholar]
- 38. Grace A, Sahu R, Owen DR, Dennis VA. 2022. Pseudomonas aeruginosa reference strains PAO1 and PA14: a genomic, phenotypic, and therapeutic review. Front Microbiol 13:1023523. doi: 10.3389/fmicb.2022.1023523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chandler CE, Horspool AM, Hill PJ, Wozniak DJ, Schertzer JW, Rasko DA, Ernst RK. 2019. Genomic and phenotypic diversity among ten laboratory isolates of Pseudomonas aeruginosa PAO1. J Bacteriol 201:e00595-18. doi: 10.1128/JB.00595-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kasetty S, Katharios-Lanwermeyer S, O’Toole GA, Nadell CD. 2021. Differential surface competition and biofilm invasion strategies of Pseudomonas aeruginosa PA14 and PAO1. J Bacteriol 203:e0026521. doi: 10.1128/JB.00265-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Holloway BW. 1955. Genetic recombination in Pseudomonas aeruginosa. Microbiol (Reading, Engl) 13:572–581. doi: 10.1099/00221287-13-3-572 [DOI] [PubMed] [Google Scholar]
- 42. He J, Baldini RL, Déziel E, Saucier M, Zhang Q, Liberati NT, Lee D, Urbach J, Goodman HM, Rahme LG. 2004. The broad host range pathogen Pseudomonas aeruginosa strain PA14 carries two pathogenicity islands harboring plant and animal virulence genes. Proc Natl Acad Sci USA 101:2530–2535. doi: 10.1073/pnas.0304622101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee DG, Urbach JM, Wu G, Liberati NT, Feinbaum RL, Miyata S, Diggins LT, He J, Saucier M, Déziel E, Friedman L, Li L, Grills G, Montgomery K, Kucherlapati R, Rahme LG, Ausubel FM. 2006. Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol 7:R90. doi: 10.1186/gb-2006-7-10-r90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Forti F, Roach DR, Cafora M, Pasini ME, Horner DS, Fiscarelli EV, Rossitto M, Cariani L, Briani F, Debarbieux L, Ghisotti D. 2018. Design of a broad-range bacteriophage cocktail that reduces Pseudomonas aeruginosa biofilms and treats acute infections in two animal models. Antimicrob Agents Chemother 62:e02573-17. doi: 10.1128/AAC.02573-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Naknaen A, Samernate T, Wannasrichan W, Surachat K, Nonejuie P, Chaikeeratisak V. 2023. Combination of genetically diverse Pseudomonas phages enhances the cocktail efficiency against bacteria. Sci Rep 13:8921. doi: 10.1038/s41598-023-36034-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Amgarten D, Martins LF, Lombardi KC, Antunes LP, de Souza APS, Nicastro GG, Kitajima EW, Quaggio RB, Upton C, Setubal JC, da Silva AM. 2017. Three novel Pseudomonas phages isolated from composting provide insights into the evolution and diversity of tailed phages. BMC Genomics 18:346. doi: 10.1186/s12864-017-3729-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Phee A, Bondy-Denomy J, Kishen A, Basrani B, Azarpazhooh A, Maxwell K. 2013. Efficacy of bacteriophage treatment on Pseudomonas aeruginosa biofilms. J Endod 39:364–369. doi: 10.1016/j.joen.2012.10.023 [DOI] [PubMed] [Google Scholar]
- 48. Cook R, Brown N, Redgwell T, Rihtman B, Barnes M, Clokie M, Stekel DJ, Hobman J, Jones MA, Millard A. 2021. INfrastructure for a PHAge REference Database: identification of large-scale biases in the current collection of cultured phage genomes. Phage (New Rochelle) 2:214–223. doi: 10.1089/phage.2021.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ceyssens P-J, Lavigne R. 2010. Bacteriophages of Pseudomonas. Future Microbiol 5:1041–1055. doi: 10.2217/fmb.10.66 [DOI] [PubMed] [Google Scholar]
- 50. Camargo AP, Nayfach S, Chen I-M, Palaniappan K, Ratner A, Chu K, Ritter SJ, Reddy TBK, Mukherjee S, Schulz F, Call L, Neches RY, Woyke T, Ivanova NN, Eloe-Fadrosh EA, Kyrpides NC, Roux S. 2023. IMG/VR v4: an expanded database of uncultivated virus genomes within a framework of extensive functional, taxonomic, and ecological metadata. Nucleic Acids Res 51:D733–D743. doi: 10.1093/nar/gkac1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Benler S, Koonin EV. 2021. Fishing for phages in metagenomes: what do we catch, what do we miss? Curr Opin Virol 49:142–150. doi: 10.1016/j.coviro.2021.05.008 [DOI] [PubMed] [Google Scholar]
- 52. Turner D, Shkoporov AN, Lood C, Millard AD, Dutilh BE, Alfenas-Zerbini P, van Zyl LJ, Aziz RK, Oksanen HM, Poranen MM, et al. 2023. Abolishment of morphology-based taxa and change to binomial species names: 2022 taxonomy update of the ICTV bacterial viruses subcommittee. Arch Virol 168:74. doi: 10.1007/s00705-022-05694-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schoch CL, Ciufo S, Domrachev M, Hotton CL, Kannan S, Khovanskaya R, Leipe D, Mcveigh R, O’Neill K, Robbertse B, Sharma S, Soussov V, Sullivan JP, Sun L, Turner S, Karsch-Mizrachi I. 2020. NCBI taxonomy: a comprehensive update on curation, resources and tools. Database (Oxf) 2020. doi: 10.1093/database/baaa062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Essoh C, Latino L, Midoux C, Blouin Y, Loukou G, Nguetta S-P, Lathro S, Cablanmian A, Kouassi AK, Vergnaud G, Pourcel C. 2015. Investigation of a large collection of Pseudomonas aeruginosa bacteriophages collected from a single environmental source in Abidjan, Côte d'Ivoire. PLoS One 10:e0130548. doi: 10.1371/journal.pone.0130548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wittmann J, Turner D, Millard AD, Mahadevan P, Kropinski AM, Adriaenssens EM. 2020. From orphan phage to a proposed new family–the diversity of N4-like viruses. Antibiotics (Basel) 9:663. doi: 10.3390/antibiotics9100663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maciejewska B, Olszak T, Drulis-Kawa Z. 2018. Applications of bacteriophages versus phage enzymes to combat and cure bacterial infections: an ambitious and also a realistic application? Appl Microbiol Biotechnol 102:2563–2581. doi: 10.1007/s00253-018-8811-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Paysan-Lafosse T, Blum M, Chuguransky S, Grego T, Pinto BL, Salazar GA, Bileschi ML, Bork P, Bridge A, Colwell L, et al. 2023. InterPro in 2022. Nucleic Acids Res 51:D418–D427. doi: 10.1093/nar/gkac993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Latka A, Maciejewska B, Majkowska-Skrobek G, Briers Y, Drulis-Kawa Z. 2017. Bacteriophage-encoded virion-associated enzymes to overcome the carbohydrate barriers during the infection process. Appl Microbiol Biotechnol 101:3103–3119. doi: 10.1007/s00253-017-8224-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rodríguez-Rubio L, Gutiérrez D, Donovan DM, Martínez B, Rodríguez A, García P. 2016. Phage lytic proteins: biotechnological applications beyond clinical antimicrobials. Crit Rev Biotechnol 36:542–552. doi: 10.3109/07388551.2014.993587 [DOI] [PubMed] [Google Scholar]
- 60. Abril AG, Carrera M, Notario V, Sánchez-Pérez Á, Villa TG. 2022. The use of bacteriophages in biotechnology and recent insights into proteomics. Antibiotics (Basel) 11:653. doi: 10.3390/antibiotics11050653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Santos SB, Costa AR, Carvalho C, Nóbrega FL, Azeredo J. 2018. Exploiting bacteriophage proteomes: the hidden biotechnological potential. Trends Biotechnol 36:966–984. doi: 10.1016/j.tibtech.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 62. Costa AR, van den Berg DF, Esser JQ, Muralidharan A, van den Bossche H, Bonilla BE, van der Steen BA, Haagsma AC, Fluit AC, Nobrega FL, Haas P-J, Brouns SJJ. 2024. Accumulation of defense systems in phage-resistant strains of Pseudomonas aeruginosa. Sci Adv 10:eadj0341. doi: 10.1126/sciadv.adj0341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bondy-Denomy J, Qian J, Westra ER, Buckling A, Guttman DS, Davidson AR, Maxwell KL. 2016. Prophages mediate defense against phage infection through diverse mechanisms. ISME J 10:2854–2866. doi: 10.1038/ismej.2016.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Craig L, Forest KT, Maier B. 2019. Type IV pili: dynamics, biophysics and functional consequences. Nat Rev Microbiol 17:429–440. doi: 10.1038/s41579-019-0195-4 [DOI] [PubMed] [Google Scholar]
- 65. Hao Y, Murphy K, Lo RY, Khursigara CM, Lam JS. 2015. Single-nucleotide polymorphisms found in the migA and wbpX glycosyltransferase genes account for the intrinsic lipopolysaccharide defects exhibited by Pseudomonas aeruginosa PA14. J Bacteriol 197:2780–2791. doi: 10.1128/JB.00337-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Horna G, Quezada K, Ramos S, Mosqueda N, Rubio M, Guerra H, Ruiz J. 2019. Specific type IV pili groups in clinical isolates of Pseudomonas aeruginosa. Int Microbiol 22:131–141. doi: 10.1007/s10123-018-00035-3 [DOI] [PubMed] [Google Scholar]
- 67. Bogiel T, Depka D, Kruszewski S, Rutkowska A, Kanarek P, Rzepka M, Leitão JH, Deptuła A, Gospodarek-Komkowska E. 2023. Comparison of virulence-factor-encoding genes and genotype distribution amongst clinical Pseudomonas aeruginosa strains. Int J Mol Sci 24:1269. doi: 10.3390/ijms24021269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hyman P. 2019. Phages for phage therapy: isolation, characterization, and host range breadth. Pharmaceuticals (Basel) 12:35. doi: 10.3390/ph12010035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rahme LG, Stevens EJ, Wolfort SF, Shao J, Tompkins RG, Ausubel FM. 1995. Common virulence factors for bacterial pathogenicity in plants and animals. Science 268:1899–1902. doi: 10.1126/science.7604262 [DOI] [PubMed] [Google Scholar]
- 70. Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, et al. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature New Biol 406:959–964. doi: 10.1038/35023079 [DOI] [PubMed] [Google Scholar]
- 71. Pelzek AJ, Schuch R, Schmitz JE, Fischetti VA. 2013. Isolation, culture, and characterization of bacteriophages. CP Ess Lab Tech 7:1–28. doi: 10.1002/9780470089941.et0404s07 [DOI] [Google Scholar]
- 72. Azeredo J, Sillankorva S. 2018. Bacteriophage therapy: from lab to clinical practice. Humana Press, New York, NY. [Google Scholar]
- 73. Luong T, Salabarria A-C, Edwards RA, Roach DR. 2020. Standardized bacteriophage purification for personalized phage therapy. Nat Protoc 15:2867–2890. doi: 10.1038/s41596-020-0346-0 [DOI] [PubMed] [Google Scholar]
- 74. Abedon ST. 2018. Basic phage mathematics, p 3–30. In Clokie MRJ, Kropinski A, Lavigne R (ed), Bacteriophages methods and protocols. Humana Press, New York. [DOI] [PubMed] [Google Scholar]
- 75. Mirzaei MK, Nilsson AS. 2015. Isolation of phages for phage therapy: a comparison of spot tests and efficiency of plating analyses for determination of host range and efficacy. PLoS One 10:e0127606. doi: 10.1371/journal.pone.0127606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature New Biol 227:680–685. doi: 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- 77. MacCoss MJ, Wu CC, Yates JR. 2002. Probability-based validation of protein identifications using a modified SEQUEST algorithm. Anal Chem 74:5593–5599. doi: 10.1021/ac025826t [DOI] [PubMed] [Google Scholar]
- 78. Perez-Riverol Y, Bai J, Bandla C, García-Seisdedos D, Hewapathirana S, Kamatchinathan S, Kundu DJ, Prakash A, Frericks-Zipper A, Eisenacher M, Walzer M, Wang S, Brazma A, Vizcaíno JA. 2022. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res 50:D543–D552. doi: 10.1093/nar/gkab1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hernandez-Montelongo J, Nicastro GG, Pereira T de O, Zavarize M, Beppu MM, Macedo WAA, Baldini RL, Cotta MA. 2021. Antibacterial effect of hyaluronan/chitosan nanofilm in the initial adhesion of Pseudomonas aeruginosa wild type, and IV pili and LPS mutant strains. Surf Interf 26:101415. doi: 10.1016/j.surfin.2021.101415 [DOI] [Google Scholar]
- 80. Newman JR, Fuqua C. 1999. Broad-host-range expression vectors that carry the L-arabinose-inducible Escherichia coli araBAD promoter and the araC regulator. Gene 227:197–203. doi: 10.1016/S0378-1119(98)00601-5 [DOI] [PubMed] [Google Scholar]
- 81. Jeong JY, Yim HS, Ryu JY, Lee HS, Lee JH, Seen DS, Kang SG. 2012. One-step sequence- and ligation-independent cloning as a rapid and versatile cloning method for functional genomics studies. Appl Environ Microbiol 78:5440–5443. doi: 10.1128/AEM.00844-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Beeton ML, Alves DR, Enright MC, Jenkins ATA. 2015. Assessing phage therapy against Pseudomonas aeruginosa using a Galleria mellonella infection model. Int J Antimicrob Agents 46:196–200. doi: 10.1016/j.ijantimicag.2015.04.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
One-step growth curve for phage ZC01.
Time-killing curves of P. aeruginosa PA14 exposed to phages ZC01 and ZC03.
ZC01 and ZC03 phage particles subjected to SDS-PAGE.
Tables S1 and S2.
Host range of phages ZC01 and ZC03 on 69 clinical or environmental P. aeruginosa isolates.
An accounting of the reviewer comments and feedback.