Abstract
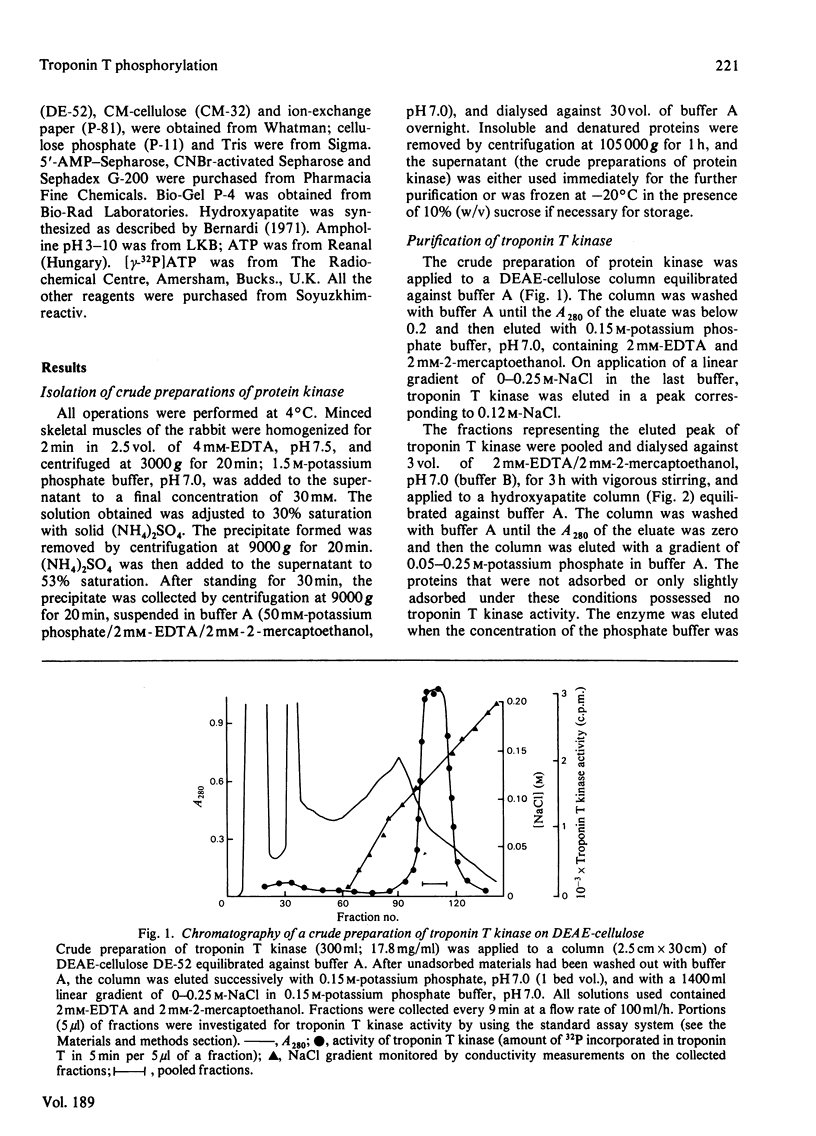
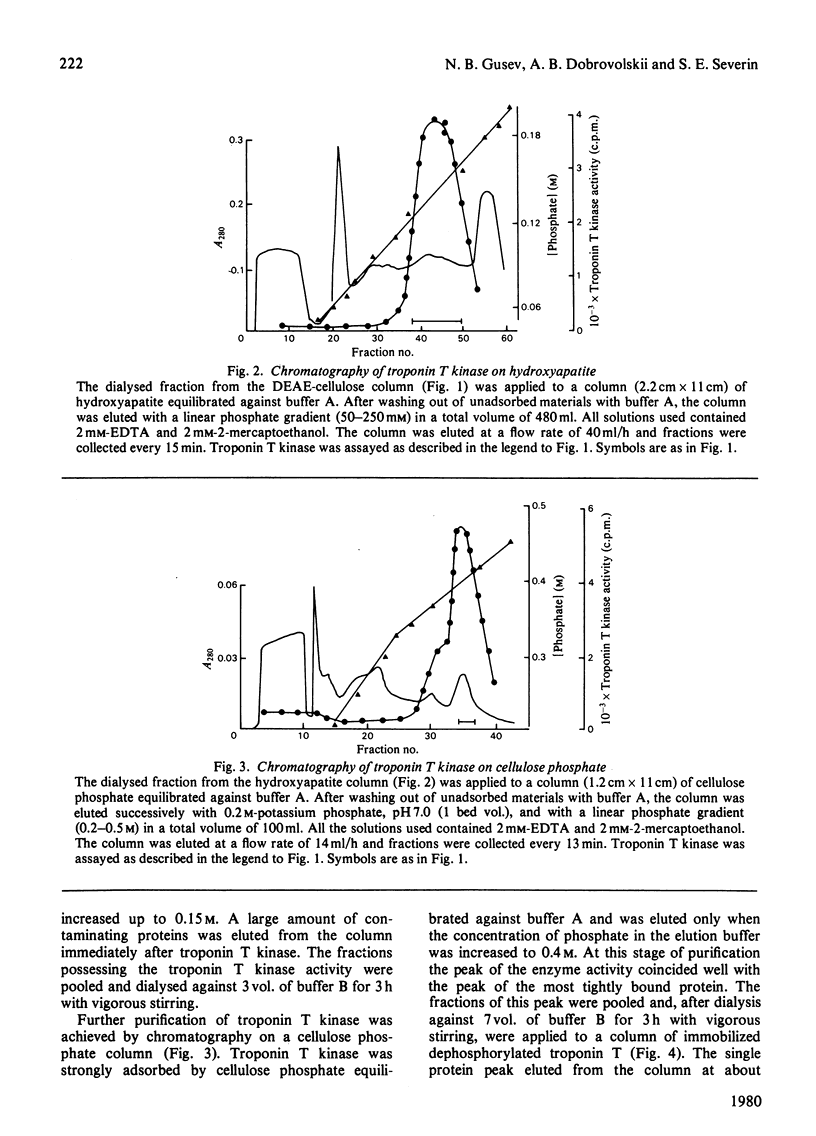
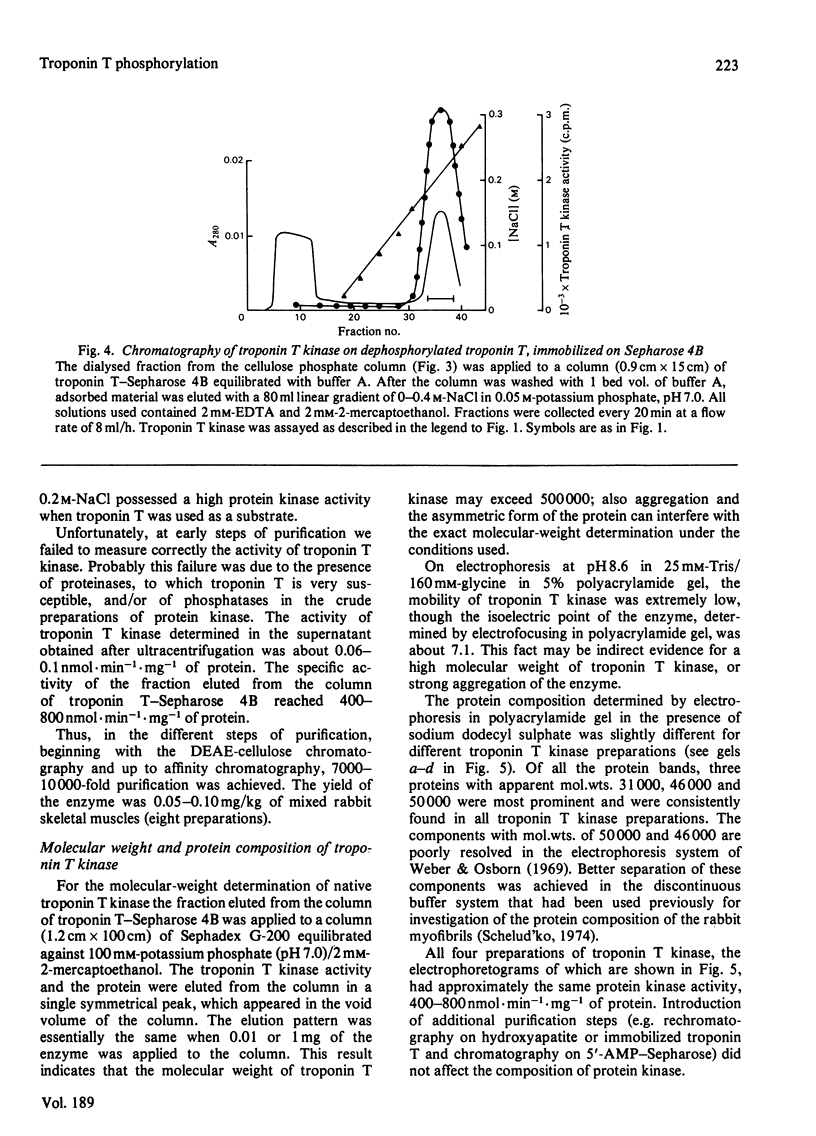
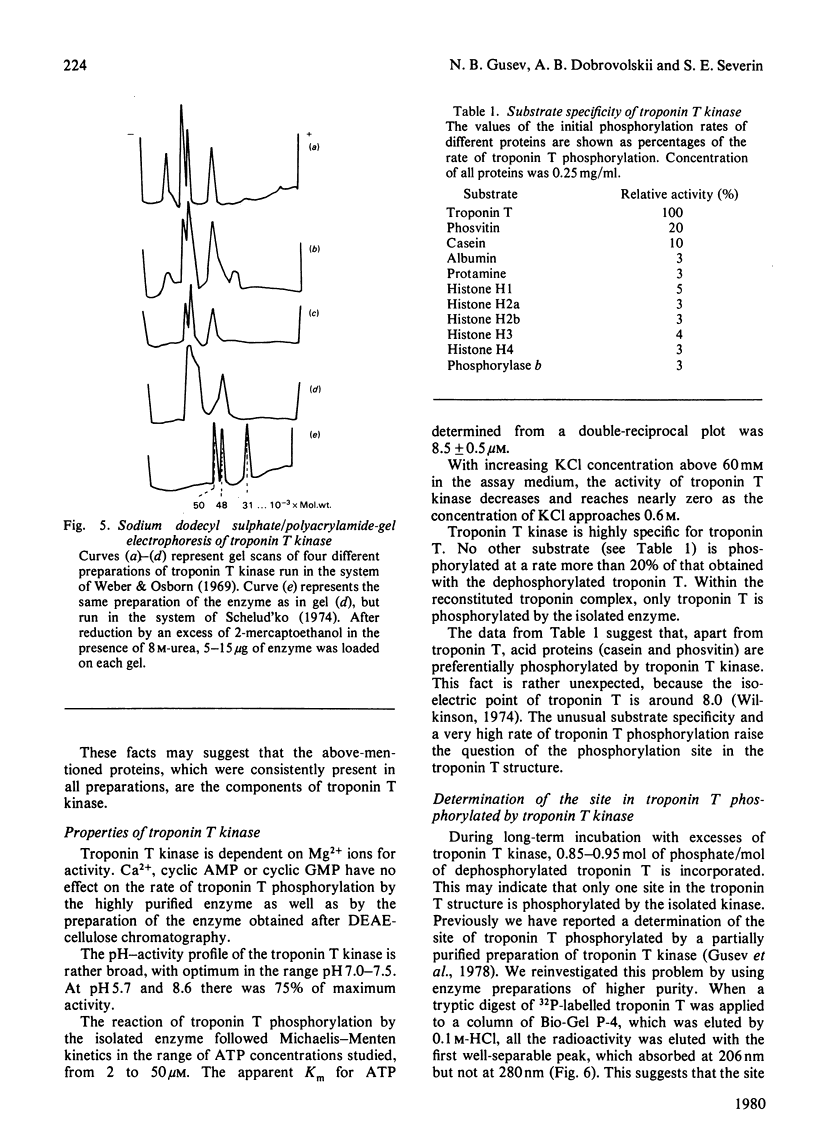
A method for isolation of troponin T kinase (ATP-protein phosphotransferase, EC 2.7.1.37) from rabbit skeletal muscles in proposed. The method gives a 7000-10 000-fold purification and results in an enzyme with specific activity of 400-800-nmol x min-1 x mg-1 of protein. The molecular weight of tropin T kinase as determined by gel filtration exceeds 500 000. Electrophoresis in polyacrylamide gel in the presence of sodium dodecyl sulphate revealed that isolated preparations of the enzyme consisted of at least three distinct proteins with apparent mol.wt. of 50 000, 46 000 and 31 000. The enzyme phosphorylates isolated troponin T at a rate which exceeds the phosphorylation rates of casein, phosvitin, histones, phosphorylase b and protamine 5-30-fold. Within the whole troponin complex, only troponin T is phosphorylated by the enzyme. The enzyme phosphorylates only the N-terminal serine residue of troponin T, i.e. the site that is normally phosphorylated in the whole troponin complex isolated from rabbit skeletal muscles.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cole H. A., Perry S. V. The phosphorylation of troponin I from cardiac muscle. Biochem J. 1975 Sep;149(3):525–533. doi: 10.1042/bj1490525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmus M. E. Stimulation of ascites tumor RNA polymerase II by protein kinase. Biochemistry. 1976 May 4;15(9):1821–1829. doi: 10.1021/bi00654a006. [DOI] [PubMed] [Google Scholar]
- Daile P., Carnegie P. R., Young J. D. Synthetic substrate for cyclic AMP-dependent protein kinase. Nature. 1975 Oct 2;257(5525):416–418. doi: 10.1038/257416a0. [DOI] [PubMed] [Google Scholar]
- Dickneite G., Jennissen H. P., Heilmeyer L. M., Jr Differentiation of two catalytic sites on phosphorylase kinase for phosphorylase b and troponin T phosphorylation. FEBS Lett. 1978 Mar 15;87(2):297–302. doi: 10.1016/0014-5793(78)80355-x. [DOI] [PubMed] [Google Scholar]
- Dobroval'skii A. B., Gusev N. B., Martynov A. V., Severin S. E. Poisk spetsificiheskoi k troponinu T proteinkinazy. Biokhimiia. 1976;41(7):1291–1296. [PubMed] [Google Scholar]
- Dobrovol'skii A. B., Gusev N. B. Rol' Ca-spetsificheskikh uchastkov sviazyvaniia v indutsirovanii konformatsionnykh izmenenii struktury troponina. Biokhimiia. 1978 Sep;43(9):1695–1703. [PubMed] [Google Scholar]
- England P. J. Correlation between contraction and phosphorylation of the inhibitory subunit of troponin in perfused rat heart. FEBS Lett. 1975 Jan 15;50(1):57–60. doi: 10.1016/0014-5793(75)81040-4. [DOI] [PubMed] [Google Scholar]
- England P. J. Studies on the phosphorylation of the inhibitory subunit of troponin during modification of contraction in perfused rat heart. Biochem J. 1976 Nov 15;160(2):295–304. doi: 10.1042/bj1600295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Hasty M. A. Phosvitin kinase from the liver of the rooster. Purification and partial characterization. J Biol Chem. 1973 Sep 25;248(18):6300–6307. [PubMed] [Google Scholar]
- Gusev N. B., Dobrovol'skii A. B., Severin S. E. Tropenin skeletnykh myshts i fosforilir vanie: uchastok troponina T, fosforiliruemyi spetisficheskoi proteinkinazoi. Biokhimiia. 1978 Feb;43(2):365–372. [PubMed] [Google Scholar]
- Huang L. C., Huang C. Rabbit skeletal muscle protein kinase. Conversion from cAMP dependent to independent form by chemical perturbations. Biochemistry. 1975 Jan 14;14(1):18–24. doi: 10.1021/bi00672a004. [DOI] [PubMed] [Google Scholar]
- JOHNS E. W., BUTLER J. A. Further fractionations of histones from calf thymus. Biochem J. 1962 Jan;82:15–18. doi: 10.1042/bj0820015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOUBERT F. J., COOK W. H. Preparation and characterization of phosvitin from hen egg yolk. Can J Biochem Physiol. 1958 Apr;36(4):399–408. [PubMed] [Google Scholar]
- Korovkin B. F., Antipenko A. E. Osobennosti fosforilirovaniia troponina skeletnykh myshts pri nekotorykh formakh patologii myshechnoi sistemy. Biokhimiia. 1979 Feb;44(2):359–363. [PubMed] [Google Scholar]
- Kumon A., Villar-Palasi C. Purification and properties of troponin T kinase from rabbit skeletal muscle. Biochim Biophys Acta. 1979 Feb 9;566(2):305–320. doi: 10.1016/0005-2744(79)90034-2. [DOI] [PubMed] [Google Scholar]
- Mackinlay A. G., West D. W., Manson W. Specific casein phosphorylation by a casein kinase from lactating bovine mammary gland. Eur J Biochem. 1977 Jun 1;76(1):233–243. doi: 10.1111/j.1432-1033.1977.tb11588.x. [DOI] [PubMed] [Google Scholar]
- Moir A. J., Cole H. A., Perry S. V. The phosphorylation sites of troponin T from white skeletal muscle and the effects of interaction with troponin C on their phosphorylation by phosphorylase kinase. Biochem J. 1977 Feb 1;161(2):371–382. doi: 10.1042/bj1610371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir A. J., Perry S. V. The sites of phosphorylation of rabbit cardiac troponin I by adenosine 3':5'-cyclic monophosphate-dependent protein kinase. Effect of interaction with troponin C. Biochem J. 1977 Nov 1;167(2):333–343. doi: 10.1042/bj1670333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstone J. R., Carpenter M. R., Johnson P., Smillie L. B. Amino-acid sequence of tropomyosin-binding component of rabbit skeletal muscle troponin. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1902–1906. doi: 10.1073/pnas.73.6.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V., Cole H. A. Phosphorylation of the "37000 component" of the troponin complex (troponin-t). Biochem J. 1973 Feb;131(2):425–428. doi: 10.1042/bj1310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V., Cole H. A. Phosphorylation of troponin and the effects of interactions between the components of the complex. Biochem J. 1974 Sep;141(3):733–743. doi: 10.1042/bj1410733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires E. M., Perry S. V. Purification and properties of myosin light-chain kinase from fast skeletal muscle. Biochem J. 1977 Oct 1;167(1):137–146. doi: 10.1042/bj1670137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratje E., Heilmeyer L. M.G. Phosphorylation of rabbit muscle troponin and actin by a 3', 5'-c-AMP-dependent protein kinase. FEBS Lett. 1972 Oct 15;27(1):89–93. doi: 10.1016/0014-5793(72)80416-2. [DOI] [PubMed] [Google Scholar]
- Ray K. P., England P. J. Phosphorylation of the inhibitory subunit of troponin and its effect on the calcium dependence of cardiac myofibril adenosine triphosphatase. FEBS Lett. 1976 Nov;70(1):11–16. doi: 10.1016/0014-5793(76)80716-8. [DOI] [PubMed] [Google Scholar]
- Reimann E. M., Walsh D. A., Krebs E. G. Purification and properties of rabbit skeletal muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1986–1995. [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Schaub M. C., Perry S. V. The relaxing protein system of striated muscle. Resolution of the troponin complex into inhibitory and calcium ion-sensitizing factors and their relationship to tropomyosin. Biochem J. 1969 Dec;115(5):993–1004. doi: 10.1042/bj1150993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaro R. J., Moir A. J., Perry S. V. Phosphorylation of troponin I and the inotropic effect of adrenaline in the perfused rabbit heart. Nature. 1976 Aug 12;262(5569):615–617. doi: 10.1038/262615a0. [DOI] [PubMed] [Google Scholar]
- Staprans I., Takahashi H., Russell M. P., Watanabe S. Skeletal and cardiac troponins and their components. J Biochem. 1972 Sep;72(3):723–735. doi: 10.1093/oxfordjournals.jbchem.a129951. [DOI] [PubMed] [Google Scholar]
- Stull J. T., Brostrom C. O., Krebs E. G. Phosphorylation of the inhibitor component of troponin by phosphorylase kinase. J Biol Chem. 1972 Aug 25;247(16):5272–5274. [PubMed] [Google Scholar]
- Varsànyi M., Gröschel-Stewart U., Heilmeyer M. G., Jr Characterization of a Ca2+ -dependent protein kinase in skeletal muscle membranes of I-strain and wild-type mice. Eur J Biochem. 1978 Jun 15;87(2):331–340. doi: 10.1111/j.1432-1033.1978.tb12382.x. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Krebs E. G. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968 Jul 10;243(13):3763–3765. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wilkinson J. M. The preparation and properties of the components of troponin B. Biochim Biophys Acta. 1974 Aug 8;359(2):379–388. doi: 10.1016/0005-2795(74)90238-4. [DOI] [PubMed] [Google Scholar]
- Williams R. E. Phosphorylated sites in substrates of intracellular protein kinases: a common feature in amino acid sequences. Science. 1976 Apr 30;192(4238):473–474. doi: 10.1126/science.1257781. [DOI] [PubMed] [Google Scholar]
- Witt J. J., Roskoski R., Jr Rapid protein kinase assay using phosphocellulose-paper absorption. Anal Biochem. 1975 May 26;66(1):253–258. doi: 10.1016/0003-2697(75)90743-5. [DOI] [PubMed] [Google Scholar]
- Wyborny L. E., Reddy Y. S. Phosphorylated cardiac myofibrils and their effect on ATPase activity. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1175–1179. doi: 10.1016/0006-291x(78)91260-3. [DOI] [PubMed] [Google Scholar]


