Abstract
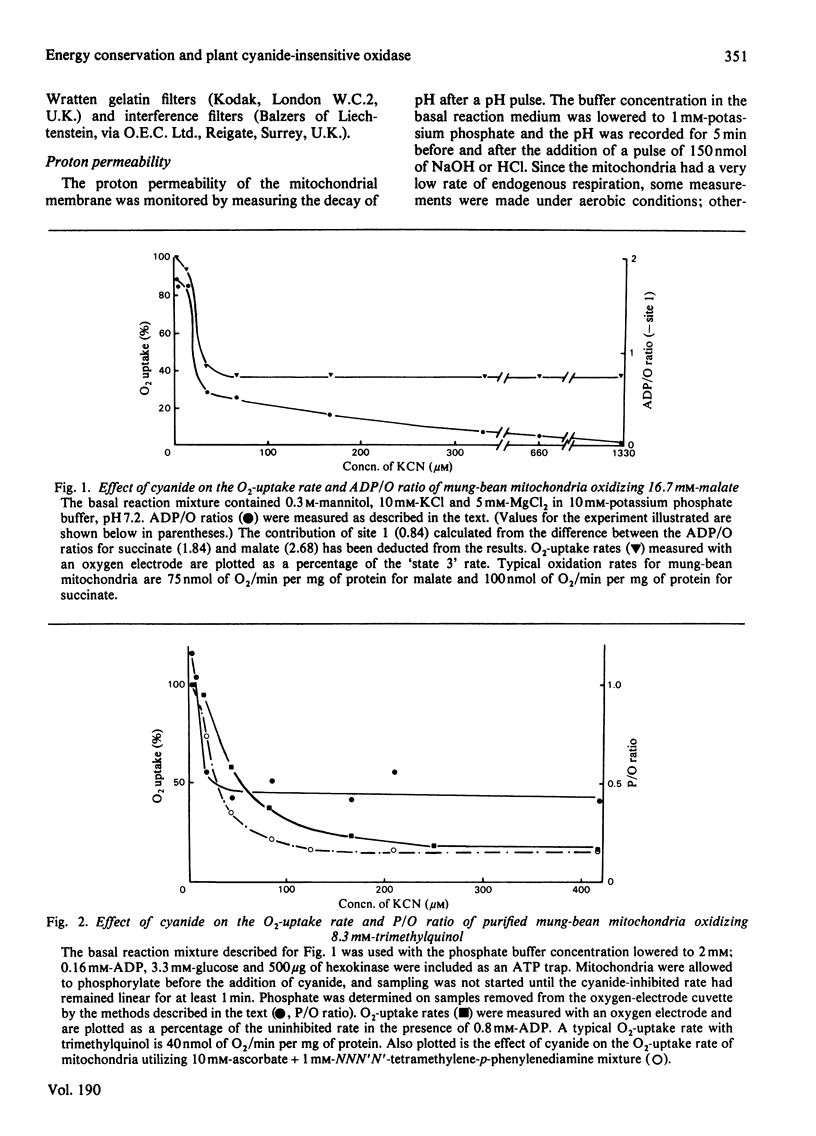
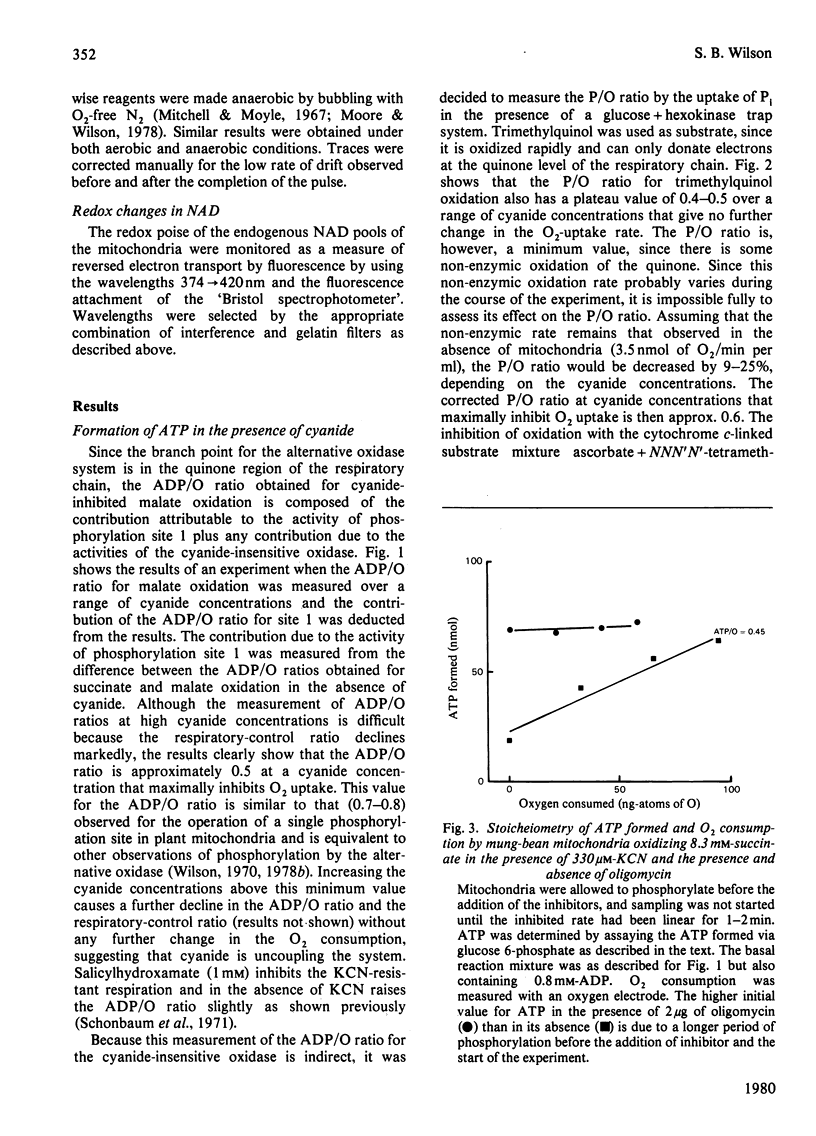
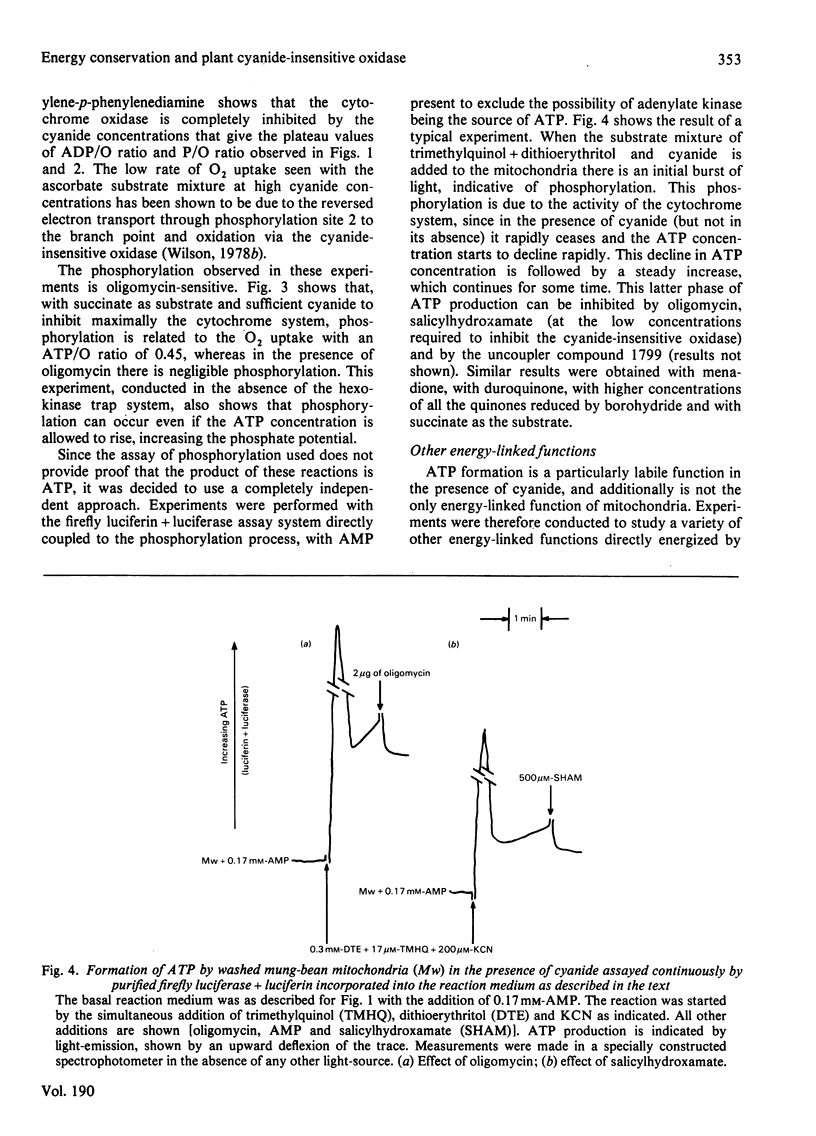
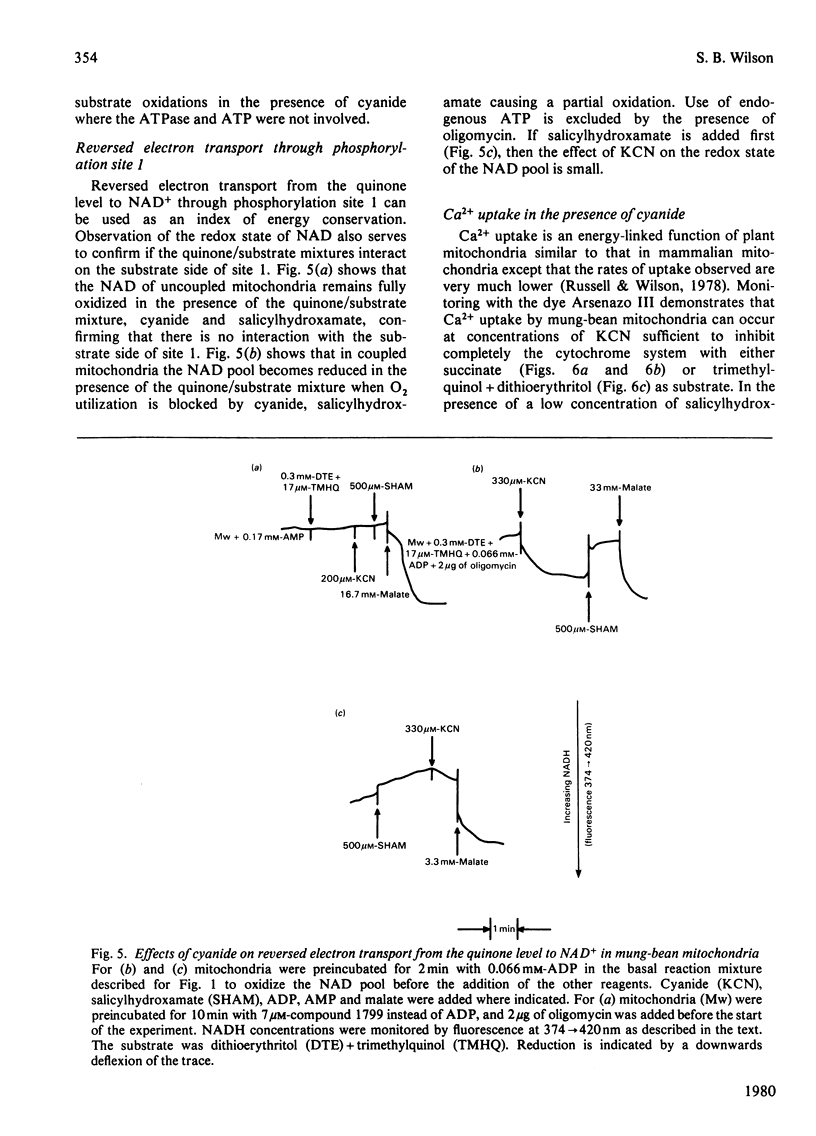
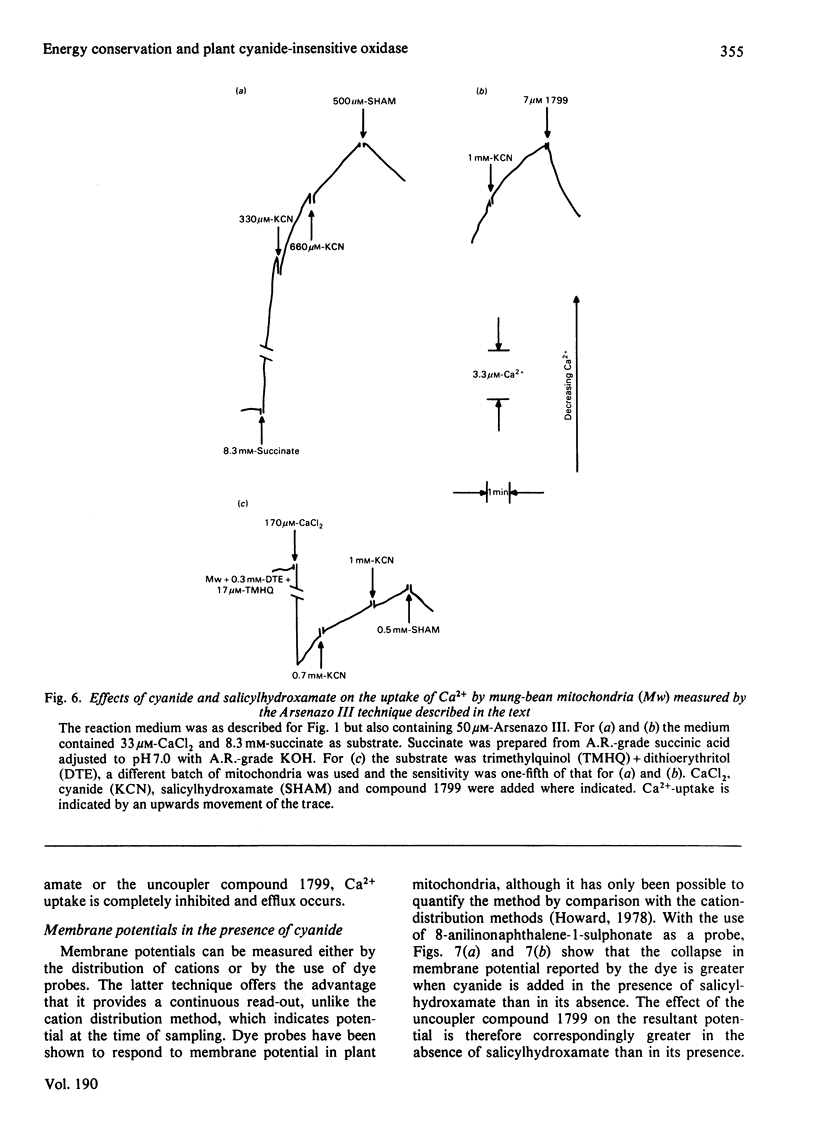
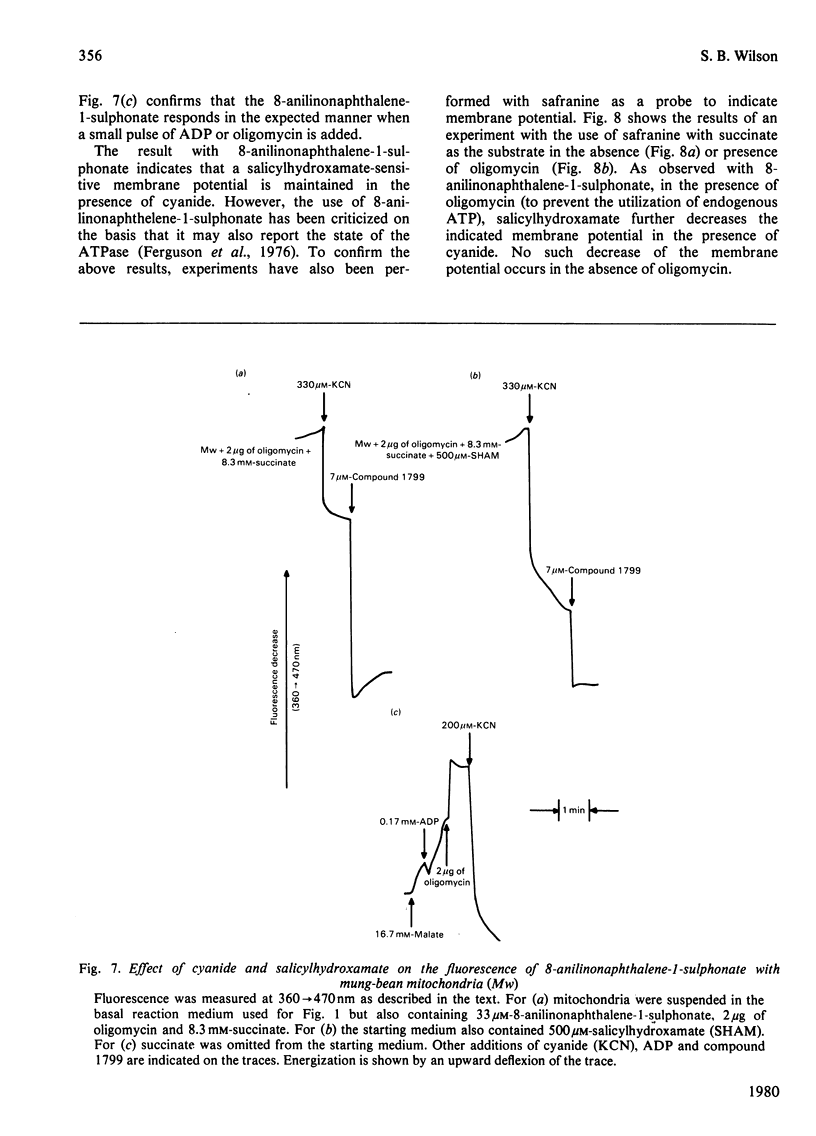
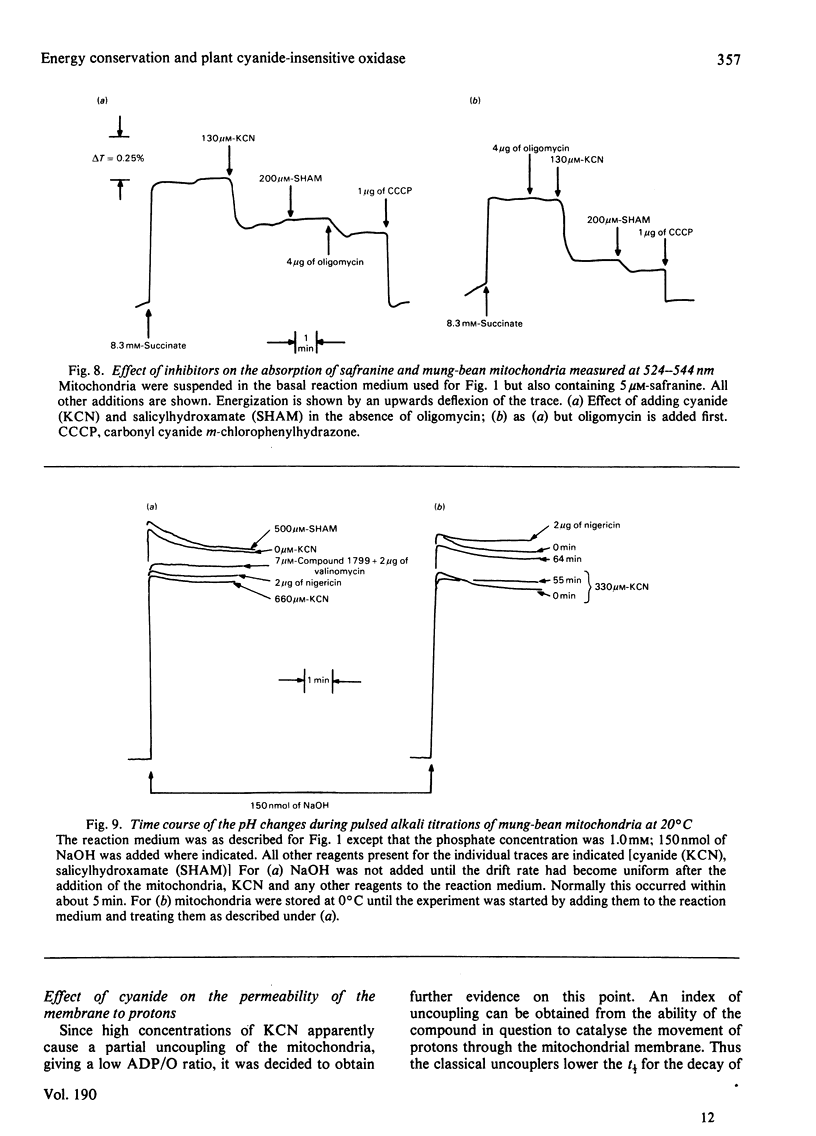
Several measures of energy conservation, namely ADP/O ratio, P/O ratio, ATP/O ratio and phosphorylation detected by continuous assay with purified firefly luciferase and luciferin, all show phosphorylation can occur with mung-bean mitochondria at cyanide concentrations sufficient to inhibit the cytochrome oxidase system. Phosphorylation in the presence of cyanide is uncoupler- oligomycin- and salicylhydroxamate-sensitive. The participation of phosphorylation site 1 is excluded, phosphorylation being attributable to a single phosphorylation site associated with the cyanide-insensitive oxidase. The cyanide-insensitive oxidase has also been shown to support a variety of other energy-linked functions, namely, Ca2+ uptake, reversed electron transport and the maintenance of a membrane potential detected by the dye probes 8-anilinonaphthalene-1-sulphonate and safranine. High concentrations of cyanide have uncoupler-like activity, decreasing the ADP/O ratio and the t 1/2 for the decay of a pH pulse through the the mitochondrial membrane. This uncoupler-like effect is most marked with aged mitochondria. The observations of energy conservation attributable to the cyanide-insensitive oxidase are compared with other reports where it is concluded that the alternative oxidase is uncoupled.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerman K. E., Wikström M. K. Safranine as a probe of the mitochondrial membrane potential. FEBS Lett. 1976 Oct 1;68(2):191–197. doi: 10.1016/0014-5793(76)80434-6. [DOI] [PubMed] [Google Scholar]
- Akimenko V. K., Golovchenko N. P., Medentsev A. G. The absence of energy conservation coupled with electron transfer via the alternative pathway in cyanide-resistant yeast mitochondria. Biochim Biophys Acta. 1979 Mar 15;545(3):398–403. doi: 10.1016/0005-2728(79)90148-8. [DOI] [PubMed] [Google Scholar]
- Azzi A., Chance B., Radda G. K., Lee C. P. A fluorescence probe of energy-dependent structure changes in fragmented membranes. Proc Natl Acad Sci U S A. 1969 Feb;62(2):612–619. doi: 10.1073/pnas.62.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall D. S., Bonner W. D. Cyanide-insensitive Respiration in Plant Mitochondria. Plant Physiol. 1971 Feb;47(2):236–245. doi: 10.1104/pp.47.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P., Gains N. Energy conservation in arum spadix mitochondria. FEBS Lett. 1969 Aug;4(3):164–166. doi: 10.1016/0014-5793(69)80224-3. [DOI] [PubMed] [Google Scholar]
- Doussière J., Sainsard-Chanet A., Vignais P. V. The respiratory chain of Paramecium tetraurelia in wild type and the mutant Cl1. II. Cyanide-insensitive respiration. Function and regulation. Biochim Biophys Acta. 1979 Nov 8;548(2):236–252. doi: 10.1016/0005-2728(79)90132-4. [DOI] [PubMed] [Google Scholar]
- Ferguson S. J., Lloyd W. J., Radda G. K. On the nature of the energised state of submitochondrial particles; investigations with N-aryl naphthalene sulphonate probes. Biochim Biophys Acta. 1976 Feb 16;423(2):174–188. doi: 10.1016/0005-2728(76)90176-6. [DOI] [PubMed] [Google Scholar]
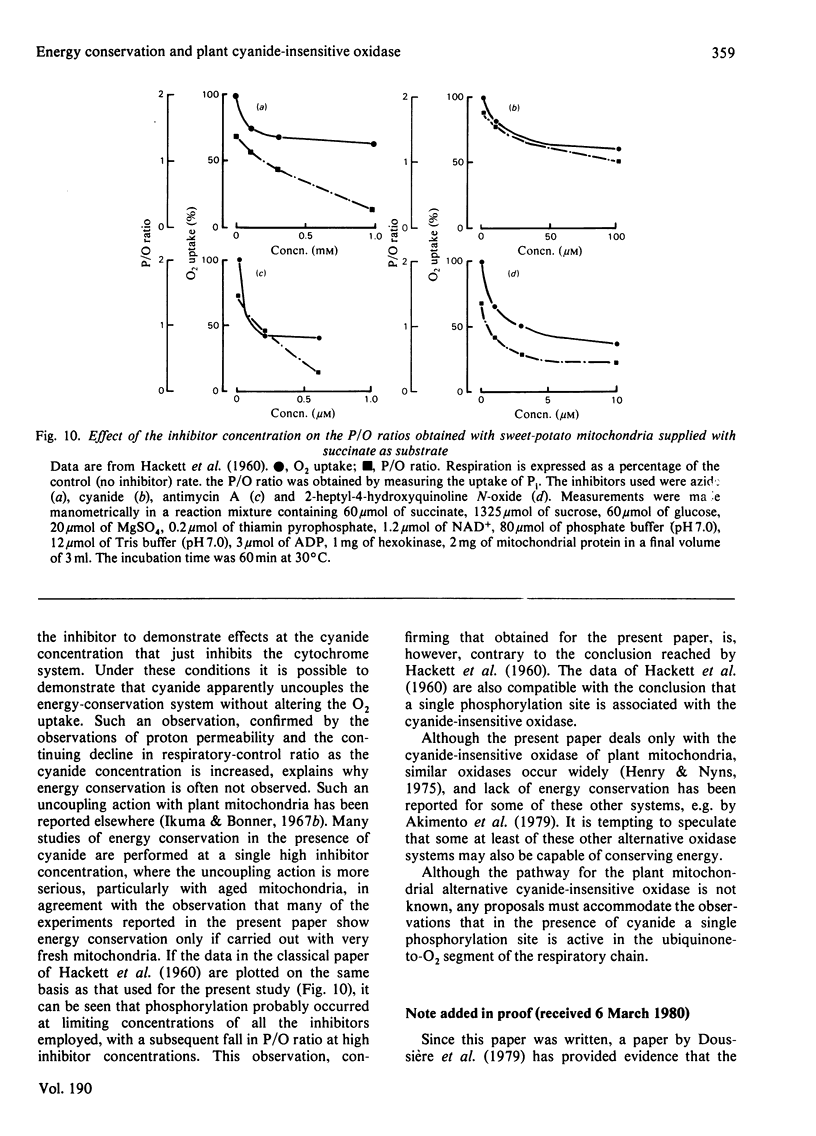
- HACKETT D. P., RICE B., SCHMID C. The partial dissociation of phosphorylation from oxidation in plant mitochondria by respiratory chain inhibitors. J Biol Chem. 1960 Jul;235:2140–2144. [PubMed] [Google Scholar]
- Henry M. F., Nyns E. D. Cyanide-insensitive respiration. An alternative mitochondrial pathway. Subcell Biochem. 1975 Mar;4(1):1–65. [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. I. Isolation and Some Characteristics of Tightly-coupled Mitochondria from Dark-grown Mung Bean Hypocotyls. Plant Physiol. 1967 Jan;42(1):67–75. doi: 10.1104/pp.42.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. III. Effects of Respiratory Inhibitors. Plant Physiol. 1967 Nov;42(11):1535–1544. doi: 10.1104/pp.42.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawford H. G., Garland P. B. Proton translocation coupled to quinol oxidation in ox heart mitochondria. Biochem J. 1973 Nov;136(3):711–720. doi: 10.1042/bj1360711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasters J. J., Hackenbrock C. E. Adenosine triphosphate: continuous measurement in mitochondrial suspension by firefly luciferase luminescence. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1262–1270. doi: 10.1016/s0006-291x(73)80030-0. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Acid-base titration across the membrane system of rat-liver mitochondria. Catalysis by uncouplers. Biochem J. 1967 Aug;104(2):588–600. doi: 10.1042/bj1040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A. L., Bonner W. D., Jr, Rich P. R. The determination of the proton-motive force during cyanide-insensitive respiration in plant mitochondria. Arch Biochem Biophys. 1978 Mar;186(2):298–306. doi: 10.1016/0003-9861(78)90439-3. [DOI] [PubMed] [Google Scholar]
- Schonbaum G. R., Bonner W. D., Jr, Storey B. T., Bahr J. T. Specific inhibition of the cyanide-insensitive respiratory pathway in plant mitochondria by hydroxamic acids. Plant Physiol. 1971 Jan;47(1):124–128. doi: 10.1104/pp.47.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. II. Oxidative phosphorylation in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):126–134. doi: 10.1104/pp.44.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart W. V., Sanadi D. R. Menadiol as an electron donor for reversed oxidative phosphorylation in submitochondrial particles. Biochim Biophys Acta. 1972 Jun 23;267(3):439–443. doi: 10.1016/0005-2728(72)90171-5. [DOI] [PubMed] [Google Scholar]
- Wilson S. B., Bonner W. D. Energy-linked Functions of Submitochondrial Particles Prepared from Mung Bean Mitochondria. Plant Physiol. 1970 Jul;46(1):31–35. doi: 10.1104/pp.46.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. B. Cyanide-insensitive oxidation of ascorbate + NNN'N'-tetramethyl-p-phenylenediamine mixture by mung-bean (Phaseolus aureus) mitochondria. An energy-linked function. Biochem J. 1978 Oct 15;176(1):129–136. doi: 10.1042/bj1760129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. B. Energy conservation associated with cyanide-insensitive respiration in plant mitochondria. Biochim Biophys Acta. 1970 Dec 8;223(2):383–387. doi: 10.1016/0005-2728(70)90195-7. [DOI] [PubMed] [Google Scholar]
- Wilson S. B. Energy conservation in isolated mung-bean (Phaseolus aureus L.) mitochondria in the presence of cyanide [proceedings]. Biochem Soc Trans. 1977;5(5):1508–1509. doi: 10.1042/bst0051508. [DOI] [PubMed] [Google Scholar]


