Abstract
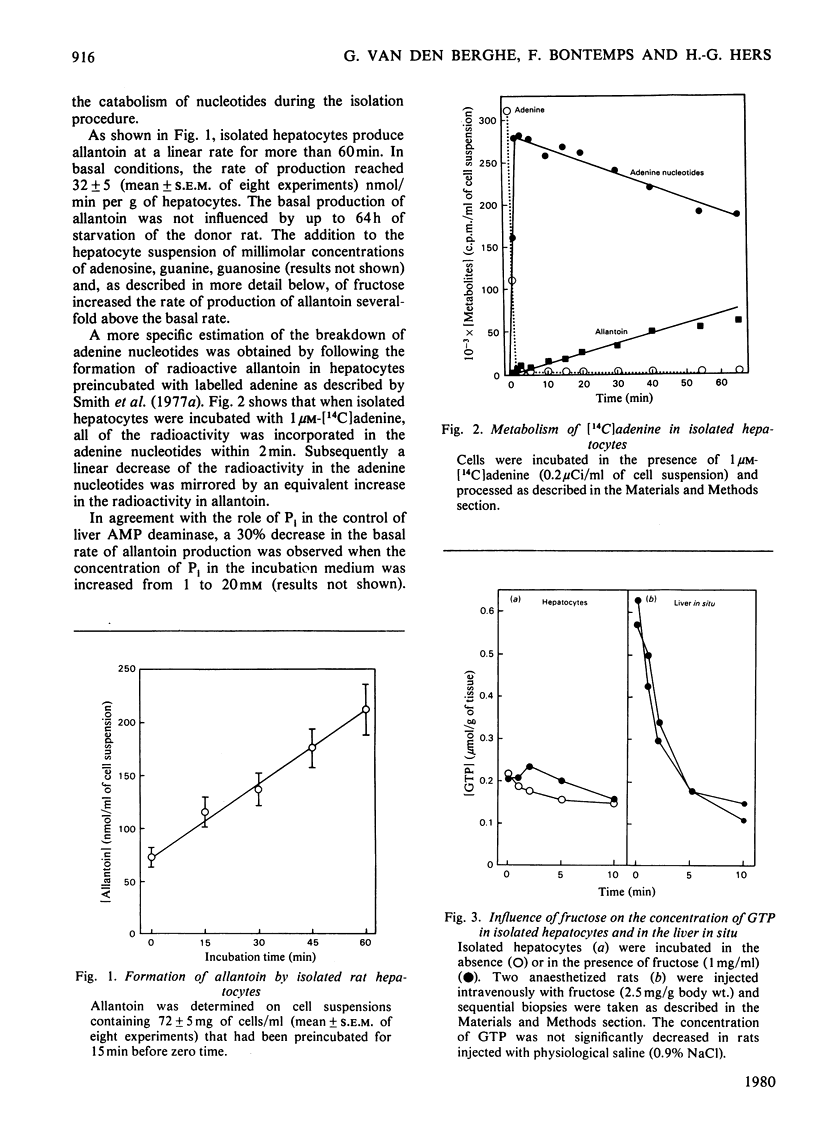
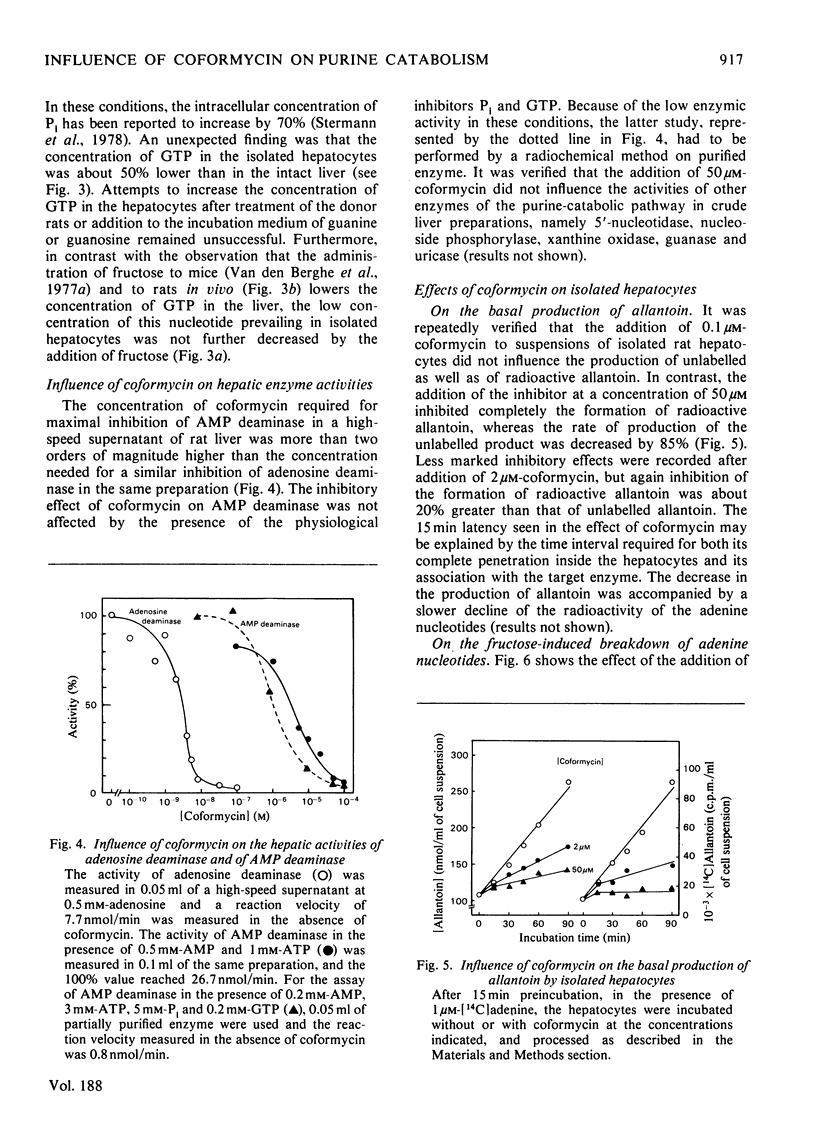
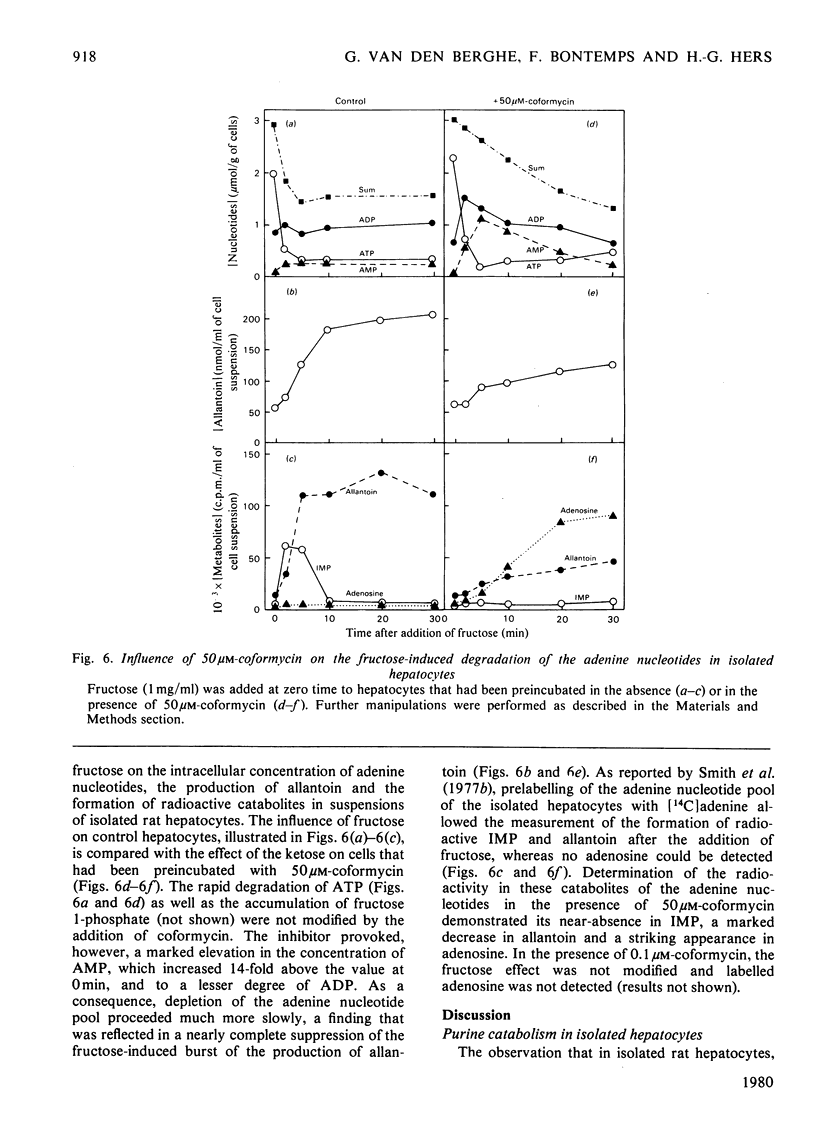
1. The catabolism of purine nucleotides was investigated by both chemical and radiochemical methods in isolated rat hepatocytes, previously incubated with [14C]adenine. The production of allantoin reached 32±5nmol/min per g of cells (mean±s.e.m.) and as much as 30% of the radioactivity incorporated in the adenine nucleotides was lost after 1h. This rate of degradation is severalfold in excess over values previously reported to occur in the liver in vivo. An explanation for this enhancement of catabolism may be the decrease in the concentration of GTP. 2. In a high-speed supernatant of rat liver, adenosine deaminase was maximally inhibited by 0.1μm-coformycin. The activity of AMP deaminase, measured in the presence of its stimulator ATP in the same preparation, as well as the activity of the partially purified enzyme, measured after addition of its physiological inhibitors GTP and Pi, required 50μm-coformycin for maximal inhibition. 3. The production of allantoin by isolated hepatocytes was not influenced by the addition of 0.1μm-coformycin, but was decreased by concentrations of coformycin that were inhibitory for AMP deaminase. With 50μm-coformycin the production of allantoin was decreased by 85% and the formation of radioactive allantoin from [14C]adenine nucleotides was completely suppressed. 4. In the presence of 0.1μm-coformycin or in its absence, the addition of fructose (1mg/ml) to the incubation medium caused a rapid degradation of ATP, without equivalent increase in ADP and AMP, followed by transient increases in IMP and in the rate of production of allantoin; adenosine was not detectable. In the presence of 50μm-coformycin, the fructose-induced breakdown of ATP was not modified, but the depletion of the adenine nucleotide pool proceeded much more slowly and the rate of production of allantoin increased only slightly. No rise in IMP concentration could be detected, but AMP increased manyfold and reached values at which a participation of soluble 5′-nucleotidase in the catabolism of adenine nucleotides is most likely. 5. These results are in agreement with the hypothesis that the formation of allantoin is controlled by AMP deaminase. They constitute further evidence that 5′-nucleotidase is inactive on AMP, unless the concentration of this nucleotide rises to unphysiological values.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal R. P., Parks R. E. Potent inhibition of muscle 5'-AMP deaminase by the nucleoside antibiotics coformycin and deoxycoformycin. Biochem Pharmacol. 1977 Apr 1;26(7):663–666. doi: 10.1016/0006-2952(77)90046-6. [DOI] [PubMed] [Google Scholar]
- Agarwal R. P., Sagar S. M., Parks R. E., Jr Adenosine deaminase from human erythrocytes: purification and effects of adenosine analogs. Biochem Pharmacol. 1975 Mar 15;24(6):693–701. doi: 10.1016/0006-2952(75)90245-2. [DOI] [PubMed] [Google Scholar]
- BENNETT E. L., KRUECKEL B. J. Renewal of nucleotides and nucleic acids in C57 mice studied with adenine-4, 6-14C1. Biochim Biophys Acta. 1955 Aug;17(4):503–514. doi: 10.1016/0006-3002(55)90413-3. [DOI] [PubMed] [Google Scholar]
- Cha S., Agarwal R. P., Parks R. E., Jr Tight-binding inhibitors-II. Non-steady state nature of inhibition of milk xanthine oxidase by allopurinol and alloxanthine and of human erythrocytic adenosine deaminase by coformycin. Biochem Pharmacol. 1975 Dec 1;24(23):2187–2197. doi: 10.1016/0006-2952(75)90051-9. [DOI] [PubMed] [Google Scholar]
- Clifford A. J., Riumallo J. A., Baliga B. S., Munro H. N., Brown P. R. Liver nucleotide metabolism in relation to amino acid supply. Biochim Biophys Acta. 1972 Sep 14;277(3):443–458. doi: 10.1016/0005-2787(72)90087-1. [DOI] [PubMed] [Google Scholar]
- Crabtree G. W., Henderson J. F. Rate-limiting steps in the interconversion of purine ribonucleotides in Ehrlich ascites tumor cells in vitro. Cancer Res. 1971 Jul;31(7):985–991. [PubMed] [Google Scholar]
- Hartwick R. A., Brown P. R. The performance of microparticle chemically-bonded anion-exchange resins in the analysis of nucleotides. J Chromatogr. 1975 Oct 29;112:650–662. doi: 10.1016/s0021-9673(00)99994-1. [DOI] [PubMed] [Google Scholar]
- Hems R., Lund P., Krebs H. A. Rapid separation of isolated hepatocytes or similar tissue fragments for analysis of cell constituents. Biochem J. 1975 Jul;150(1):47–50. doi: 10.1042/bj1500047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson J. F., Brox L., Zombor G., Hunting D., Lomax C. A. Specificity of adenosine deaminase inhibitors. Biochem Pharmacol. 1977 Nov 1;26(21):1967–1972. doi: 10.1016/0006-2952(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Hers H. G., Van Den Berghe G. Enzyme defect in primary gout. Lancet. 1979 Mar 17;1(8116):585–586. doi: 10.1016/s0140-6736(79)91010-9. [DOI] [PubMed] [Google Scholar]
- Hue L., Bontemps F., Hers H. The effects of glucose and of potassium ions on the interconversion of the two forms of glycogen phosphorylase and of glycogen synthetase in isolated rat liver preparations. Biochem J. 1975 Oct;152(1):105–114. doi: 10.1042/bj1520105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIDDLE L., SEEGMILLER J. E., LASTER L. The enzymatic spectrophotometric method for determination of uric acid. J Lab Clin Med. 1959 Dec;54:903–913. [PubMed] [Google Scholar]
- Mäenpä P. H., Raivio K. O., Kekomäki M. P. Liver adenine nucleotides: fructose-induced depletion and its effect on protein synthesis. Science. 1968 Sep 20;161(3847):1253–1254. doi: 10.1126/science.161.3847.1253. [DOI] [PubMed] [Google Scholar]
- NIKIFORUK G., COLOWICK S. P. The purification and properties of 5-adenylic acid deaminase from muscle. J Biol Chem. 1956 Mar;219(1):119–129. [PubMed] [Google Scholar]
- Perheentupa J., Raivio K. Fructose-induced hyperuricaemia. Lancet. 1967 Sep 9;2(7515):528–531. doi: 10.1016/s0140-6736(67)90494-1. [DOI] [PubMed] [Google Scholar]
- Rowe P. B., Wyngaarden J. B. The mechanism of dietary alterations in rat hepatic xanthine oxidase levels. J Biol Chem. 1966 Dec 10;241(23):5571–5576. [PubMed] [Google Scholar]
- SEGAL H. L., BRENNER B. M. 5'-Nucleotidase of rat liver microsomes. J Biol Chem. 1960 Feb;235:471–474. [PubMed] [Google Scholar]
- Sawa T., Fukagawa Y., Homma I., Takeuchi T., Umezawa H. Mode of inhibition of coformycin on adenosine deaminase. J Antibiot (Tokyo) 1967 Jul;20(4):227–231. [PubMed] [Google Scholar]
- Schultz V., Lowenstein J. M. Purine nucleotide cycle. Evidence for the occurrence of the cycle in brain. J Biol Chem. 1976 Jan 25;251(2):485–492. [PubMed] [Google Scholar]
- Setlow B., Burger R., Lowenstein J. M. Adenylate deaminase. I. The effects of adenosine and guanosine triphosphates on activity and the organ distribution of the regulated enzyme. J Biol Chem. 1966 Mar 10;241(5):1244–1245. [PubMed] [Google Scholar]
- Smith C. M., Rovamo L. M., Kekomäki M. P., Raivio K. O. Purine metabolism in isolated rat hepatocytes. Can J Biochem. 1977 Nov;55(11):1134–1139. doi: 10.1139/o77-169. [DOI] [PubMed] [Google Scholar]
- Smith C. M., Rovamo L. M., Raivio K. O. Fructose-induced adenine nucleotide catabolism in isolated rat hepatocytes. Can J Biochem. 1977 Dec;55(12):1237–1240. doi: 10.1139/o77-185. [DOI] [PubMed] [Google Scholar]
- Smith L. D., Kizer D. E. Purification and properties of rat liver AMP deaminase. Biochim Biophys Acta. 1969 Nov 4;191(2):415–424. doi: 10.1016/0005-2744(69)90260-5. [DOI] [PubMed] [Google Scholar]
- Snyder F. F., Henderson J. F. Alternative pathways of deoxyadenosine and adenosine metabolism. J Biol Chem. 1973 Aug 25;248(16):5899–5904. [PubMed] [Google Scholar]
- Stermann R., Wagle S. R., Decker K. Inverse effects of D-galactosamine and inorganic phosphate on glycogenolysis in isolated rat hepatocytes. Eur J Biochem. 1978 Jul 17;88(1):79–85. doi: 10.1111/j.1432-1033.1978.tb12424.x. [DOI] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Woods H. F., Eggleston L. V., Krebs H. A. The cause of hepatic accumulation of fructose 1-phosphate on fructose loading. Biochem J. 1970 Sep;119(3):501–510. doi: 10.1042/bj1190501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn D., Klinger R., Frunder H. Enzymic flux rates within the mononucleotides of the mouse liver. Eur J Biochem. 1969 Dec;11(3):549–553. doi: 10.1111/j.1432-1033.1969.tb00807.x. [DOI] [PubMed] [Google Scholar]
- van den Berghe G., Bronfman M., Vanneste R., Hers H. G. The mechanism of adenosine triphosphate depletion in the liver after a load of fructose. A kinetic study of liver adenylate deaminase. Biochem J. 1977 Mar 15;162(3):601–609. doi: 10.1042/bj1620601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berghe G., van Pottelsberghe C., Hers H. G. A kinetic study of the soluble 5'-nucleotidase of rat liver. Biochem J. 1977 Mar 15;162(3):611–616. doi: 10.1042/bj1620611. [DOI] [PMC free article] [PubMed] [Google Scholar]


