Abstract
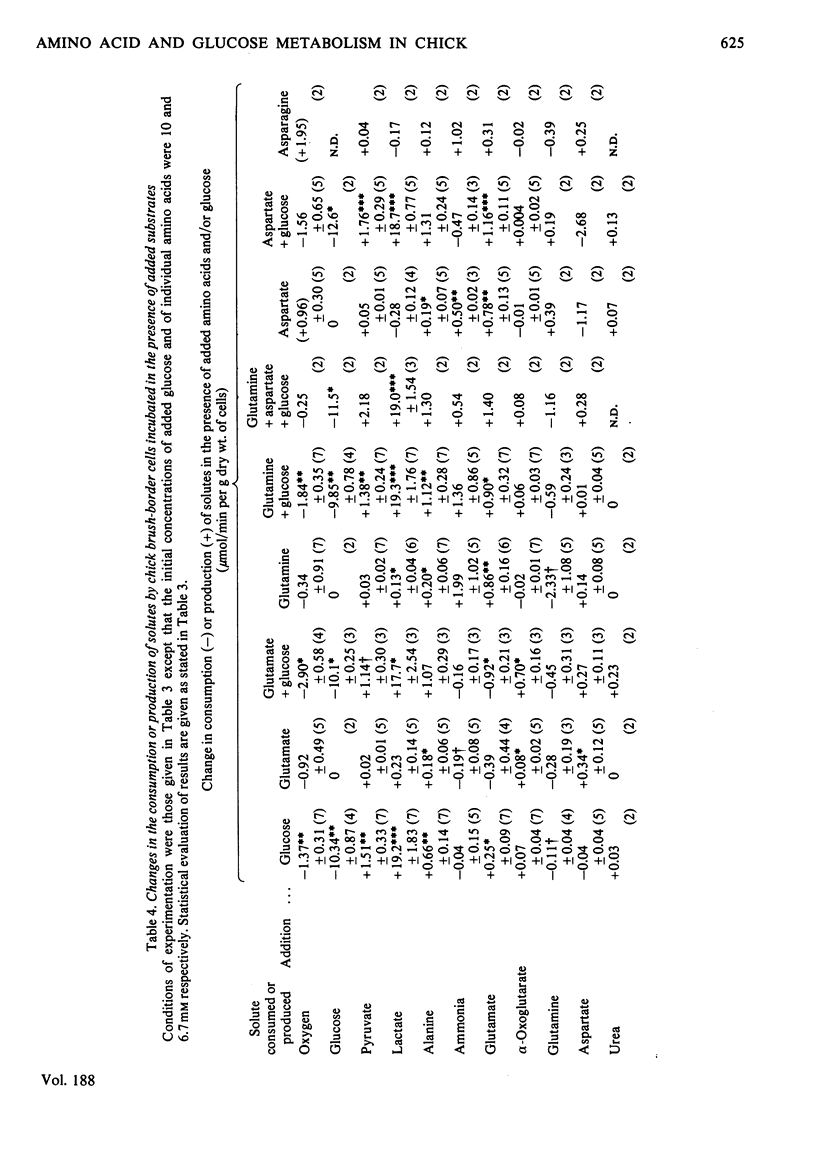
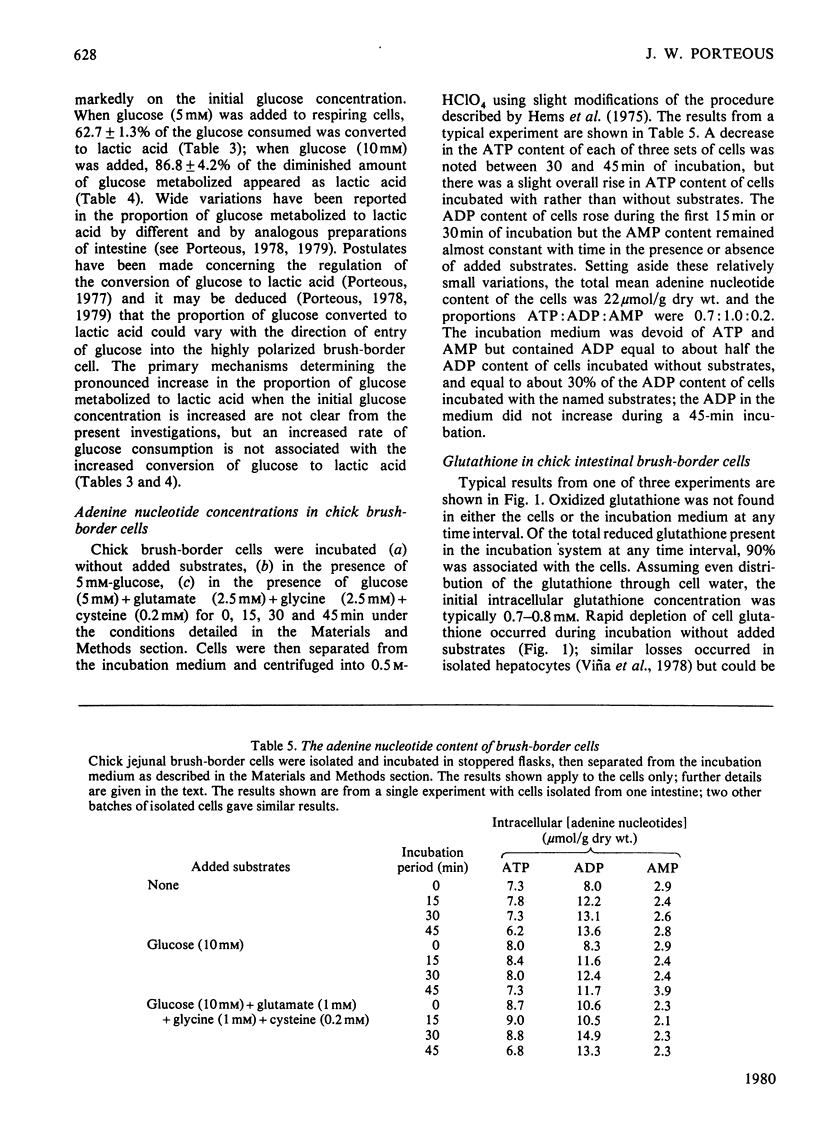
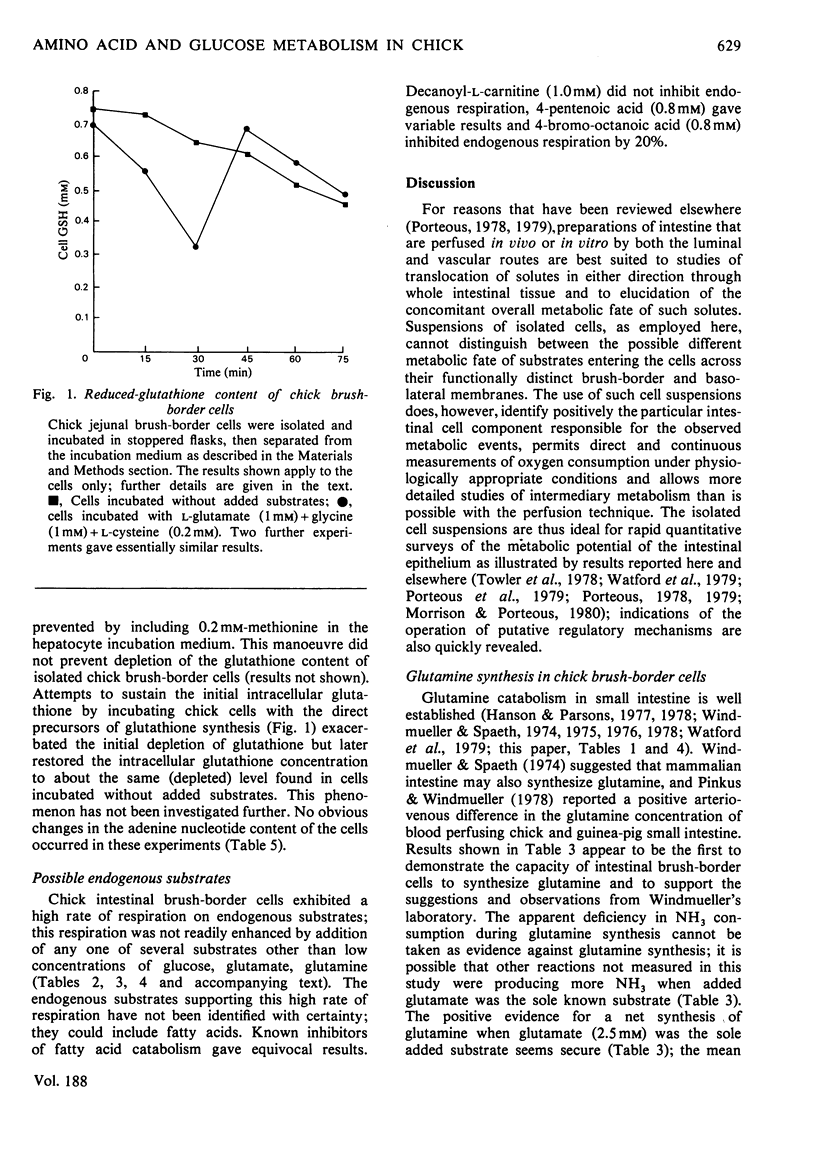
1. Suspensions of isolated chick jejunal columnar absorptive (brush-border) cells respired on endogenous substrates at a rate 40% higher than that shown by rat brush-border cells. 2. Added d-glucose (5 or 10mm), l-glutamine (2.5mm) and l-glutamate (2.5mm) were the only individual substrates which stimulated respiration by chick cells; l-aspartate (2.5 or 6.7mm), glutamate (6.7mm), glutamine (6.7mm), l-alanine (1 or 10mm), pyruvate (1 or 2mm), l-lactate (5 or 10mm), butyrate (10mm) and oleate (1mm) did not stimulate chick cell respiration; l-asparagine (6.7mm) inhibited slightly; glucose (5mm) stimulated more than did 10mm-glucose. 3. Acetoacetate (10mm) and d-3-hydroxybutyrate (10mm) were rapidly consumed but, in contrast to rat brush-border cells, did not stimulate respiration. 4. Glucose (10mm) was consumed more slowly than 5mm-glucose; the dominant product of glucose metabolism during vigorous respiration was lactate; the proportion of glucose converted to lactate was greater with 10mm- than with 5mm-glucose. 5. Glutamate and aspartate consumption rates decreased, and alanine and glutamine consumption rates increased when their initial concentrations were raised from 2.5 to 6.7 or 10mm. 6. The metabolic fate of glucose was little affected by concomitant metabolism of any one of aspartate, glutamate or glutamine except for an increased production of alanine; the glucose-stimulated respiration rate was unaffected by concomitant metabolism of these individual amino acids. 7. Chick cells produced very little alanine from aspartate and, in contrast to rat cells, likewise produced very little alanine from glutamate or glutamine; in chick cells alanine appeared to be predominantly a product of transmination of pyruvate derived from glucose metabolism. 8. In chick cells, glutamate and glutamine were formed from aspartate (2.5 or 6.7mm); aspartate and glutamine were formed from glutamate (2.5mm) but only aspartate from 6.7mm-glutamate; glutamate was the dominant product formed from glutamine (6.7mm) but aspartate only was formed from 2.5mm-glutamine. 9. Chick brush-border cells can thus both catabolize and synthesize glutamine; glutamine synthesis is always diminished by concomitant metabolism of glucose, presumably by allosteric inhibition of glutamine synthetase by alanine. 10. Proline was formed from glutamine (2.5mm) but not from glutamine (2.5mm)+glucose (5mm) and not from 2.5mm-glutamate; ornithine was formed from glutamine (2.5mm)+glucose (5.0mm) but not from glutamine alone; serine was formed from glutamine (2.5mm)+glucose (5mm) and from these two substrates plus aspartate (2.5mm). 11. Total intracellular adenine nucleotides (22μmol/g dry wt.) remained unchanged during incubation of chick cells with glucose. 12. Intracellular glutathione (0.7–0.8mm) was depleted by 40% during incubation of respiring chick cells without added substrates for 75min at 37°C; partial restoration of the lost glutathione was achieved by incubating cells with l-glutamate+l-cysteine+glycine.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hanson P. J., Parsons D. S. Factors affecting the utilization of ketone bodies and other substrates by rat jejunum: effects of fasting and of diabetes. J Physiol. 1978 May;278:55–67. doi: 10.1113/jphysiol.1978.sp012292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson P. J., Parsons D. S. The utilization of glucose and production of lactate by in vitro preparations of rat small intestine: effects of vascular perfusion. J Physiol. 1976 Mar;255(3):775–795. doi: 10.1113/jphysiol.1976.sp011307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson P. J., Parsons S. Metabolism and transport of glutamine and glucose in vascularly perfused small intestine rat. Biochem J. 1977 Sep 15;166(3):509–519. doi: 10.1042/bj1660509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems R., Lund P., Krebs H. A. Rapid separation of isolated hepatocytes or similar tissue fragments for analysis of cell constituents. Biochem J. 1975 Jul;150(1):47–50. doi: 10.1042/bj1500047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A., Tate S. S. Glutathione and related gamma-glutamyl compounds: biosynthesis and utilization. Annu Rev Biochem. 1976;45:559–604. doi: 10.1146/annurev.bi.45.070176.003015. [DOI] [PubMed] [Google Scholar]
- Morrison A., Porteous J. W. Changes in the synthesis of ribosomal ribonucleic acid and of poly(A)-containing ribonucleic acid during the differentiation of intestinal epithelial cells in the rat and in the chick. Biochem J. 1980 Jun 15;188(3):609–618. doi: 10.1042/bj1880609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEPTUNE E. M., Jr RESPIRATION AND OXIDATION OF VARIOUS SUBSTRATES BY ILEUM IN VITRO. Am J Physiol. 1965 Aug;209:329–332. doi: 10.1152/ajplegacy.1965.209.2.329. [DOI] [PubMed] [Google Scholar]
- Pinkus L. M., Windmueller H. G. Phosphate-dependent glutaminase of small intestine: localization and role in intestinal glutamine metabolism. Arch Biochem Biophys. 1977 Aug;182(2):506–517. doi: 10.1016/0003-9861(77)90531-8. [DOI] [PubMed] [Google Scholar]
- Porteous J. W., Furneaux H. M., Pearson C. K., Lake C. M., Morrison A. Poly(adenosine diphosphate ribose) synthetase activity in nuclei of dividing and of non-dividing but differentiating intestinal epithelial cells. Biochem J. 1979 Jun 15;180(3):455–461. doi: 10.1042/bj1800455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteous J. W. Glucose as a fuel for small intestine. Biochem Soc Trans. 1978;6(3):534–539. doi: 10.1042/bst0060534. [DOI] [PubMed] [Google Scholar]
- Pritchard P. J., Porteous J. W. Steady-state metabolism and transport of D-glucose by rat small intestine in vitro. Biochem J. 1977 Apr 15;164(1):1–14. doi: 10.1042/bj1640001a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler C. M., Pugh-Humphreys G. P., Porteous J. W. Characterization of columnar absorptive epithelial cells isolated from rat jejunum. J Cell Sci. 1978 Feb;29:53–75. doi: 10.1242/jcs.29.1.53. [DOI] [PubMed] [Google Scholar]
- Viña J., Hems R., Krebs H. A. Maintenance of glutathione content is isolated hepatocyctes. Biochem J. 1978 Mar 15;170(3):627–630. doi: 10.1042/bj1700627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watford M., Lund P., Krebs H. A. Isolation and metabolic characteristics of rat and chicken enterocytes. Biochem J. 1979 Mar 15;178(3):589–596. doi: 10.1042/bj1780589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Identification of ketone bodies and glutamine as the major respiratory fuels in vivo for postabsorptive rat small intestine. J Biol Chem. 1978 Jan 10;253(1):69–76. [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Intestinal metabolism of glutamine and glutamate from the lumen as compared to glutamine from blood. Arch Biochem Biophys. 1975 Dec;171(2):662–672. doi: 10.1016/0003-9861(75)90078-8. [DOI] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Metabolism of absorbed aspartate, asparagine, and arginine by rat small intestine in vivo. Arch Biochem Biophys. 1976 Aug;175(2):670–676. doi: 10.1016/0003-9861(76)90558-0. [DOI] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Uptake and metabolism of plasma glutamine by the small intestine. J Biol Chem. 1974 Aug 25;249(16):5070–5079. [PubMed] [Google Scholar]


