Abstract
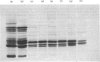
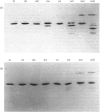
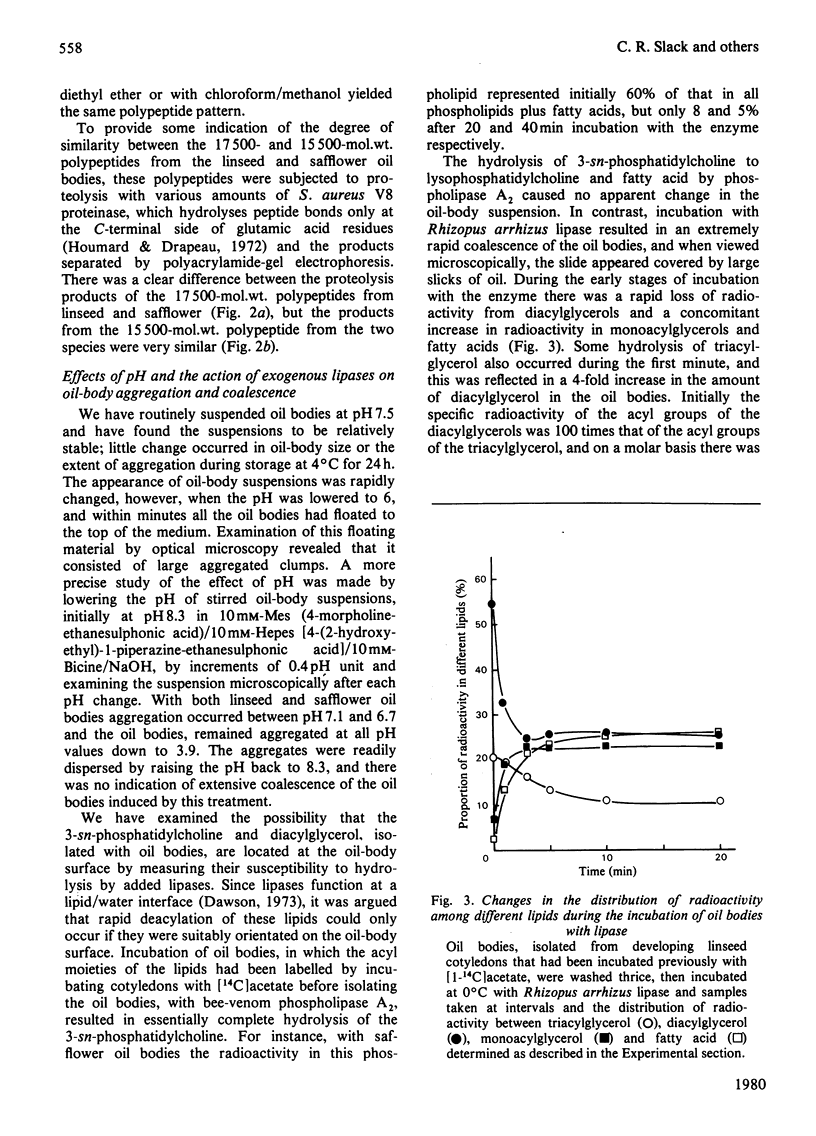
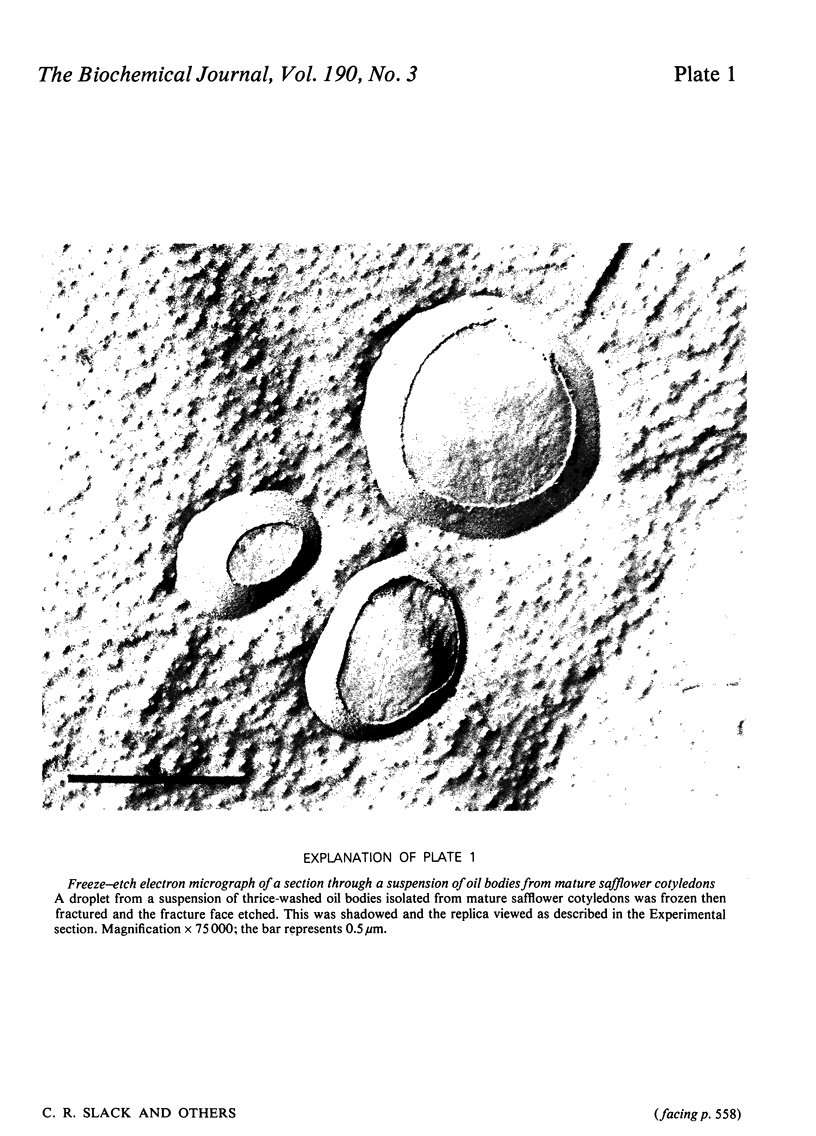
1. The average oil-body diameter in intact cells of developing linseed (Linum usitatissimum) and safflower (Carthamus tinctorius) cotyledons was similar (about 1.4 micrometer), and there was little change in size after oil bodies were isolated and repeatedly washed. 2. The glycerolipid composition of washed oil bodies from both developing and mature cotyledons of the two species was similar; oil bodies from ten different batches of cotyledons contained 4.3 +/- 0.16 mumol of 3-sn-phosphatidylcholine and 25.2 +/- 1.7 mumol of diacylglycerol per 1000 mumol of triacylglycerol. During four successive washings of a once-washed oil-body preparation, the proportion of diacylglycerol to triacylglycerol remained constant and that of 3-sn-phosphatidylcholine to triacylglycerol decreased by only 20%. 3. The protein content of thrice-washed oil bodies from the two species was similar, about 2.4% of the weight of glycerolipids, and appeared to be independent of the stage of cotyledon maturity. Sodium dodecyl sulphate/polyacrylamide-gel electrophoresis indicated that the protein of purified oil bodies from the two species consisted mainly of only four polypeptides and that two of the polypeptides from each species had apparent mol.wts. of 17500 and 15500. Similar patterns of polypeptides were obtained after the hydrolysis of the 15500-mol.wt. polypeptides from linseed and safflower oil bodies by Staphylococcus aureus V8 proteinase, whereas the proteolysis of the 17500-mol.wt. polypeptides from the two species produced different patterns of polypeptides. 4. The 3-sn-phosphatidylcholine in oil-body preparations was hydrolysed about 85% by bee-venom phospholipase A2 without any apparent coalescence of the oil bodies. Incubation with lipase from Rhizopus arrhizus caused rapid coalescence of the oil bodies, and this lipase appeared to initially hydrolyse diacylglycerols in preference to triacylglycerol. 5. Oil bodies from both species were almost completely dispersed in suspensions of pH between 7.1 and 8.3, but formed large aggregates at pH values between 6.7 and 3.9; pH-induced aggregation caused no coalescence. Aggregates formed under acidic conditions were dispersed by re-adjusting the pH of suspensions to 8.3. 6. A freeze-etch electron-microscopic examination of isolated oil bodies indicated that these organelles were bounded by some form of membrane with a particle-free outer surface.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bierbaum T. J., Bouma S. R., Huestis W. H. A mechanism of erythrocyte lysis by lysophosphatidylcholine. Biochim Biophys Acta. 1979 Jul 19;555(1):102–110. doi: 10.1016/0005-2736(79)90075-0. [DOI] [PubMed] [Google Scholar]
- Cashmore A. R. Protein synthesis in plant leaf tissue. The sites of synthesis of the major proteins. J Biol Chem. 1976 May 10;251(9):2848–2853. [PubMed] [Google Scholar]
- Christiansen K. Triacylglycerol synthesis in lipid particles from baker's yeast (Saccharomyces cerevisiae). Biochim Biophys Acta. 1978 Jul 25;530(1):78–90. doi: 10.1016/0005-2760(78)90128-5. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- GREIG M. E., GIBBONS A. J. The effect of phenothiazine drugs on the removal of cholinesterase from erythrocytes and on hemolysis by lysolecithin. Arch Biochem Biophys. 1956 Apr;61(2):335–342. doi: 10.1016/0003-9861(56)90355-1. [DOI] [PubMed] [Google Scholar]
- Gurr M. I., Blades J., Appleby R. S., Smith C. G., Robinson M. P., Nichols B. W. Studies on seed-oil triglycerides. Triglyceride biosynthesis and storage in whole seeds and oil bodies of Crambe abyssinica. Eur J Biochem. 1974 Apr 1;43(2):281–290. doi: 10.1111/j.1432-1033.1974.tb03411.x. [DOI] [PubMed] [Google Scholar]
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T. J., Yatsu L. Y., Altschul A. M. Isolation and characterization of peanut spherosomes. Plant Physiol. 1967 Apr;42(4):585–597. doi: 10.1104/pp.42.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Schumaker M. F. A program which automatically quantitates gel electrophoretic autoradiograms. Anal Biochem. 1978 Dec;91(2):375–393. doi: 10.1016/0003-2697(78)90523-7. [DOI] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Balasingham N. Labelling of glycerolipids in the cotyledons of developing oilseeds by [1-14C] acetate and [2-3H] glycerol. Biochem J. 1978 Feb 15;170(2):421–433. doi: 10.1042/bj1700421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Balasingham N. Labelling studies in vivo on the metabolism of the acyl and glycerol moieties of the glycerolipids in the developing maize leaf. Biochem J. 1977 Feb 15;162(2):289–296. doi: 10.1042/bj1620289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Terpstra J. Some properties of a microsomal oleate desaturase from leaves. Biochem J. 1976 Apr 1;155(1):71–80. doi: 10.1042/bj1550071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G. The kinetics of incorporation in vivo of (14C)acetate and (14C)carbon dioxide into the fatty acids of glycerolipids in developing leaves. Biochem J. 1975 Nov;152(2):217–228. doi: 10.1042/bj1520217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsu L. Y., Jacks T. J., Hensarling T. P. Isolation of spherosomes (oleosomes) from onion, cabbage, and cottonseed tissues. Plant Physiol. 1971 Dec;48(6):675–682. doi: 10.1104/pp.48.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsu L. Y., Jacks T. J. Spherosome membranes: half unit-membranes. Plant Physiol. 1972 Jun;49(6):937–943. doi: 10.1104/pp.49.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]