Abstract
Tryptophan 2,3-dioxygenase [L-tryptophan--oxygen 2,3-oxidoreductase (decyclizing), EC 1.13.11.11] has been reported to act solely on the L-isomer of tryptophan. However, by using a sensitive assay method with D- and L-[ring-2-14C]tryptophan and improved assay conditions, we were able to demonstrate that both the D- and L-stereoisomers of tryptophan were cleaved by the supernatant fraction (30000 g, 30 min) of liver homogenates of several species of mammals, including rat, mouse, rabbit and human. The ratio of activities toward D- and L-tryptophan was species variable, the highest (0.67) in ox liver and the lowest (0.07) in rat liver, the latter being hitherto exclusively used for the study of hepatic tryptophan 2,3-dioxygenase. In the supernatant fraction from mouse liver, the ratio was 0.23 but the specific activity with D-tryptophan was by far the highest of all the species tested. To identify the D-tryptophan cleaving enzyme activity, the enzyme was purified from mouse liver to apparent homogeneity. The specific activities toward D- and L-tryptophan showed a parallel rise with each purification step. The electrophoretically homogeneous protein had specific activities of 0.55 and 2.13 mumol/min per mg of protein at 25 degrees C toward D- and L-tryptophan, respectively. Additional evidence from heat treatment, inhibition and kinetic studies indicated that the same active site of a single enzyme was responsible for both activities. The molecular weight (150000), subunit structure (alpha 2 beta 2) and haem content (1.95 mol/mol) of the purified enzyme from mouse liver were similar to those of rat liver tryptophan 2,3-dioxygenase. The assay conditions employed in the previous studies on the stereospecificity of hepatic tryptophan 2,3-dioxygenase were apparently inadequate for determination of the D-tryptophan cleaving activity. Under the assay conditions in the present study, the purified enzyme from rat liver also acted on D-tryptophan, whereas the pseudomonad enzyme was strictly specific for the L-isomer.
Full text
PDF



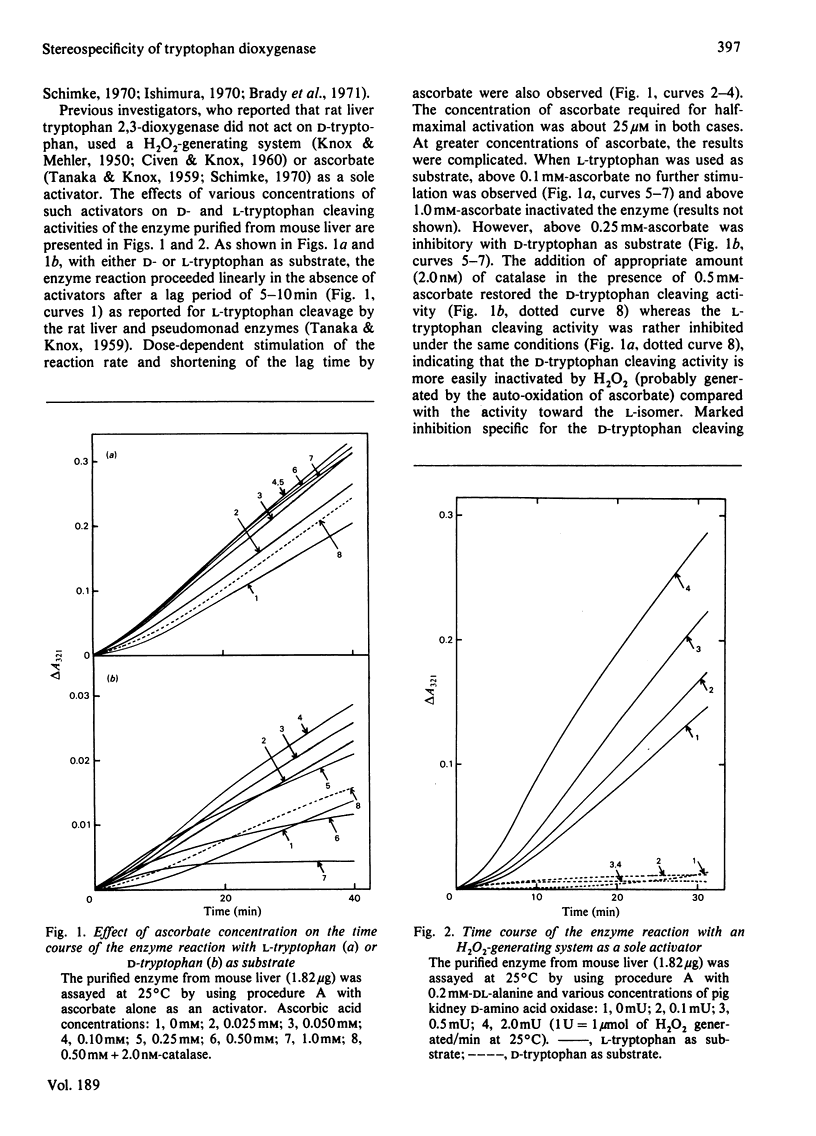
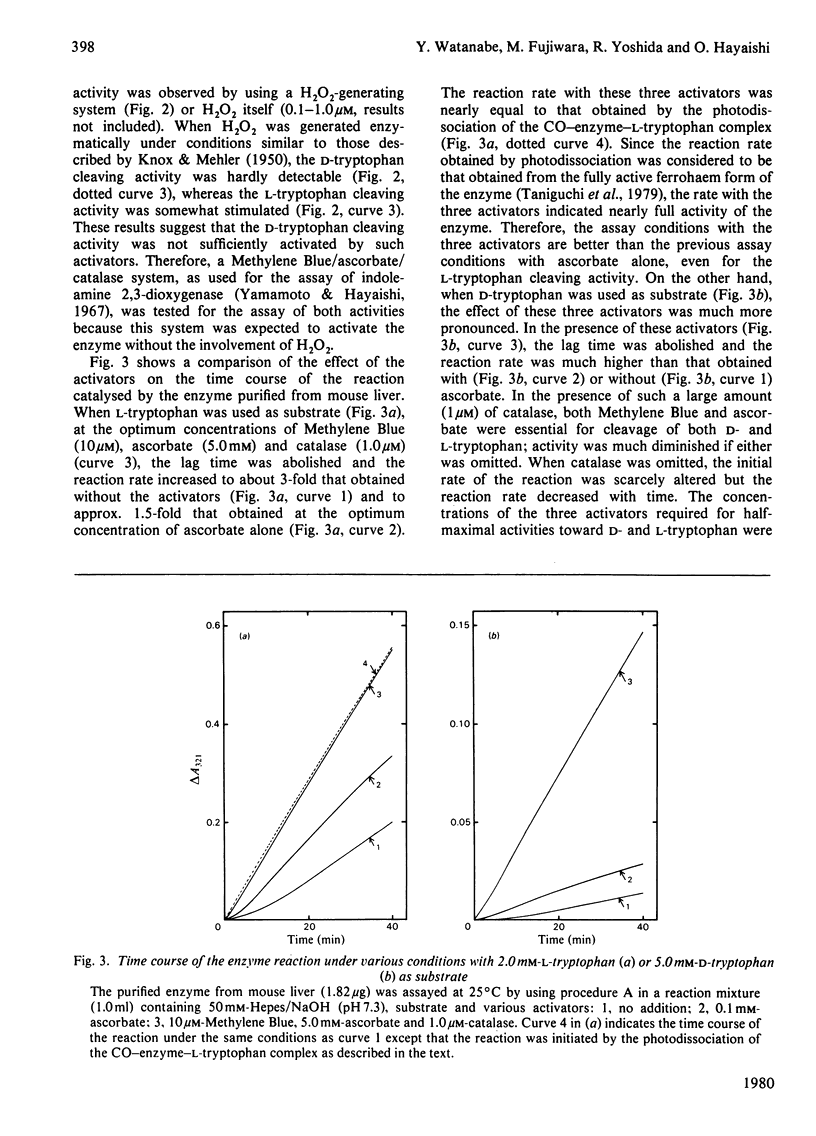
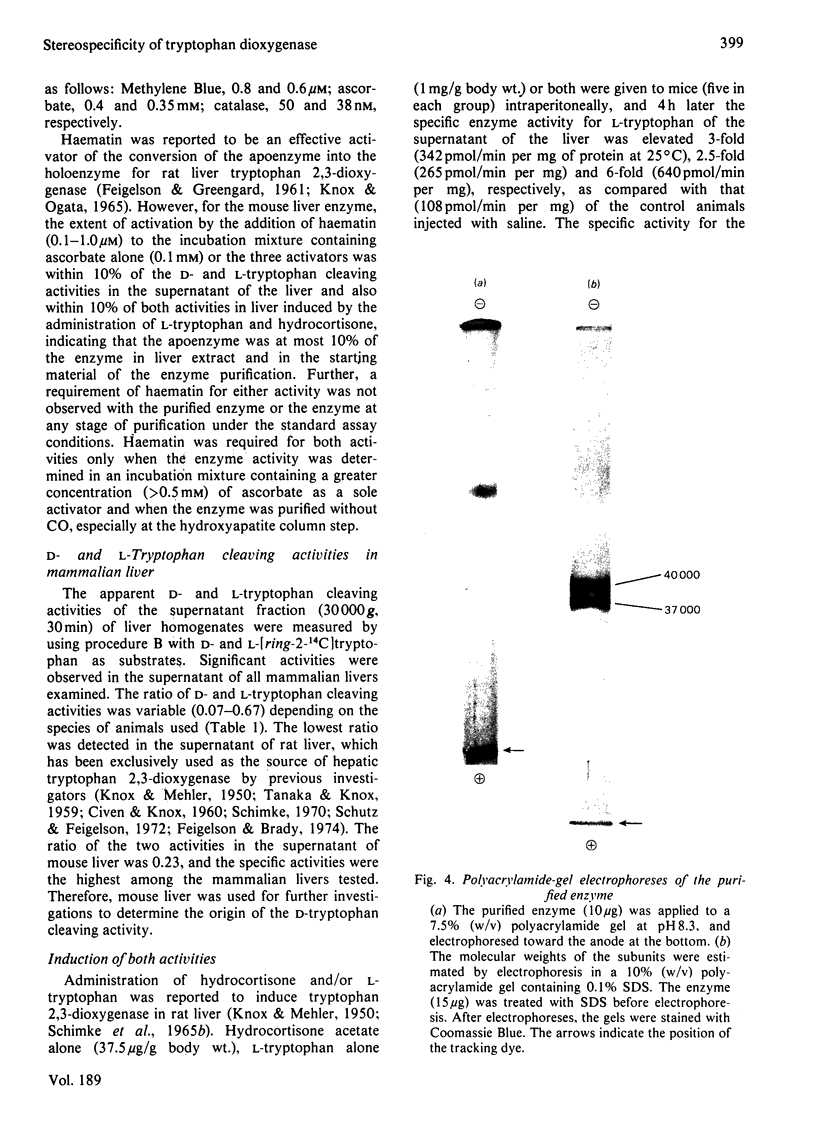
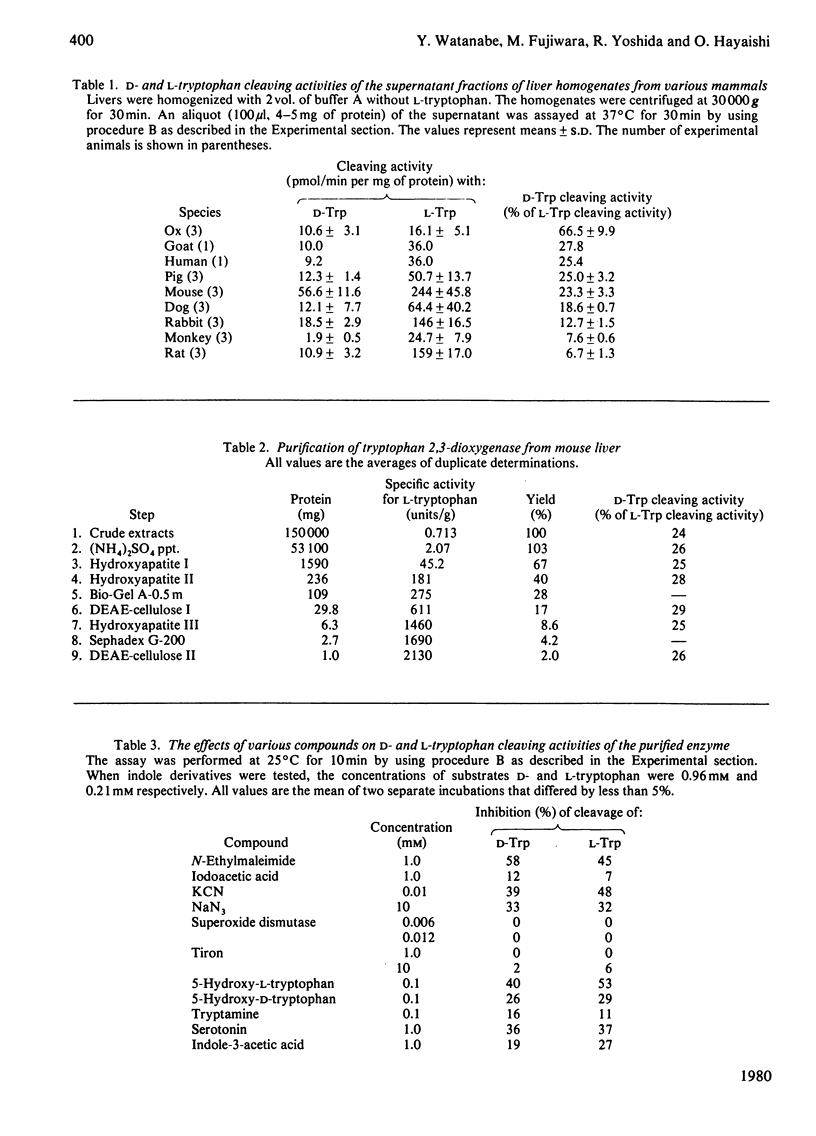
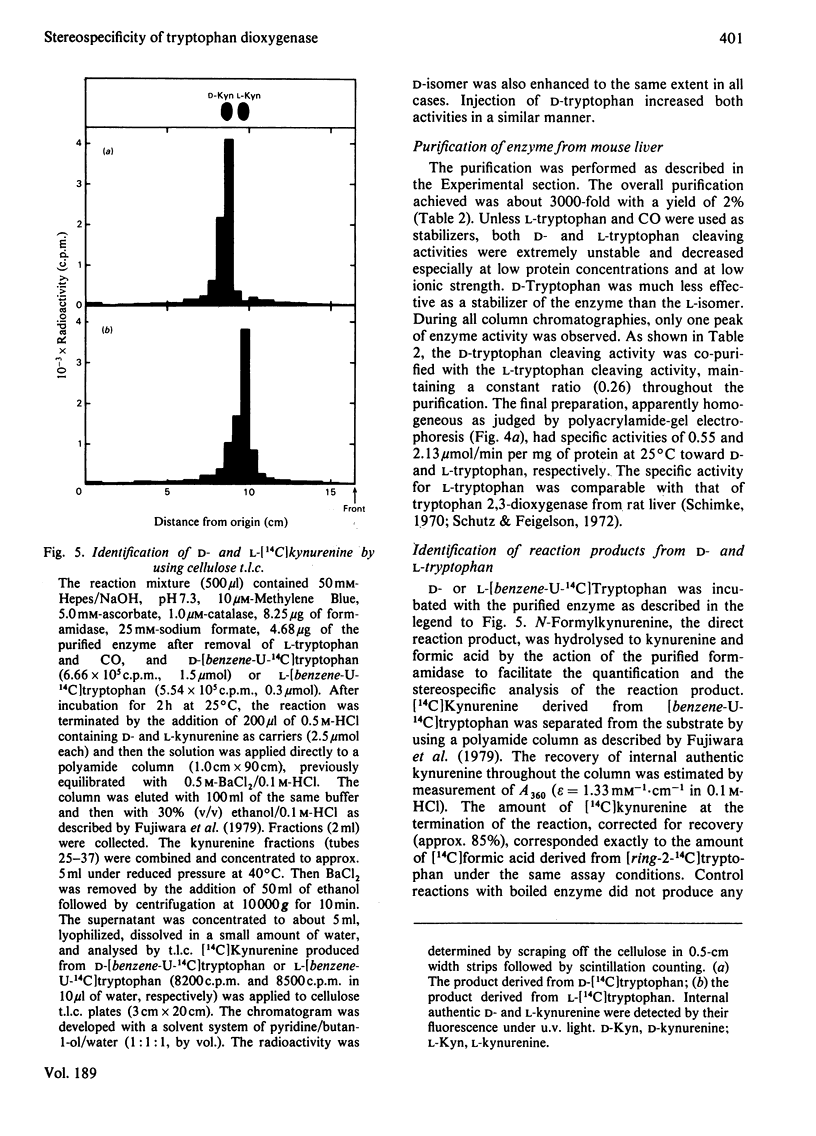
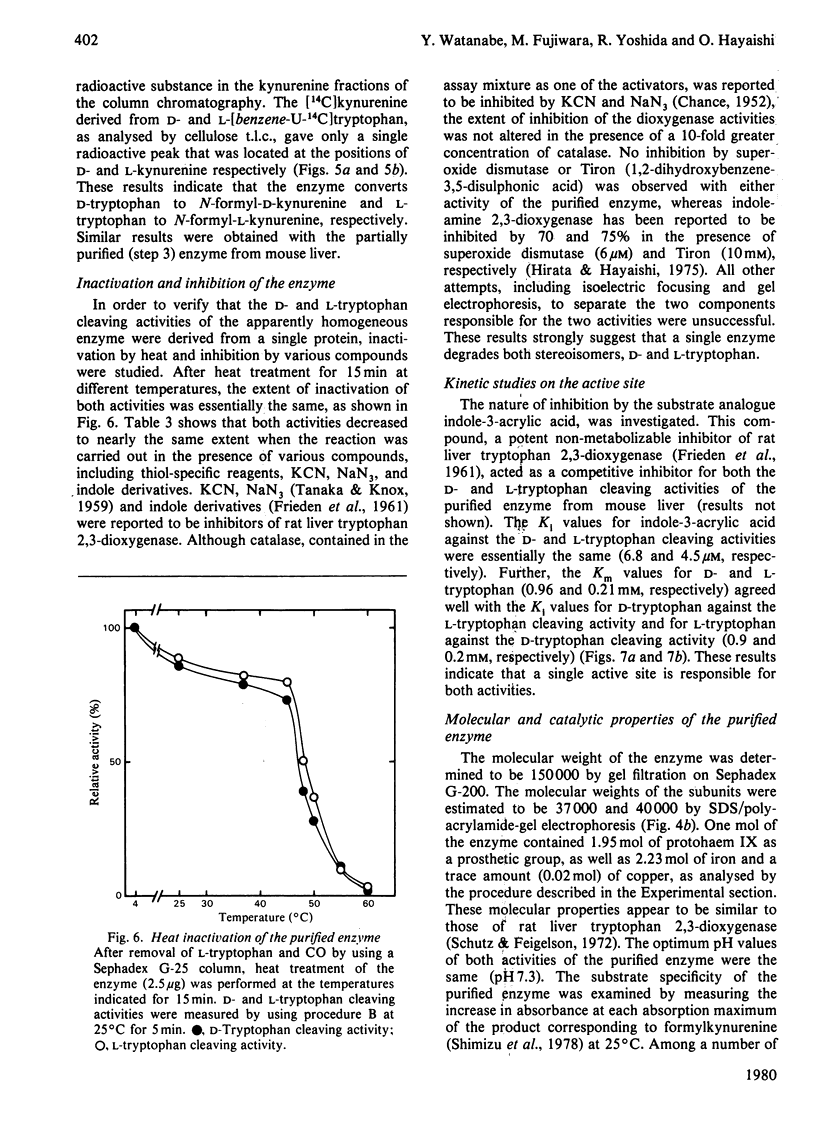
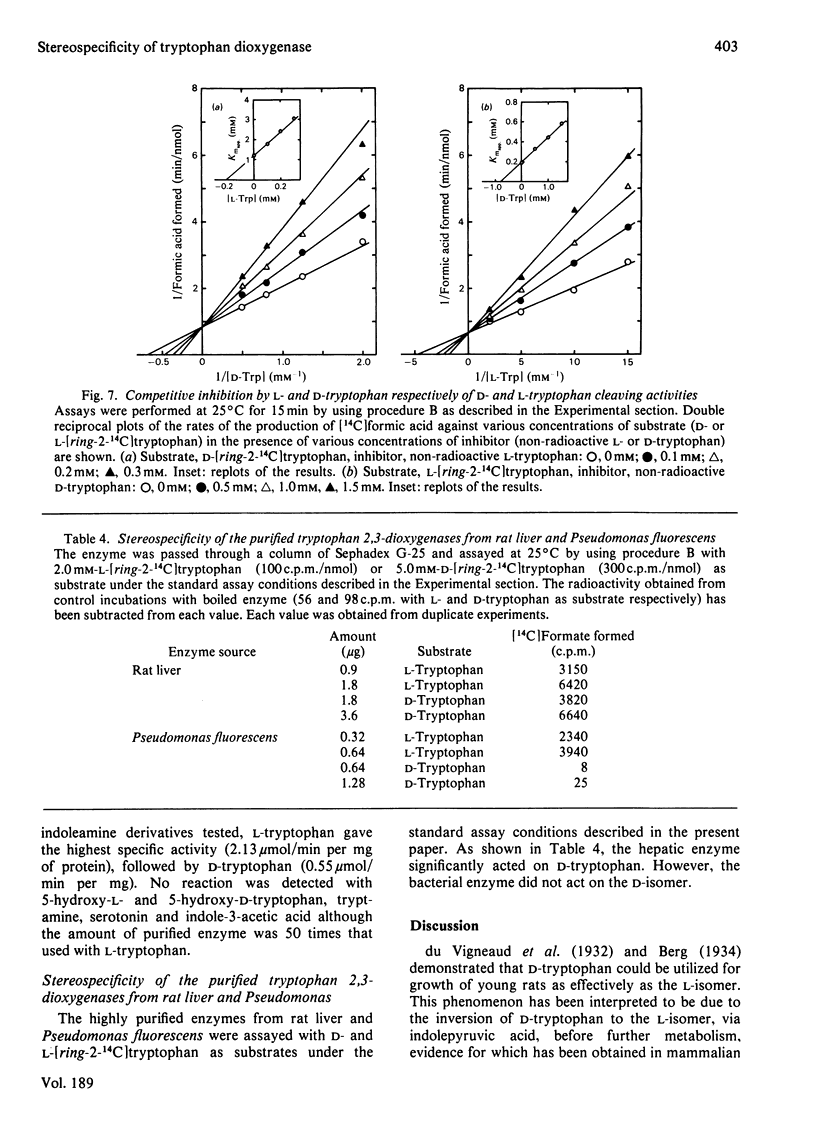


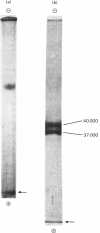
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady F. O., Forman H. J., Feigelson P. The role of superoxide and hydroperoxide in the reductive activation of tryptophan-2,3-dioxygenase. J Biol Chem. 1971 Dec 10;246(23):7119–7124. [PubMed] [Google Scholar]
- CHANCE B. The effect of pH upon the equilibria of catalase compounds. J Biol Chem. 1952 Feb;194(2):483–496. [PubMed] [Google Scholar]
- CIVEN M., KNOX W. E. The specificity of tryptophan analogues as inducers, substrates, inhibitors, and stabilizers of liver tryptophan pyrrolase. J Biol Chem. 1960 Jun;235:1716–1718. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- FEIGELSON P., GREENGARD O. A microsomal iron-porphyrin activator of rat liver tryptophan pyrrolase. J Biol Chem. 1961 Jan;236:153–157. [PubMed] [Google Scholar]
- FRIEDEN E., WESTMARK G. W., SCHOR J. M. Inhibition of tryptophan pyrrolase by serotonin, epinephrine and tryptophan analogs. Arch Biochem Biophys. 1961 Jan;92:176–182. doi: 10.1016/0003-9861(61)90233-8. [DOI] [PubMed] [Google Scholar]
- Fujiwara M., Shibata M., Nomiyama Y., Sugimoto T., Hirata F., Tokuyama T., Senoh S., Hayaishi O. Formation of 5-hydroxykynurenine and 5-hydroxykynurenamine from 5-hydroxytryptophan in rabbit small intestine. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1145–1149. doi: 10.1073/pnas.76.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON Q. H. The photochemical formation of a quickly reacting form of haemoglobin. Biochem J. 1959 Feb;71(2):293–303. doi: 10.1042/bj0710293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYAISHI O., STANIER R. Y. The bacterial oxidation of tryptophan. III. Enzymatic activities of cell-free extracts from bacteria employing the aromatic pathway. J Bacteriol. 1951 Dec;62(6):691–709. doi: 10.1128/jb.62.6.691-709.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi K., Hayaishi O. Enzymic formation of D-kynurenine from D-tryptophan. Arch Biochem Biophys. 1967 May;120(2):397–403. doi: 10.1016/0003-9861(67)90256-1. [DOI] [PubMed] [Google Scholar]
- Hirata F., Hayaishi O. New degradative routes of 5-hydroxytryptophan and serotonin by intestinal tryptophan 2,3-dioxygenase. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1112–1119. doi: 10.1016/0006-291x(72)90949-7. [DOI] [PubMed] [Google Scholar]
- Hirata F., Hayaishi O. Possible participation of superoxide anion in the intestinal tryptophan 2,3-dioxygenase reaction. J Biol Chem. 1971 Dec 25;246(24):7825–7826. [PubMed] [Google Scholar]
- Hirata F., Hayaishi O. Studies on indoleamine 2,3-dioxygenase. I. Superoxide anion as substrate. J Biol Chem. 1975 Aug 10;250(15):5960–5966. [PubMed] [Google Scholar]
- Hirata F., Hayaishi O., Tokuyama T., Seno S. In vitro and in vivo formation of two new metabolites of melatonin. J Biol Chem. 1974 Feb 25;249(4):1311–1313. [PubMed] [Google Scholar]
- Hirata F., Ohnishi T., Hayaishi O. Indoleamine 2,3-dioxygenase. Characterization and properties of enzyme. O2- complex. J Biol Chem. 1977 Jul 10;252(13):4637–4642. [PubMed] [Google Scholar]
- KNOX W. E., MEHLER A. H. The conversion of tryptophan to kynurenine in liver. I. The coupled tryptophan peroxidase-oxidase system forming formylkynurenine. J Biol Chem. 1950 Nov;187(1):419–430. [PubMed] [Google Scholar]
- KNOX W. E., OGATA M. EFFECTS OF PEROXIDE, CATALASE, AND HEMATIN IN THE ASSAY OF LIVER TRYPTOPHAN PYRROLASE. J Biol Chem. 1965 May;240:2216–2221. [PubMed] [Google Scholar]
- Knox W. E., Piras M. M. Tryptophan pyrrolase of liver. 3. Conjugation in vivo during cofactor induction by tryptophan analogues. J Biol Chem. 1967 Jun 25;242(12):2959–2965. [PubMed] [Google Scholar]
- LANGNER R. R., BERG C. P. Metabolism of D-tryptophan in the normal human subject. J Biol Chem. 1955 Jun;214(2):699–707. [PubMed] [Google Scholar]
- Loh H. H., Berg C. P. Intestinal pyrrolase and formamidase activities in the rabbit. J Nutr. 1973 Mar;103(3):397–406. doi: 10.1093/jn/103.3.397. [DOI] [PubMed] [Google Scholar]
- Loh H. H., Berg C. P. Inversion in the metabolism of D-tryptophan in the rabbit and the rat. J Nutr. 1971 Oct;101(10):1351–1358. doi: 10.1093/jn/101.10.1351. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Ohnishi T., Hirata F., Hayaish O. Indoleamine 2,3-dioxygenase. Potassium superoxide as substrate. J Biol Chem. 1977 Jul 10;252(13):4643–4647. [PubMed] [Google Scholar]
- Peterkofsky B. Use of a new radioassay for tryptophan oxygenase to study the development of the enzyme in chick embryos. Arch Biochem Biophys. 1968 Dec;128(3):637–645. doi: 10.1016/0003-9861(68)90073-8. [DOI] [PubMed] [Google Scholar]
- Poillon W. N., Maeno H., Koike K., Feigelson P. Tryptophan oxygenase of Pseudomonas acidovorans. Purification, composition, and subunit structure. J Biol Chem. 1969 Jul 10;244(13):3447–3456. [PubMed] [Google Scholar]
- Rodden F. A., Berg C. P. Enzymatic conversion of L- and D-tryptophan to kynurenine by rat liver. J Nutr. 1974 Feb;104(2):227–238. doi: 10.1093/jn/104.2.227. [DOI] [PubMed] [Google Scholar]
- SCHIMKE R. T., SWEENEY E. W., BERLIN C. M. THE ROLES OF SYNTHESIS AND DEGRADATION IN THE CONTROL OF RAT LIVER TRYPTOPHAN PYRROLASE. J Biol Chem. 1965 Jan;240:322–331. [PubMed] [Google Scholar]
- Schimke R. T., Sweeney E. W., Berlin C. M. Studies of the stability in vivo and in vitro of rat liver tryptophan pyrrolase. J Biol Chem. 1965 Dec;240(12):4609–4620. [PubMed] [Google Scholar]
- Schutz G., Feigelson P. Purification and properties of rat liver tryptophan oxygenase. J Biol Chem. 1972 Sep 10;247(17):5327–5332. [PubMed] [Google Scholar]
- Shimizu T., Nomiyama S., Hirata F., Hayaishi O. Indoleamine 2,3-dioxygenase. Purification and some properties. J Biol Chem. 1978 Jul 10;253(13):4700–4706. [PubMed] [Google Scholar]
- Snell E. E., Strong F. M., Peterson W. H. Growth factors for bacteria: Fractionation and properties of an accessory factor for lactic acid bacteria. Biochem J. 1937 Oct;31(10):1789–1799. doi: 10.1042/bj0311789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANAKA T., KNOX W. E. The nature and mechanism of the tryptophan pyrrolase (peroxidase-oxidase) reaction of Pseudomonas and of rat liver. J Biol Chem. 1959 May;234(5):1162–1170. [PubMed] [Google Scholar]
- Taniguchi T., Hirata F., Hayaishi O. Intracellular utilization of superoxide anion by indoleamine 2,3-dioxygenase of rabbit enterocytes. J Biol Chem. 1977 Apr 25;252(8):2774–2776. [PubMed] [Google Scholar]
- Taniguchi T., Sono M., Hirata F., Hayaishi O., Tamura M., Hayashi K., Iizuka T., Ishimura Y. Indoleamine 2,3-dioxygenase. Kinetic studies on the binding of superoxide anion and molecular oxygen to enzyme. J Biol Chem. 1979 May 10;254(9):3288–3294. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yamamoto S., Hayaishi O. Tryptophan pyrrolase of rabbit intestine. D- and L-tryptophan-cleaving enzyme or enzymes. J Biol Chem. 1967 Nov 25;242(22):5260–5266. [PubMed] [Google Scholar]