Abstract
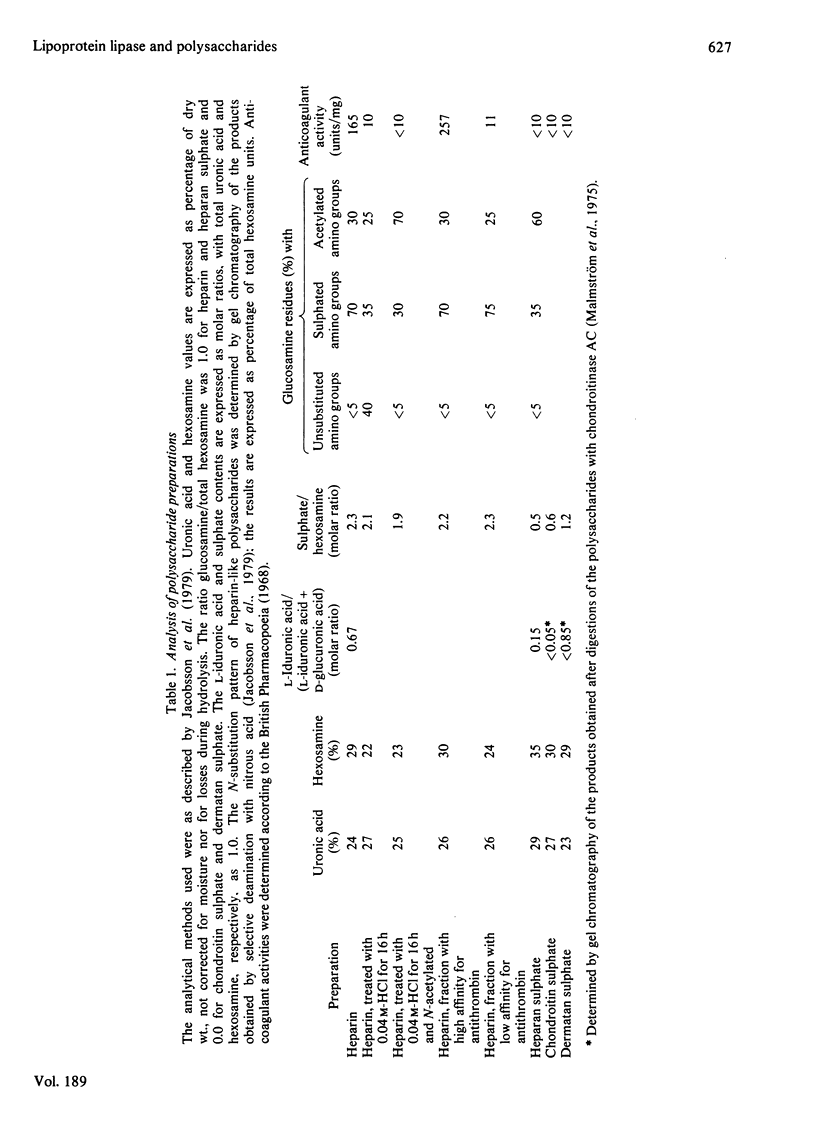
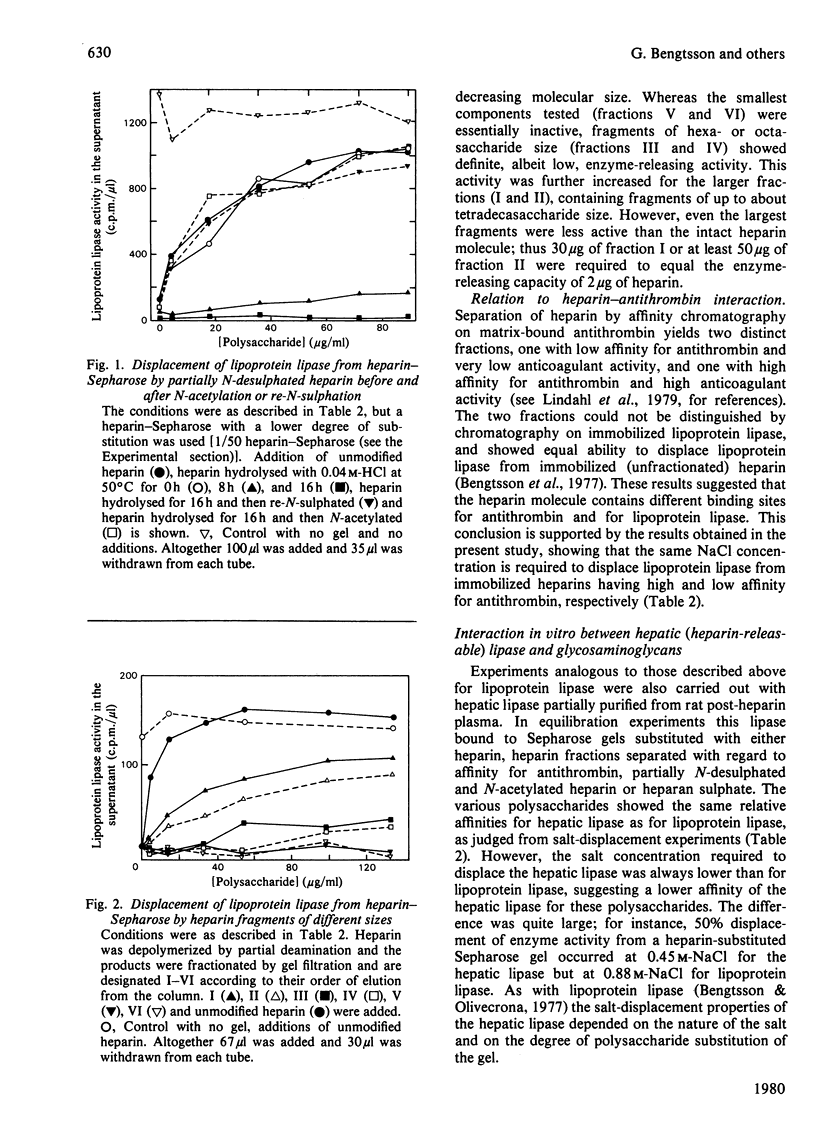
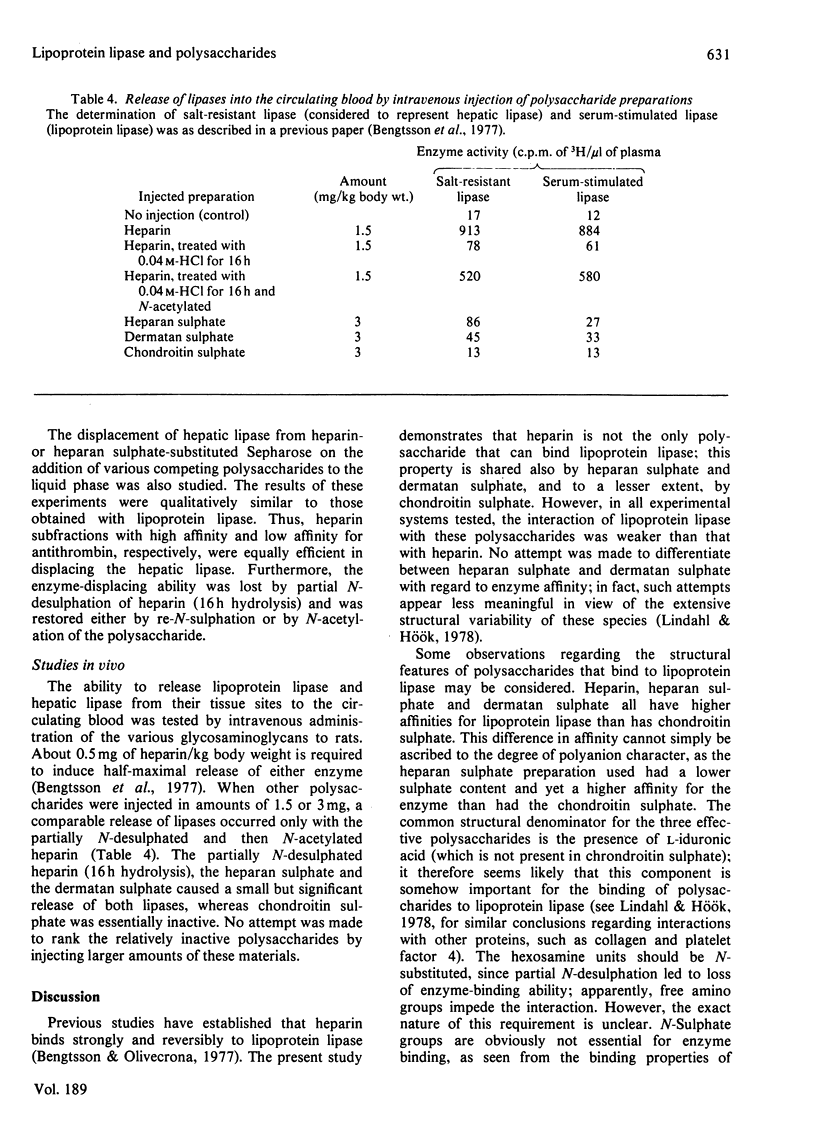
1. Lipoprotein lipase (EC 3.1.1.34), which was previously shown to bind to immobilized heparin, was now found to bind also to heparan sulphate and dermatan sulphate and to some extent to chondroitin sulphate. 2. The relative binding affinities were compared by determining (a) the concentration of NaCl required to release the enzyme from polysaccharide-substituted Sepharose; (b) the concentration of free polysaccharides required to displace the enzyme from immobilized polysaccharides; and (c) the total amounts of enzyme bound after saturation of immobilized polysaccharides. By each of these criteria heparin bound the enzyme most efficiently, followed by heparan sulphate and dermatan sulphate, which were more efficient than chondroitin sulphate. 3. Heparin fractions with high and low affinity for antithrombin, respectively, did not differ with regard to affinity for lipoprotein lipase. 4. Partially N-desulphated heparin (40–50% of N-unsubstituted glucosamine residues) was unable to displace lipoprotein lipase from immobilized heparin. This ability was restored by re-N-sulphation or by N-acetylation; the N-acetylated product was essentially devoid of anticoagulant activity. 5. Partial depolymerization of heparin led to a decrease in ability to displace lipoprotein lipase from heparin–Sepharose; however, even fragments of less than decasaccharide size showed definite enzyme-releasing activity. 6. Studies with hepatic lipase (purified from rat post-heparin plasma) gave results similar to those obtained with milk lipoprotein lipase. However, the interaction between the hepatic lipase and the glycosaminoglycans was weaker and was abolished at lower concentrations of NaCl. 7. The ability of the polysaccharides to release lipoprotein lipase to the circulating blood after intravenous injection into rats essentially conformed to their affinity for the enzyme as evaluated by the experiments in vitro.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akesson B., Gronowitz S., Herslöf B. Stereospecificity of hepatic lipases. FEBS Lett. 1976 Dec 1;71(2):241–244. doi: 10.1016/0014-5793(76)80941-6. [DOI] [PubMed] [Google Scholar]
- Bengtsson G., Olivecrona T. Interaction of heparin with proteins. Demonstration of different binding sites for antithrombin and lipoprotein lipase. FEBS Lett. 1977 Jul 1;79(1):59–63. doi: 10.1016/0014-5793(77)80350-5. [DOI] [PubMed] [Google Scholar]
- Bengtsson G., Olivecrona T. Interaction of lipoprotein lipase with heparin-Sepharose. Evaluation of conditions for affinity binding. Biochem J. 1977 Oct 1;167(1):109–119. doi: 10.1042/bj1670109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckström G., Hök M., Lindahl U., Feingold D. S., Malmström A., Rodén L., Jacobsson I. Biosynthesis of heparin. Assay and properties of the microsomal uronosyl C-5 epimerase. J Biol Chem. 1979 Apr 25;254(8):2975–2982. [PubMed] [Google Scholar]
- DANISHEFSKY I., STEINER H. INVESTIGATIONS ON THE CHEMISTRY OF HEPARIN. V. DISACCHARIDES OBTAINED AFTER PARTIAL HYDROLYSIS. Biochim Biophys Acta. 1965 Mar 1;101:37–45. doi: 10.1016/0926-6534(65)90028-x. [DOI] [PubMed] [Google Scholar]
- Ehnholm C., Greten H., Brown W. V. A comparative study of post-heparin lipolytic activity and a purified human plasma triacylglycerol lipase. Biochim Biophys Acta. 1974 Jul 26;360(1):68–77. doi: 10.1016/0005-2760(74)90180-5. [DOI] [PubMed] [Google Scholar]
- Ehnholm C., Shaw W., Greten H., Brown W. V. Purification from human plasma of a heparin-released lipase with activity against triglyceride and phospholipids. J Biol Chem. 1975 Sep 10;250(17):6756–6761. [PubMed] [Google Scholar]
- Hahn P. F. ABOLISHMENT OF ALIMENTARY LIPEMIA FOLLOWING INJECTION OF HEPARIN. Science. 1943 Jul 2;98(2531):19–20. doi: 10.1126/science.98.2531.19. [DOI] [PubMed] [Google Scholar]
- Hernell O., Egelrud T., Olivecrona T. Serum-stimulated lipases (lipoprotein lipases). Immunological crossreaction between the bovine and the human enzymes. Biochim Biophys Acta. 1975 Feb 13;381(2):233–241. [PubMed] [Google Scholar]
- Inoue Y., Nagasawa K. Selective N-desulfation of heparin with dimethyl sulfoxide containing water or methanol. Carbohydr Res. 1976 Jan;46(1):87–95. doi: 10.1016/s0008-6215(00)83533-8. [DOI] [PubMed] [Google Scholar]
- Iverius P. H. Coupling of glycosaminoglycans to agarose beads (sepharose 4B). Biochem J. 1971 Oct;124(4):677–683. doi: 10.1042/bj1240677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverius P. H., Lindahl U., Egelrud T., Olivecrona T. Effects of heparin on lipoprotein lipase from bovine milk. J Biol Chem. 1972 Oct 25;247(20):6610–6616. [PubMed] [Google Scholar]
- Iverius P. H., Ostlund-Lindqvist A. M. Lipoprotein lipase from bovine milk. Isolation procedure, chemical characterization, and molecular weight analysis. J Biol Chem. 1976 Dec 25;251(24):7791–7795. [PubMed] [Google Scholar]
- Jansson L., Ogren S., Lindahl U. Macromolecular properties and end-group analysis of heparin isolated from bovine liver capsule. Biochem J. 1975 Jan;145(1):53–62. doi: 10.1042/bj1450053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORN E. D. Clearing factor, a heparin-activated lipoprotein lipase. I. Isolation and characterization of the enzyme from normal rat heart. J Biol Chem. 1955 Jul;215(1):1–14. [PubMed] [Google Scholar]
- LEVY L., PETRACEK F. J. Chemical and pharmacological studies on N-resulfated heparin. Proc Soc Exp Biol Med. 1962 Apr;109:901–905. doi: 10.3181/00379727-109-27372. [DOI] [PubMed] [Google Scholar]
- LINDAHL U., CIFONELLI J. A., LINDAHL B., RODEN L. THE ROLE OF SERINE IN THE LINKAGE OF HEPARIN TO PROTEIN. J Biol Chem. 1965 Jul;240:2817–2820. [PubMed] [Google Scholar]
- LaRosa J. C., Levy R. I., Windmueller H. G., Fredrickson D. S. Comparison of the triglyceride lipase of liver, adipose tissue, and postheparin plasma. J Lipid Res. 1972 May;13(3):356–363. [PubMed] [Google Scholar]
- Laurent T. C., Tengblad A., Thunberg L., Hök M., Lindahl U. The molecular-weight-dependence of the anti-coagulant activity of heparin. Biochem J. 1978 Nov 1;175(2):691–701. doi: 10.1042/bj1750691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Hök M., Thunberg L., Fransson L. A., Linker A. Structure of the antithrombin-binding site in heparin. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3198–3202. doi: 10.1073/pnas.76.7.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Jansson L., Hallén A. Biosynthesis of heparin. II. Formation of sulfamino groups. J Biol Chem. 1973 Oct 25;248(20):7234–7241. [PubMed] [Google Scholar]
- Lindahl U., Hök M. Glycosaminoglycans and their binding to biological macromolecules. Annu Rev Biochem. 1978;47:385–417. doi: 10.1146/annurev.bi.47.070178.002125. [DOI] [PubMed] [Google Scholar]
- Malström A., Carlstedt I., Aberg L., Fransson L. A. The copolymeric structure of dermatan sulphate produced by cultured human fibroblasts. Different distribution of iduronic acid and glucuronic acid-containing units in soluble and cell-associated glycans. Biochem J. 1975 Dec;151(3):477–489. doi: 10.1042/bj1510477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa K., Tokuyasu T., Inoue Y. Studies of the influence of N-substitution in heparin on its anticoagulant activity. J Biochem. 1977 Apr;81(4):989–993. doi: 10.1093/oxfordjournals.jbchem.a131565. [DOI] [PubMed] [Google Scholar]
- Olivecrona T., Bengtsson G., Marklund S. E., Lindahl U., Hök M. Heparin-lipoprotein lipase interactions. Fed Proc. 1977 Jan;36(1):60–65. [PubMed] [Google Scholar]
- Olivecrona T., Egelrud T., Iverius P. H., Lindahl U. Evidence for an ionic binding of lipoprotein lipase to heparin. Biochem Biophys Res Commun. 1971 May 7;43(3):524–529. doi: 10.1016/0006-291x(71)90645-0. [DOI] [PubMed] [Google Scholar]
- Ostlung-Lindqvist A. M., Boberg J. Purification of salt resistant lipase and lipoprotein lipase from human post-heparin plasma. FEBS Lett. 1977 Nov 15;83(2):231–236. doi: 10.1016/0014-5793(77)81011-9. [DOI] [PubMed] [Google Scholar]
- Riensenfeld J., Hök M., Björk I., Lindahl U., Ajaxon B. Structural requirements for the interaction of heparin with antithrombin III. Fed Proc. 1977 Jan;36(1):39–43. [PubMed] [Google Scholar]
- Teien A. N., Abildgaard U., Hök M. The anticoagulant effect of heparan sulfate and dermatan sulfate. Thromb Res. 1976 Jun;8(6):859–867. doi: 10.1016/0049-3848(76)90014-1. [DOI] [PubMed] [Google Scholar]


