Abstract
The total weight percentage glycosaminoglycan content of rat liber was found to increase by 50% in the first 30 h after partial hepatectomy. The content returned to near normal by the third day, but then increased again to a second maximum at 5-6 days, only to gradually decline to normal by the ninth day, when regeneration was nearly complete. This biphasic pattern was most marked in the chondroitin sulphate A/C component, with a 6-fold increase by the sixth day. Dermatan sulphate showed the same temporal trend, whereas heparan sulphate remained relatively unaltered. No such changes were detected in the livers of rats subjected to sham operation. The possible molecular mechanisms underlying the apparent link between cellular glycosaminoglycan content and proliferative tendency are discussed.
Full text
PDF

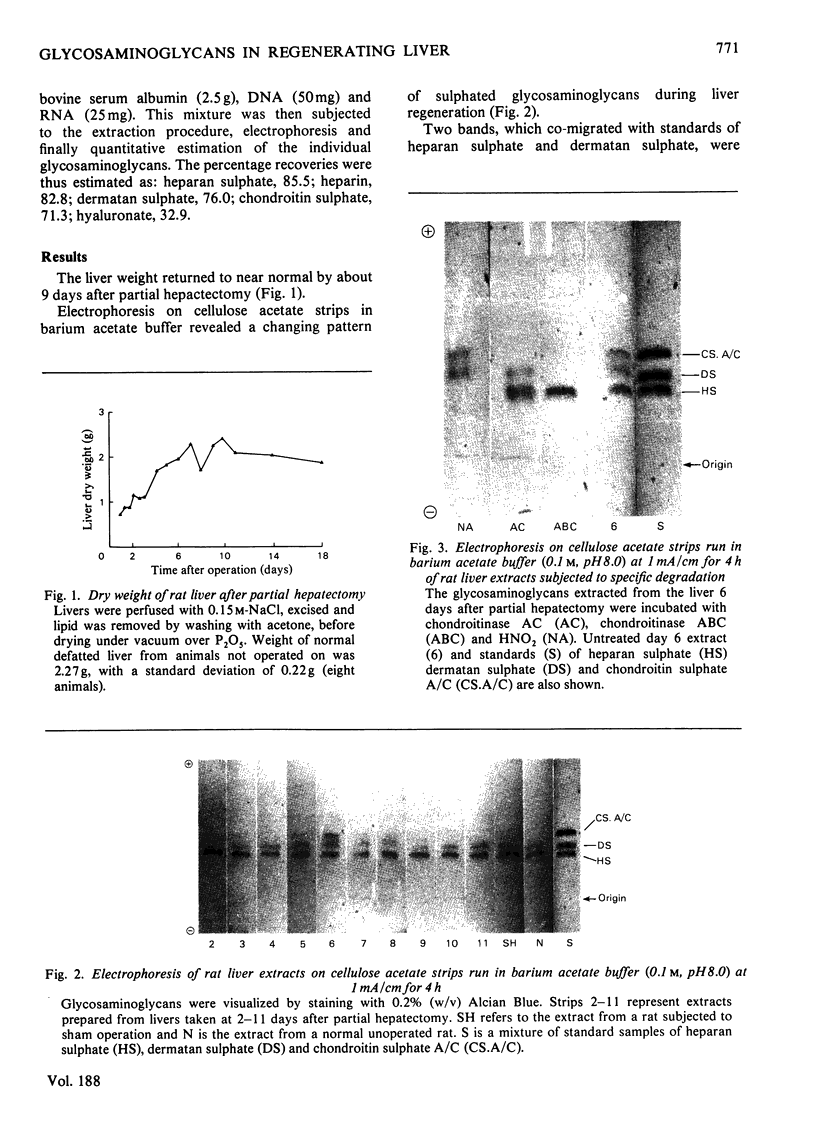
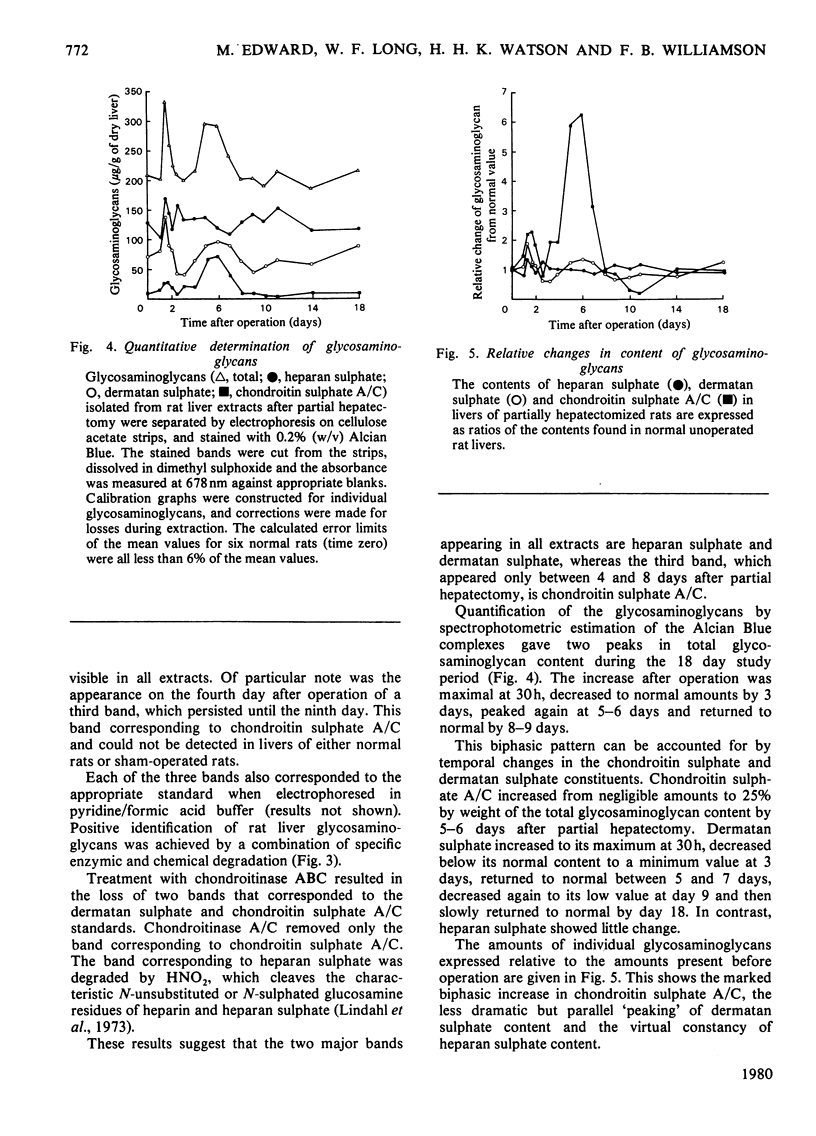

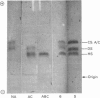
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augusti-Tocco G., Chiarugi V. P. Surface glycosaminoglycans as a differentiation cofactor in neuroblastoma cell cultures. Cell Differ. 1976 Oct;5(3):161–170. doi: 10.1016/0045-6039(76)90018-x. [DOI] [PubMed] [Google Scholar]
- BUDDECKE E., DRZENIEK R. [Stability constants of the calcium complexes of acid mucopolysaccharides]. Hoppe Seylers Z Physiol Chem. 1962 May 4;327:49–64. doi: 10.1515/bchm2.1962.327.1.49. [DOI] [PubMed] [Google Scholar]
- Balk S. D., Whitfield J. F., Youdale T., Braun A. C. Roles of calcium, serum, plasma, and folic acid in the control of proliferation of normal and Rous sarcoma virus-infected chicken fibroblasts. Proc Natl Acad Sci U S A. 1973 Mar;70(3):675–679. doi: 10.1073/pnas.70.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C. H., Hassels B. F., Reutter W. G. Galactose metabolism in regenerating rat liver. Biochem J. 1976 Jan 15;154(1):141–147. doi: 10.1042/bj1540141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. The interaction of cyclic nucleotides and calcium in the control of cellular activity. Adv Cyclic Nucleotide Res. 1975;6:1–98. [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Chiarugi V. P., Vannucchi S. Surface heparan sulphate as a control element in eukariotic cells: a working model. J Theor Biol. 1976 Sep 21;61(2):459–475. doi: 10.1016/0022-5193(76)90030-8. [DOI] [PubMed] [Google Scholar]
- Cássaro C. M., Dietrich C. P. Distribution of sulfated mucopolysaccharides in invertebrates. J Biol Chem. 1977 Apr 10;252(7):2254–2261. [PubMed] [Google Scholar]
- Dietrich C. P., Sampaio L. O., Toledo O. M., Cássaro C. M. Cell recognition and adhesiveness: a possible biological role for the sulfated mucopolysaccharides. Biochem Biophys Res Commun. 1977 Mar 21;75(2):329–336. doi: 10.1016/0006-291x(77)91046-4. [DOI] [PubMed] [Google Scholar]
- Kindness G., Long W. F., Williamson F. B., Boyd J. Effects of carrageenans on the aggregation of human blood platelets. Thromb Res. 1979;15(1-2):3–15. doi: 10.1016/0049-3848(79)90047-1. [DOI] [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Jansson L., Hallén A. Biosynthesis of heparin. II. Formation of sulfamino groups. J Biol Chem. 1973 Oct 25;248(20):7234–7241. [PubMed] [Google Scholar]
- McGowan J. A., Fausto N. Ornithine decarboxylase activity and the onset of deoxyribonucleic acid synthesis in regenerating liver. Biochem J. 1978 Jan 15;170(1):123–127. doi: 10.1042/bj1700123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton D. J., Scott J. E., Whiteman P. The estimation of acid glycosaminoglycan-Alcian blue complexes eluted from electrophoretic strips. Anal Biochem. 1974 Nov;62(1):268–273. doi: 10.1016/0003-2697(74)90386-8. [DOI] [PubMed] [Google Scholar]
- Okamoto Y., Ito E., Akamatsu N. UDP-N-acetylglucosamine-glycoprotein N-acetylglucosaminyltransferase in regenerating rat liver. Biochim Biophys Acta. 1978 Aug 3;542(1):21–27. doi: 10.1016/0304-4165(78)90228-3. [DOI] [PubMed] [Google Scholar]
- Rabes H. M., Brändle H. Synthesis of RNA, protein, and DNA in the liver of normal and hypophysectomized rats after partial hepatectomy. Cancer Res. 1969 Apr;29(4):817–822. [PubMed] [Google Scholar]
- Sampaio L. O., Dietrich C. P., Filho O. G. Changes in sulfated mucopolysaccharide composition of mammalian tissues during growth and in cancer tissues. Biochim Biophys Acta. 1977 Jun 23;498(1):123–131. doi: 10.1016/0304-4165(77)90093-9. [DOI] [PubMed] [Google Scholar]
- Takeuchi J., Sobue M., Sato E., Shamoto M., Miura K. Variation in glycosaminoglycan components of breast tumors. Cancer Res. 1976 Jul;36(7 Pt 1):2133–2139. [PubMed] [Google Scholar]
- Vannucchi S., Del Rosso M., Cella C., Urbano P., Chiarugi V. Surface glycosaminoglycans and calcium distribution in 3T3 cells. Biochem J. 1978 Jan 15;170(1):185–187. doi: 10.1042/bj1700185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Terayama H. Comparison of cell coat acid mucopolysaccharides of normal liver and various ascites hepatoma cells. Cancer Res. 1973 Oct;33(10):2257–2264. [PubMed] [Google Scholar]