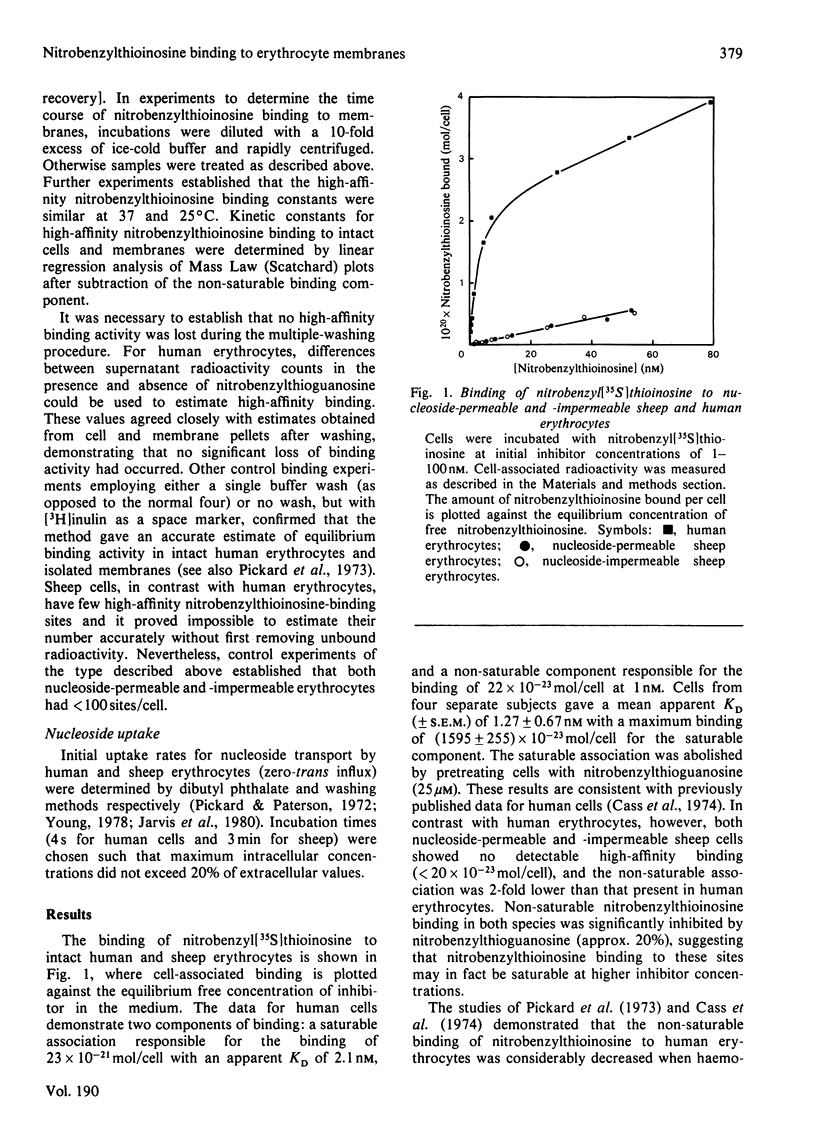
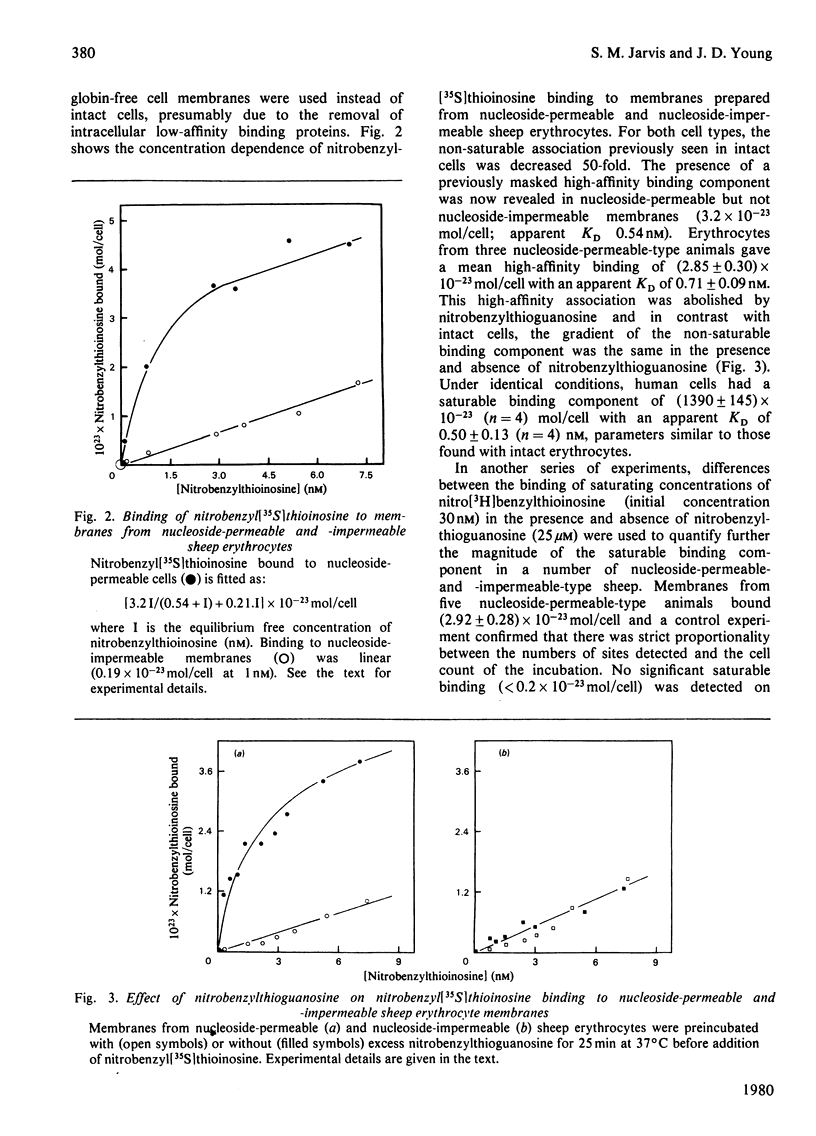
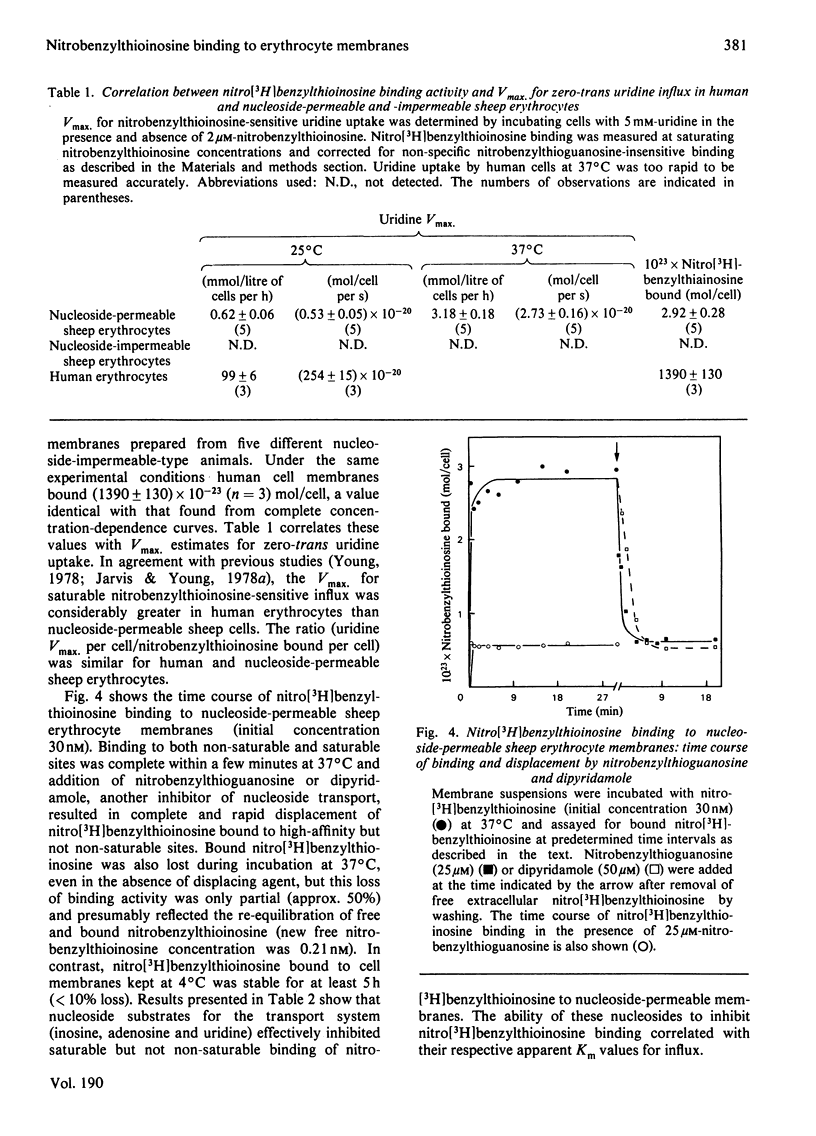
Abstract
Nitrobenzyl[35S]thioinosine binding and nitro[3H]benzylthioinosine binding to nucleoside-permeable and nucleoside-impermeable sheep erythrocyte membranes was investigated, and compared with that found for human erythrocytes. High-affinity nitrobenzylthioinosine-binding sites (apparent KD congruent to 1 nM) were present on human and nucleoside-permeable but not nucleoside-impermeable sheep erythrocyte membranes (8400 and 18 sites/cell for human and sheep nucleoside-permeable sheep erythrocytes was displaced by nitrobenzylthioguanosine and dipyridamole. Uridine, inosine and adenosine inhibited binding. The smaller number of nitrobenzylthioinosine sites on nucleoside-permeable cells compared with human erythrocytes corresponded to a considerably lower Vmax. for uridine influx in these cells (0.53 X 10(-20) mol/cell per s at 25 degrees C compared with 254 X 10(-20) mol/cell per s). It is suggested that high-affinity nitrobenzylthioinosine binding represents a specific interaction with functional nucleoside-transport sites. The uridine-translocation capacity for each transport site at 25 degrees C is 180 molecules/site per s for both nucleoside-permeable sheep cells and human erythrocytes (assuming a 1:1 interaction between nitrobenzylthioinosine and the nucleoside-transport system).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berlin R. D., Oliver J. M. Membrane transport of purine and pyrimidine bases and nucleosides in animal cells. Int Rev Cytol. 1975;42:287–336. doi: 10.1016/s0074-7696(08)60983-3. [DOI] [PubMed] [Google Scholar]
- Cass C. E., Dahlig E., Lau E. Y., Lynch T. P., Paterson A. R. Fluctuations in nucleoside uptake and binding of the inhibitor of nucleoside transport, nitrobenzylthioinosine, during the replication cycle of HeLa cells. Cancer Res. 1979 Apr;39(4):1245–1252. [PubMed] [Google Scholar]
- Cass C. E., Gaudette L. A., Paterson A. R. Mediated transport of nucleosides in human erythrocytes. Specific binding of the inhibitor nitrobenzylthioinosine to nucleoside transport sites in the erythrocyte membrane. Biochim Biophys Acta. 1974 Apr 12;345(1):1–10. doi: 10.1016/0005-2736(74)90239-9. [DOI] [PubMed] [Google Scholar]
- Cass C. E., Paterson A. R. Inhibition of thymidine uptake in asynchronous HeLa cells by nitrobenzylthioinosine. Exp Cell Res. 1977 Mar 15;105(2):427–435. doi: 10.1016/0014-4827(77)90139-2. [DOI] [PubMed] [Google Scholar]
- Cass C. E., Paterson A. R. Mediated transport of nucleosides by human erythrocytes. Specificity toward purine nucleosides as permeants. Biochim Biophys Acta. 1973 Feb 16;291(3):734–746. doi: 10.1016/0005-2736(73)90477-x. [DOI] [PubMed] [Google Scholar]
- Cass C. E., Paterson A. R. Nitrobenzylthionionosine binding sites in the erythrocyte membrane. Biochim Biophys Acta. 1976 Jan 21;419(2):285–294. doi: 10.1016/0005-2736(76)90354-0. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Eilam Y., Cabantchik Z. I. Nucleoside transport in mammalian cell membranes: a specific inhibitory mechanism of high affinity probes. J Cell Physiol. 1977 Aug;92(2):185–201. doi: 10.1002/jcp.1040920207. [DOI] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D., Ansay M., Archibald A. L., Harkness R. A., Simmonds R. J. Is inosine the physiological energy source of pig erythrocytes? Biochim Biophys Acta. 1980 Mar 27;597(1):183–188. doi: 10.1016/0005-2736(80)90162-5. [DOI] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D. Genetic control of nucleoside transport in sheep erythrocytes. Biochem Genet. 1978 Oct;16(9-10):1035–1043. doi: 10.1007/BF00483754. [DOI] [PubMed] [Google Scholar]
- Jung C. Y., Rampal A. L. Cytochalasin B binding sites and glucose transport carrier in human erythrocyte ghosts. J Biol Chem. 1977 Aug 10;252(15):5456–5463. [PubMed] [Google Scholar]
- Lauzon G. J., Paterson A. R. Binding of the nucleoside transport inhibitor nitrobenzylthioinosine to HeLa cells. Mol Pharmacol. 1977 Sep;13(5):883–891. [PubMed] [Google Scholar]
- Lin S., Snyder C. E., Jr High affinity cytochalasin B binding to red cell membrane proteins which are unrelated to sugar transport. J Biol Chem. 1977 Aug 10;252(15):5464–5471. [PubMed] [Google Scholar]
- Lum C. T., Marz R., Plagemann P. G., Wohlhueter R. M. Adenosine transport and metabolism in mouse leukemia cells and in canine thymocytes and peripheral blood leukocytes. J Cell Physiol. 1979 Nov;101(2):173–200. doi: 10.1002/jcp.1041010202. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Mawby W. J., Findlay J. B. Some transport properties of resealed washed human erythrocyte membranes. Biochem J. 1978 Jun 15;172(3):605–611. doi: 10.1042/bj1720605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson A. R., Babb L. R., Paran J. H., Cass C. E. Inhibition by nitrobenzylthioinosine of adenosine uptake by asynchronous HeLa cells. Mol Pharmacol. 1977 Nov;13(6):1147–1158. [PubMed] [Google Scholar]
- Paterson A. R., Naik S. R., Cass C. E. Inhibition of uridine uptake in HeLa cells by nitrobenzylthioinosine and related compounds. Mol Pharmacol. 1977 Nov;13(6):1014–1023. [PubMed] [Google Scholar]
- Paul B., Chen M. F., Paterson A. R. Inhibitors of nucleoside transport. A structure-activity study using human erythrocytes. J Med Chem. 1975 Oct;18(10):968–973. doi: 10.1021/jm00244a003. [DOI] [PubMed] [Google Scholar]
- Pickard M. A., Brown R. R., Paul B., Paterson A. R. Binding of the nucleoside transport inhibitor 4-nitrobenzylthioinosine to erythrocyte membranes. Can J Biochem. 1973 May;51(5):666–672. doi: 10.1139/o73-083. [DOI] [PubMed] [Google Scholar]
- Pickard M. A., Paterson A. R. Fractionation of human erythrocyte membranes. Presence of the nucleoside transport complex in an insoluble residue. Biochim Biophys Acta. 1976 Dec 14;455(3):817–823. doi: 10.1016/0005-2736(76)90051-1. [DOI] [PubMed] [Google Scholar]
- Pickard M. A., Paterson A. R. Use of 4-nitrobenzylthioinosine in the measurement of rates of nucleoside transport in human erythrocytes. Can J Biochem. 1972 Jul;50(7):839–840. doi: 10.1139/o72-116. [DOI] [PubMed] [Google Scholar]
- Taketa P., Attermeier M. H., Mauk G. A. Acetylated hemoglobins in feline blood. J Biol Chem. 1972 Jan 10;247(1):33–35. [PubMed] [Google Scholar]
- Wohlhueter R. M., Marz R., Plagemann P. G. Properties of the thymidine transport system of Chinese hamster ovary cells as probed by nitrobenzylthioinosine. J Membr Biol. 1978 Sep 19;42(3):247–264. doi: 10.1007/BF01870361. [DOI] [PubMed] [Google Scholar]
- Wohlhueter R. M., Marz R., Plagemann P. G. Thymidine transport in cultured mammalian cells. Kinetic analysis, temperature dependence and specificity of the transport system. Biochim Biophys Acta. 1979 May 17;553(2):262–283. doi: 10.1016/0005-2736(79)90231-1. [DOI] [PubMed] [Google Scholar]
- Young J. D. Nucleoside transport in sheep erythrocytes: genetically controlled transport variation and its influence on erythrocyte ATP concentrations. J Physiol. 1978 Apr;277:325–339. doi: 10.1113/jphysiol.1978.sp012274. [DOI] [PMC free article] [PubMed] [Google Scholar]


