Abstract
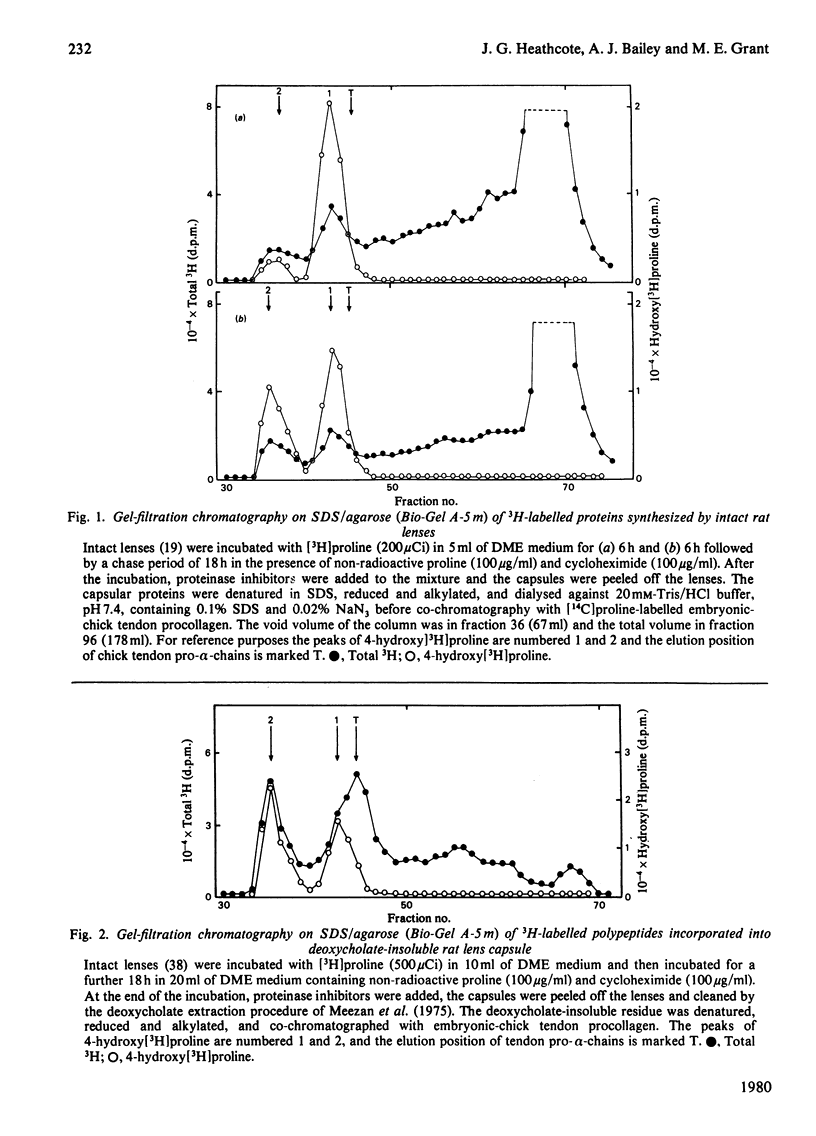
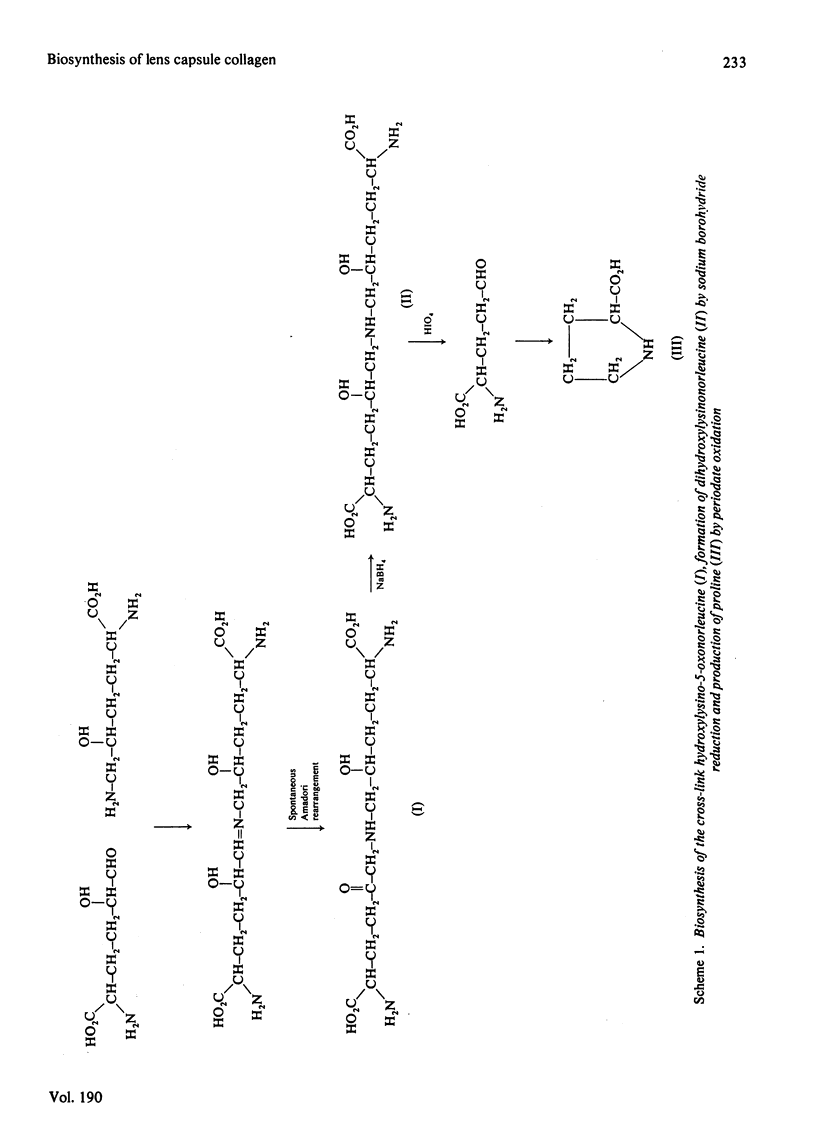
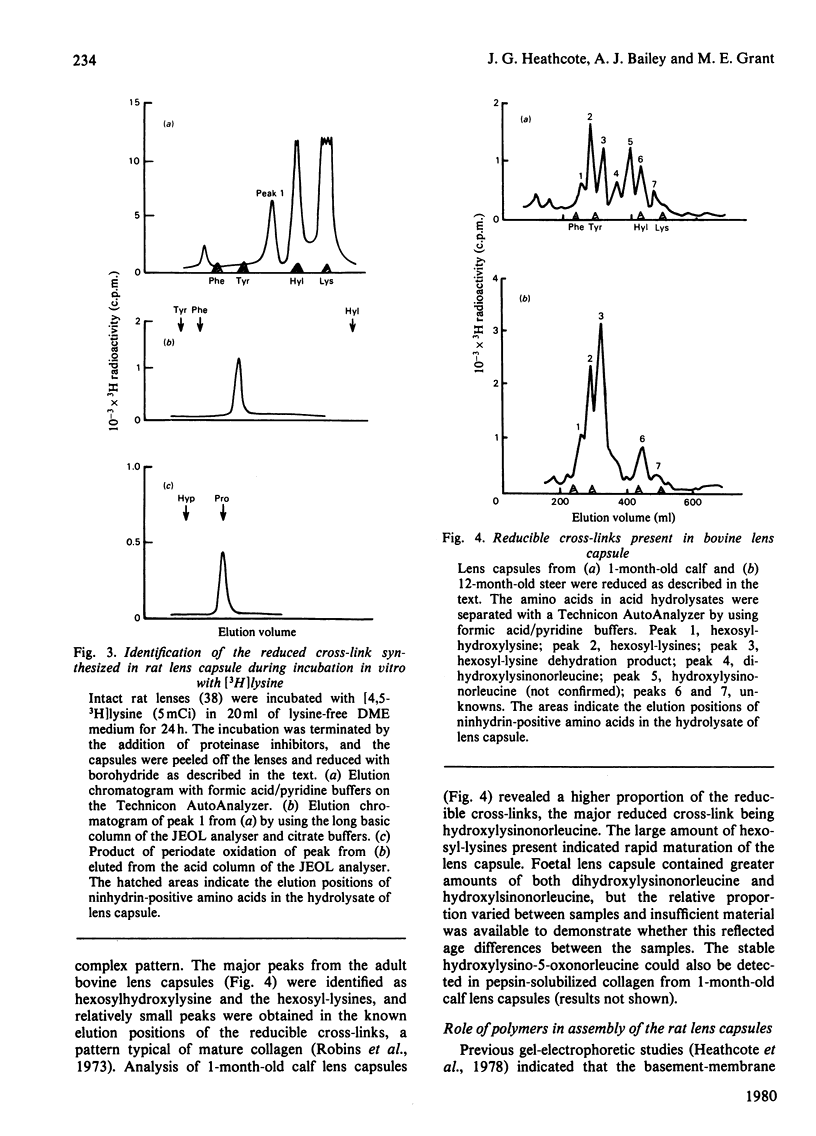
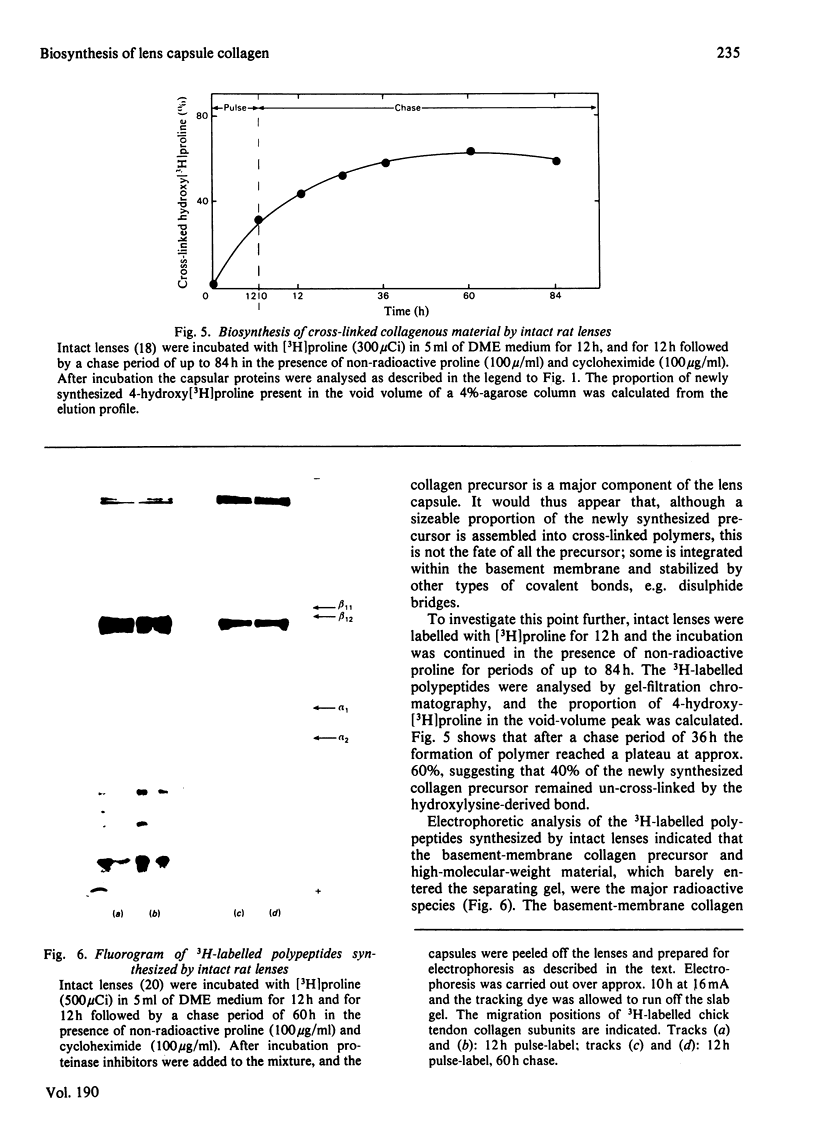
1. Intact rat lenses in tissue culture synthesize hydroxy[3H]proline-containing polypeptides of apparent mol.wt. approx. 180000, which become assembled into aggregates of higher molecular weight with time. 2. Both the 180000-mol.wt. species and the aggregates are components of the deoxycholate-insoluble base-membrane matrix. 3. Formation of the high-molecular-weight aggregate is accompanied by the biosynthesis of the reducible hydroxylysine-derived cross-link hydroxylysino-5-oxo-norleucine. 4. Hydroxylysino-5-oxonorleucine and dehydrohydroxylysinonorleucine are the major reducible cross-links present in intact foetal and 1-month-old calf lens capsules.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey A. J., Robins S. P., Balian G. Biological significance of the intermolecular crosslinks of collagen. Nature. 1974 Sep 13;251(5471):105–109. doi: 10.1038/251105a0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cheah K. S., Grant M. E., Jackson D. S. Translation of embryonic-chick tendon procollagen messenger ribonucleic acid in two cell-free protein-synthesizing systems. Biochem J. 1979 Jul 15;182(1):81–93. doi: 10.1042/bj1820081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch E., Bornstein P. Characterization of a type IV procollagen synthesized by human amniotic fluid cells in culture. J Biol Chem. 1979 May 25;254(10):4197–4204. [PubMed] [Google Scholar]
- Fisher R. F., Wakely J. The elastic constants and ultrastructural organization of a basement membrane (lens capsule). Proc R Soc Lond B Biol Sci. 1976 Jun 30;193(1113):335–358. doi: 10.1098/rspb.1976.0051. [DOI] [PubMed] [Google Scholar]
- Harwood R., Merry A. H., Woolley D. E., Grant M. E., Jackson D. S. The disulphide-bonded nature of procollagen and the role of the extension peptides in the assembly of the molecule. Biochem J. 1977 Feb 1;161(2):405–418. doi: 10.1042/bj1610405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathcote G., Sear C. H., Grant M. E. Studies on the assembly of the rat lens capsule. Biosynthesis and partial characterization of the collagenous components. Biochem J. 1978 Oct 15;176(1):283–294. doi: 10.1042/bj1760283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B. V., Macarak E. J., Gunson D., Kefalides N. A. Characterization of the collagen synthesized by endothelial cells in culture. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2361–2364. doi: 10.1073/pnas.73.7.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson B. G., Spiro R. G. Fractionation of glycoprotein components of the reduced alkylated renal glomerular basement membrane. J Biol Chem. 1972 Jul 10;247(13):4239–4247. [PubMed] [Google Scholar]
- Hudson B. G., Spiro R. G. Studies on the native and reduced alkylated renal glomerular basement membrane. Solubility, subunit size, and reaction with cyanogen bromide. J Biol Chem. 1972 Jul 10;247(13):4229–4238. [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A., Alper R., Clark C. C. Biochemistry and metabolism of basement membranes. Int Rev Cytol. 1979;61:167–228. doi: 10.1016/s0074-7696(08)61998-1. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A., Cameron J. D., Tomichek E. A., Yanoff M. Biosynthesis of basement membrane collagen by rabbit corneal endothelium in vitro. J Biol Chem. 1976 Feb 10;251(3):730–733. [PubMed] [Google Scholar]
- Kefalides N. A. Structure and biosynthesis of basement membranes. Int Rev Connect Tissue Res. 1973;6:63–104. doi: 10.1016/b978-0-12-363706-2.50008-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Meezan E., Hjelle J. T., Brendel K., Carlson E. C. A simple, versatile, nondisruptive method for the isolation of morphologically and chemically pure basement membranes from several tissues. Life Sci. 1975 Dec 1;17(11):1721–1732. doi: 10.1016/0024-3205(75)90119-8. [DOI] [PubMed] [Google Scholar]
- Moczar M., Moczar E., Robert L. Structural glycoprotein from the media of pig aorta. Aggregation of the S-carboxamidomethyl subunits. Biochimie. 1977;59(2):141–151. doi: 10.1016/s0300-9084(77)80285-x. [DOI] [PubMed] [Google Scholar]
- Robins S. P., Bailey A. J. The chemistry of the collagen cross-links. The mechanism of stabilization of the reducible intermediate cross-links. Biochem J. 1975 Aug;149(2):381–385. doi: 10.1042/bj1490381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins S. P., Shimokomaki M., Bailey A. J. The chemistry of the collagen cross-links. Age-related changes in the reducible components of intact bovine collagen fibres. Biochem J. 1973 Apr;131(4):771–780. doi: 10.1042/bj1310771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer M. L., Kefalides N. A. Collagen crosslinks: occurrence in basement membrane collagens. Biochem Biophys Res Commun. 1973 Apr 2;51(3):775–780. doi: 10.1016/0006-291x(73)91382-x. [DOI] [PubMed] [Google Scholar]


