Abstract
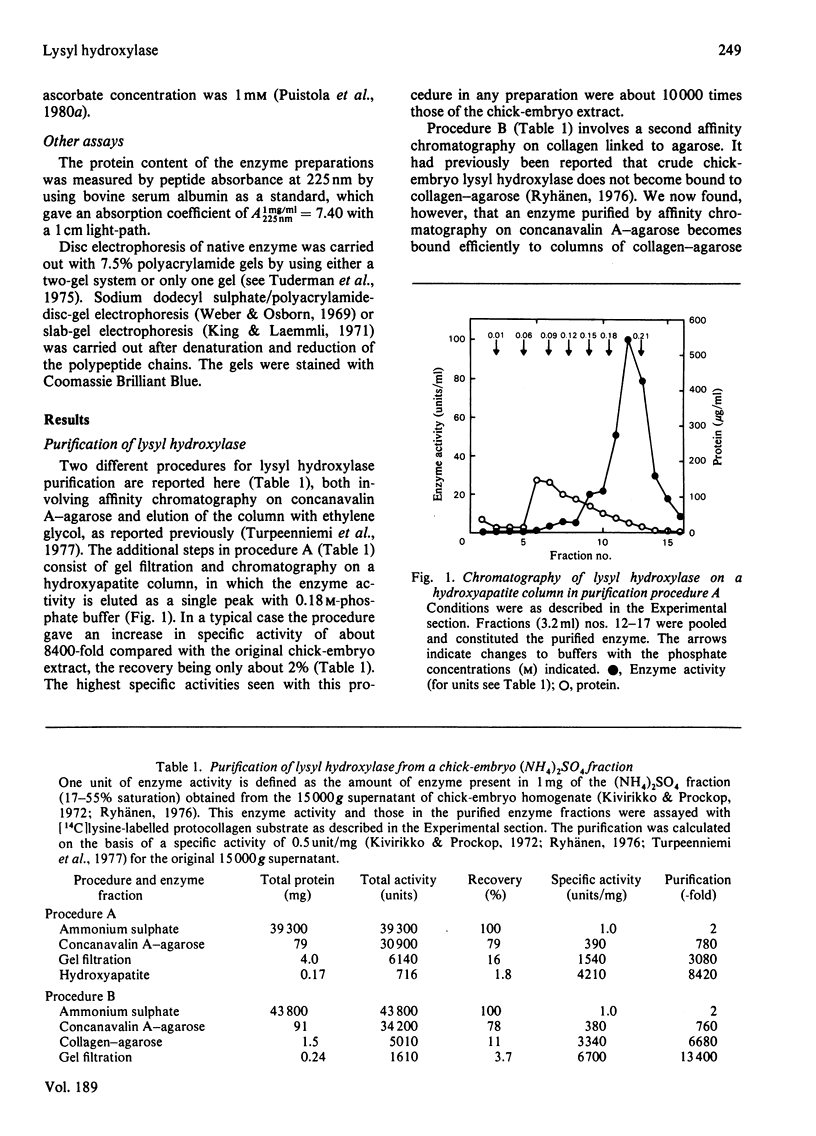
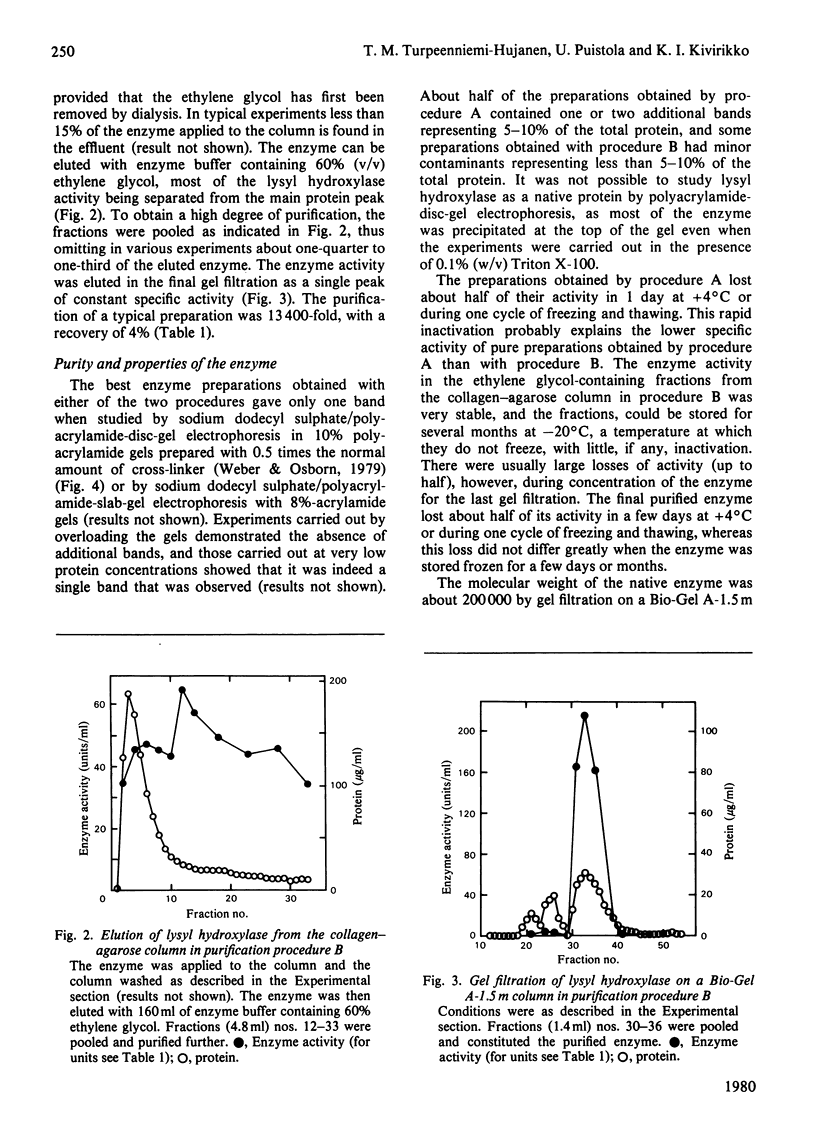
Two procedures are reported for the purification of lysyl hydroxylase, both procedures involving (NH4)2SO4 fractionation, affinity chromatography on concanavalin A-agarose and elution of the column with ethylene glycol. The additional steps in procedure A consist of gel filtration and chromatography on a hydroxyapatite column, and in procedure B of affinity chromatography on collagen linked to agarose and gel filtration. The best preparations obtained with either of the two procedures were pure when examined by sodium dodecyl sulphate-polyacrylamide-disc-gel or slab-gel electrophoresis, but about half of the preparations obtained by procedure A had minor contaminants. The specific activity of a typical preparation purified by procedure B was 13 4000 times that of the 15 000 g supernatant of the chick-embryo homogenate, with a recovery of about 4%. The molecular weight of the pure enzyme was bout 200 000 by gel filtration, and that of the enzyme subunit about 85 000 by sodium dodecyl sulphate/polyacrylamide-disc-gel or slab-gel electrophoresis. It is suggested that the active enzyme is a dimer consisting of only one type of monomer, and that a previously described enzyme form with an apparent molecular weight of about 550 000 is a polymeric form of this dimer. The catalytic-centre activity of the pure enzyme, as determined with a saturating concentration of a synthetic peptide substrate and under conditions specified, was about 3-4 mol/s per mol.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey A. J., Robins S. P., Balian G. Biological significance of the intermolecular crosslinks of collagen. Nature. 1974 Sep 13;251(5471):105–109. doi: 10.1038/251105a0. [DOI] [PubMed] [Google Scholar]
- Berg R. A., Kedersha N. L., Guzman N. A. Purification and partial characterization of the two nonidentical subunits of prolyl hydroxylase. J Biol Chem. 1979 Apr 25;254(8):3111–3118. [PubMed] [Google Scholar]
- Berg R. A., Kishida Y., Sakakibara S., Prockop D. J. Hydroxylation of (Pro-Pro-Gly)5 and (Pro-Pro-Gly)10 by prolyl hydroxylase. Evidence for an asymmetric active site in the enzyme. Biochemistry. 1977 Apr 19;16(8):1615–1621. doi: 10.1021/bi00627a014. [DOI] [PubMed] [Google Scholar]
- Berg R. A., Prockop D. J. Affinity column purification of protocollagen proline hydroxylase from chick embryos and further characterization of the enzyme. J Biol Chem. 1973 Feb 25;248(4):1175–1182. [PubMed] [Google Scholar]
- Guzman N. A., Berg R. A., Prockop D. J. Concanavalin A binds of purified prolyl hydroxylase and partially inhibits its enzymic activity. Biochem Biophys Res Commun. 1976 Nov 22;73(2):279–285. doi: 10.1016/0006-291x(76)90704-x. [DOI] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Collagen glycosyltransferases. Int Rev Connect Tissue Res. 1979;8:23–72. doi: 10.1016/b978-0-12-363708-6.50008-4. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Prockop D. J. Partial purification and characterization of protocollagen lysine hydroxylase from chick embryos. Biochim Biophys Acta. 1972 Feb 28;258(2):366–379. doi: 10.1016/0005-2744(72)90228-8. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Risteli L. Biosynthesis of collagen and its alterations in pathological states. Med Biol. 1976 Jun;54(3):159–186. [PubMed] [Google Scholar]
- Kivirikko K. I., Shudo K., Sakakibara S., Prockop D. J. Studies on protocollagen lysine hydroxylase. Hydroxylation of synthetic peptides and the stoichiometric decarboxylation of -ketoglutarate. Biochemistry. 1972 Jan 4;11(1):122–129. doi: 10.1021/bi00751a021. [DOI] [PubMed] [Google Scholar]
- Kuutti E. R., Tuderman L., Kivirikko K. I. Human prolyl hydroxylase. Purification, partial characterization and preparation of antiserum to the enzyme. Eur J Biochem. 1975 Sep 1;57(1):181–188. doi: 10.1111/j.1432-1033.1975.tb02289.x. [DOI] [PubMed] [Google Scholar]
- Myllylä R., Kuutti-Savolainen E. R., Kivirikko K. I. The role of ascorbate in the prolyl hydroxylase reaction. Biochem Biophys Res Commun. 1978 Jul 28;83(2):441–448. doi: 10.1016/0006-291x(78)91010-0. [DOI] [PubMed] [Google Scholar]
- Myllylä R., Schubotz L. M., Weser U., Kivirikko K. I. Involvement of superoxide in the prolyl and lysyl hydroxylase reactions. Biochem Biophys Res Commun. 1979 Jul 12;89(1):98–102. doi: 10.1016/0006-291x(79)90948-3. [DOI] [PubMed] [Google Scholar]
- Myllylä R., Tuderman L., Kivirikko K. I. Mechanism of the prolyl hydroxylase reaction. 2. Kinetic analysis of the reaction sequence. Eur J Biochem. 1977 Nov 1;80(2):349–357. doi: 10.1111/j.1432-1033.1977.tb11889.x. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (first of two parts). N Engl J Med. 1979 Jul 5;301(1):13–23. doi: 10.1056/NEJM197907053010104. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (second of two parts). N Engl J Med. 1979 Jul 12;301(2):77–85. doi: 10.1056/NEJM197907123010204. [DOI] [PubMed] [Google Scholar]
- Puistola U., Turpeenniemi-Hujanen T. M., Myllylä R., Kivirikko K. I. Studies on the lysyl hydroxylase reaction. I. Initial velocity kinetics and related aspects. Biochim Biophys Acta. 1980 Jan 11;611(1):40–50. doi: 10.1016/0005-2744(80)90040-6. [DOI] [PubMed] [Google Scholar]
- Puistola U., Turpeenniemi-Hujanen T. M., Myllylä R., Kivirikko K. I. Studies on the lysyl hydroxylase reaction. II. Inhibition kinetics and the reaction mechanism. Biochim Biophys Acta. 1980 Jan 11;611(1):51–60. doi: 10.1016/0005-2744(80)90041-8. [DOI] [PubMed] [Google Scholar]
- Pänkäläinen M., Aro H., Simons K., Kivirikko K. I. Protocollagen proline hydroxylase: molecular weight, subunits and isoelectric point. Biochim Biophys Acta. 1970 Dec 22;221(3):559–565. doi: 10.1016/0005-2795(70)90227-8. [DOI] [PubMed] [Google Scholar]
- Risteli J., Kivirikko K. I. Intracellular enzymes of collagen biosynthesis in rat liver as a function of age and in hepatic injury induced by dimethylnitrosamine. Changes in prolyl hydroxylase, lysyl hydroxylase, collagen galactosyltransferase and collagen glucosyltransferase activities. Biochem J. 1976 Aug 15;158(2):361–367. doi: 10.1042/bj1580361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risteli L., Myllylä R., Kivirikko K. I. Affinity chromatography of collagen glycosyltransferases on collagen linked to agarose. Eur J Biochem. 1976 Aug 1;67(1):197–202. doi: 10.1111/j.1432-1033.1976.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Ryhänen L. Lysyl hydroxylase. Further purification and characterization of the enzyme from chick embryos and chick embryo cartilage. Biochim Biophys Acta. 1976 Jun 7;438(1):71–89. doi: 10.1016/0005-2744(76)90224-2. [DOI] [PubMed] [Google Scholar]
- Tryggvason K., Majamaa K., Risteli J., Kivirikko K. I. Partial purification and characterization of chick-embryo prolyl 3-hydroxylase. Biochem J. 1979 Nov 1;183(2):303–307. doi: 10.1042/bj1830303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryggvason K., Risteli J., Kivirikko K. I. Separation of prolyl 3-hydroxylase and 4-hydroxylase activities and the 4-hydroxyproline requirement for synthesis of 3-hydroxyproline. Biochem Biophys Res Commun. 1976 May 23;76(2):275–281. doi: 10.1016/0006-291x(77)90722-7. [DOI] [PubMed] [Google Scholar]
- Tuderman L., Kuutti E. R., Kivirikko K. I. An affinity-column procedure using poly(L-proline) for the purification of prolyl hydroxylase. Purification of the enzyme from chick embryos. Eur J Biochem. 1975 Mar 3;52(1):9–16. doi: 10.1111/j.1432-1033.1975.tb03967.x. [DOI] [PubMed] [Google Scholar]
- Tuderman L., Myllylä R., Kivirikko K. I. Mechanism of the prolyl hydroxylase reaction. 1. Role of co-substrates. Eur J Biochem. 1977 Nov 1;80(2):341–348. doi: 10.1111/j.1432-1033.1977.tb11888.x. [DOI] [PubMed] [Google Scholar]
- Turpeenniemi T. M., Puistola U., Anttinen H., Kivirikko K. I. Affinity chromatography of lysyl hydroxylase on concanavalin A-agarose. Biochim Biophys Acta. 1977 Jul 8;483(1):215–219. doi: 10.1016/0005-2744(77)90024-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



