Abstract
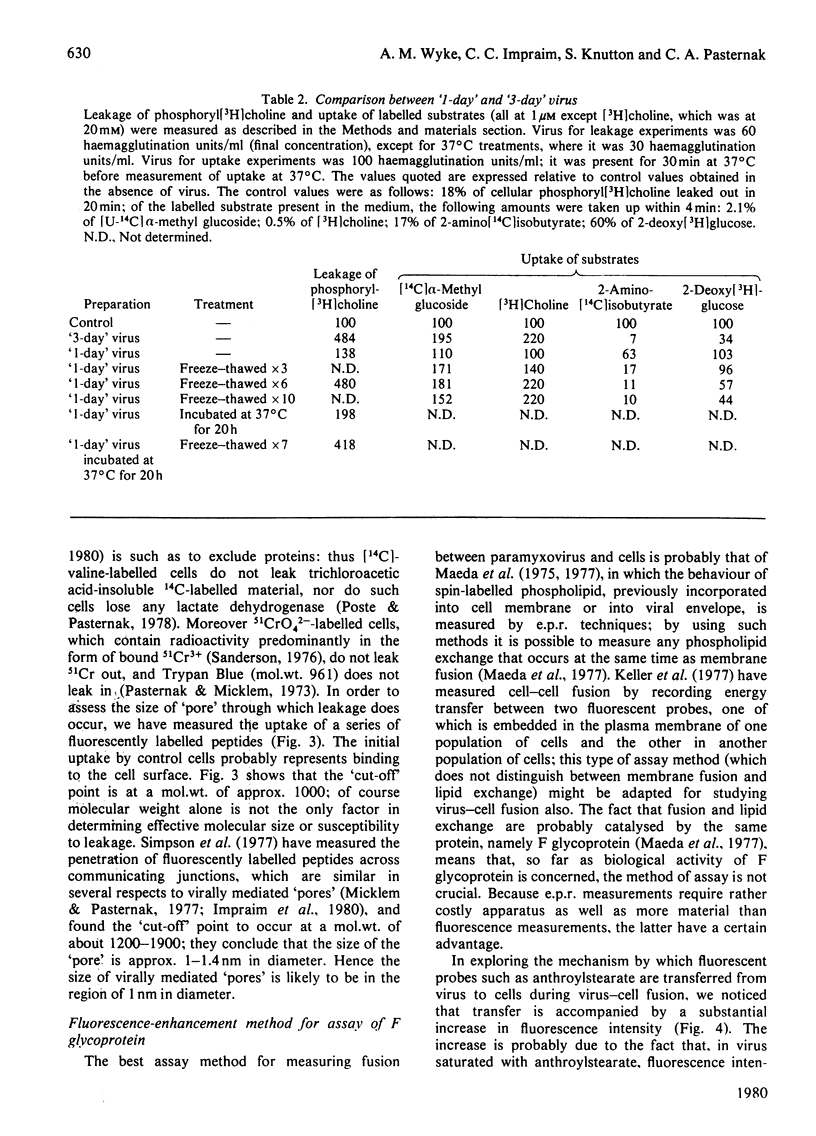
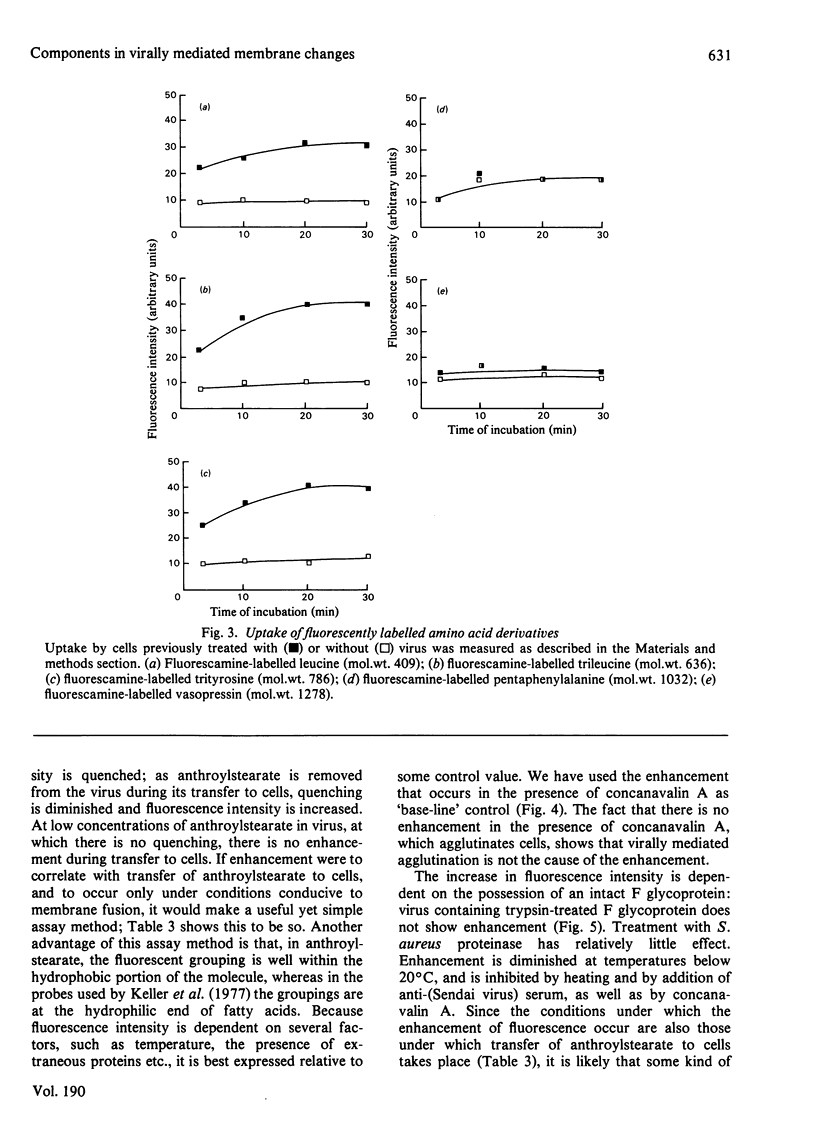
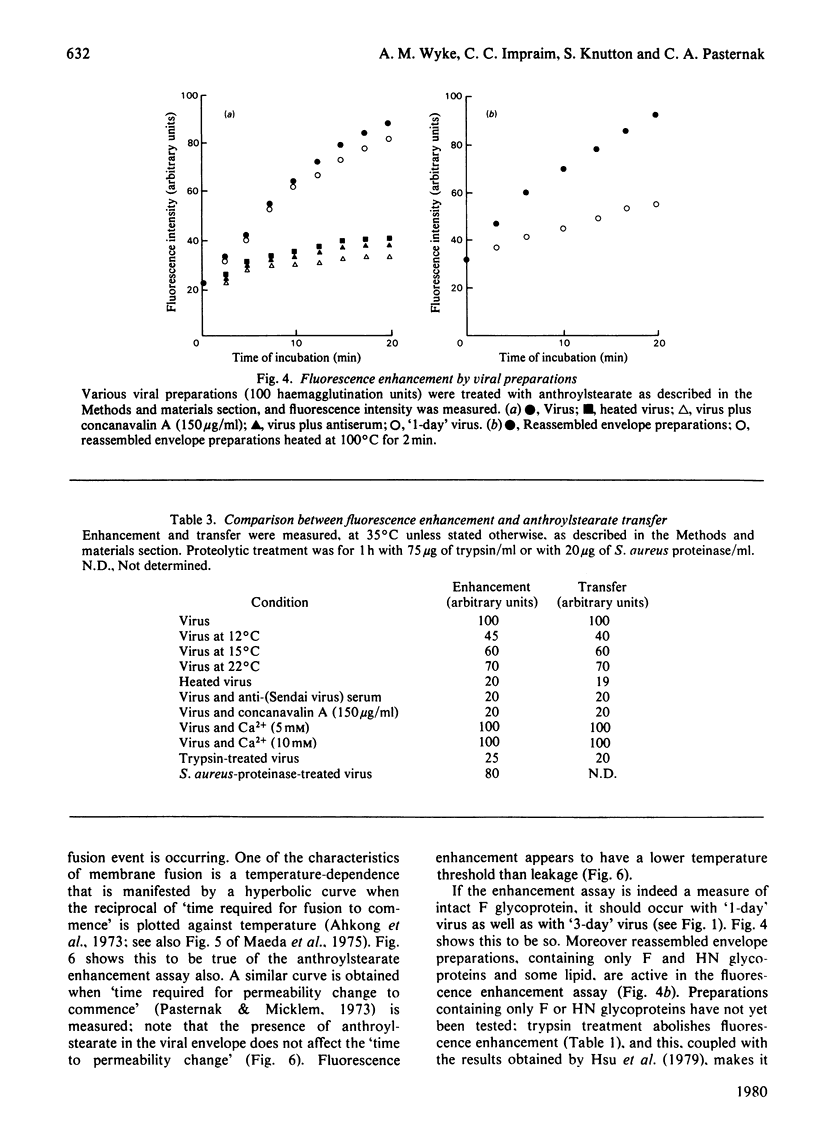
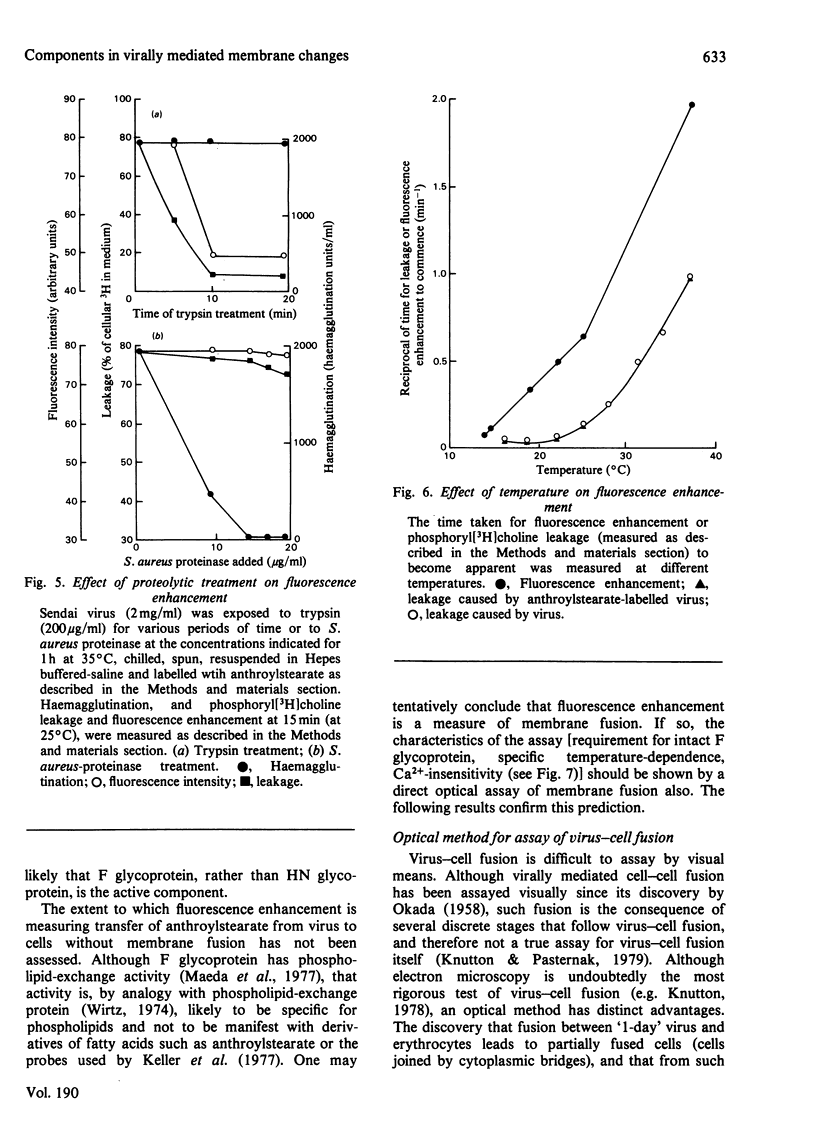
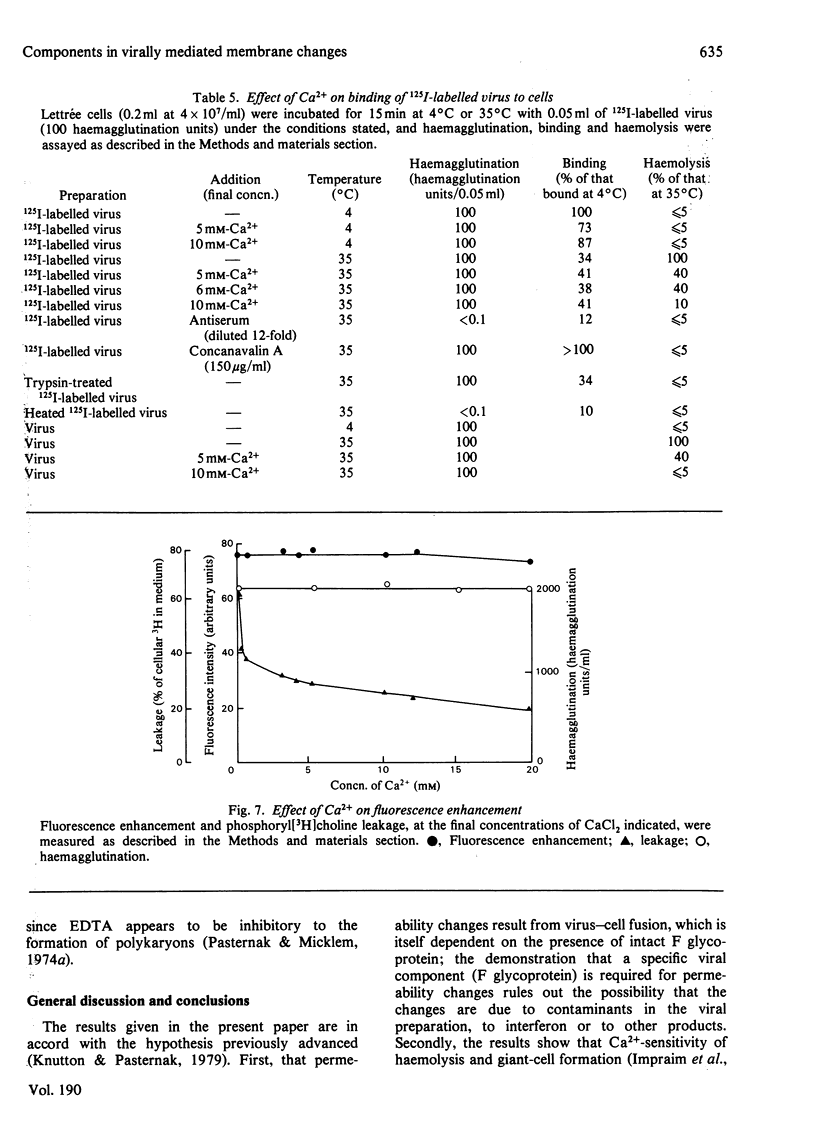
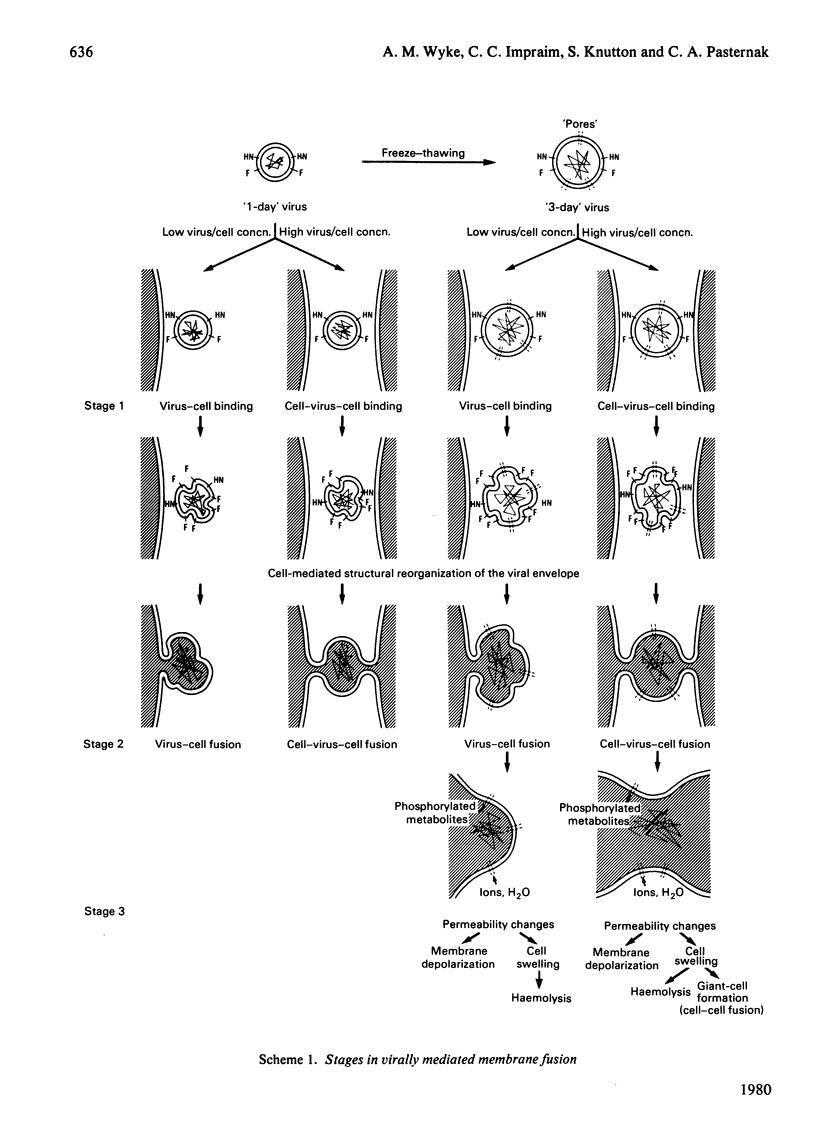
1. Intact F glycoprotein is required to induce permeability changes in Lettrée cells or in erythrocytes. Some HN glycoproteins may also be required. Permeability changes thus offer a simple, accurate and rapid means of assaying the integrity of F glycoprotein in certain viral preparations. 2. The '1-day' virus (which contains intact F glycoprotein but which differs morphologically from '3 day' virus) does not cause permeability changes; it can be rendered active by various physical treatments. It is concluded that the environment in which F glycoprotein is embedded is a determining factor for permeability changes. 3. The entry of fluorescently labelled peptides into cells made permeable by virus has been measured. Peptides having a molecular weight in excess of 1000 enter poorly, suggesting a 'pore' size of approx. 1 nm in diameter. 4. Two novel assay methods concerned with virus--cell fusion are described. The first measures the fluorescence enhancement that occurs when anthroylstearate is transferred from anthroylstearate-labelled virus to cells. The second measures the giant-cell formation that occurs when partially fused erythrocytes are exposed to hypo-osmotic treatment. The '1-day' virus is active in these assays. In contrast with permeability changes, virus--cell fusion is insensitive to changes in external Ca2+-concentration. 5. The results are compatible with a model [Knutton & Pasternak (1979) Trends Biochem. Sci. 4, 220--223; Impraim, Foster, Micklem & Pasternak (1980) Biochem. J. 186, 847--860] in which virus--cell fusion is a prerequisite for permeability changes, and in which permeability changes are the cause of haemolysis and giant-cell (polykaryon) formation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahkong Q. F., Cramp F. C., Fisher D., Howell J. I., Tampion W., Verrinder M., Lucy J. A. Chemically-induced and thermally-induced cell fusion: lipid-lipid interactions. Nat New Biol. 1973 Apr 18;242(120):215–217. doi: 10.1038/newbio242215a0. [DOI] [PubMed] [Google Scholar]
- Blow A. M., Botham G. M., Lucy J. A. Calcium ions and cell fusion. Effects of chemical fusogens on the permeability of erythrocytes to calcium and other ions. Biochem J. 1979 Aug 15;182(2):555–563. doi: 10.1042/bj1820555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bächi T., Eichenberger G., Hauri H. P. Sendai virus hemolysis: influence of lectins and analysis by immune fluorescence. Virology. 1978 Apr;85(2):518–530. doi: 10.1016/0042-6822(78)90458-0. [DOI] [PubMed] [Google Scholar]
- Carrasco L. The inhibition of cell functions after viral infection. A proposed general mechanism. FEBS Lett. 1977 Apr 1;76(1):11–15. doi: 10.1016/0014-5793(77)80110-5. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham A. C. Do viruses use calcium ions to shut off host cell functions? Nature. 1977 May 26;267(5609):375–376. doi: 10.1038/267375c0. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Holloway P. W. A simple procedure for removal of Triton X-100 from protein samples. Anal Biochem. 1973 May;53(1):304–308. doi: 10.1016/0003-2697(73)90436-3. [DOI] [PubMed] [Google Scholar]
- Homma M., Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973 Dec;12(6):1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M., Shimizu K., Shimizu Y. K., Ishida N. On the study of Sendai virus hemolysis. I. Complete Sendai virus lacking in hemolytic activity. Virology. 1976 May;71(1):41–47. doi: 10.1016/0042-6822(76)90092-1. [DOI] [PubMed] [Google Scholar]
- Hosaka Y., Shimizu Y. K. Artificial assembly of envelope particles of HVJ (Sendai virus). II. Lipid components for formation of the active hemolysin. Virology. 1972 Sep;49(3):640–646. doi: 10.1016/0042-6822(72)90520-x. [DOI] [PubMed] [Google Scholar]
- Hsu M. C., Scheid A., Choppin P. W. Reconstitution of membranes with individual paramyxovirus glycoproteins and phospholipid in cholate solution. Virology. 1979 Jun;95(2):476–491. doi: 10.1016/0042-6822(79)90502-6. [DOI] [PubMed] [Google Scholar]
- Impraim C. C., Foster K. A., Micklem K. J., Pasternak C. A. Nature of virally mediated changes in membrane permeability to small molecules. Biochem J. 1980 Mar 15;186(3):847–860. doi: 10.1042/bj1860847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impraim C. C., Micklem K. J., Pasternak C. A. Calcium, cells and virus--alterations caused by paramyxoviruses. Biochem Pharmacol. 1979 Jun 15;28(12):1963–1969. doi: 10.1016/0006-2952(79)90652-x. [DOI] [PubMed] [Google Scholar]
- Ishida N., Homma M. Sendai virus. Adv Virus Res. 1978;23:349–383. doi: 10.1016/s0065-3527(08)60103-7. [DOI] [PubMed] [Google Scholar]
- Keller P. M., Person S., Snipes W. A fluorescence enhancement assay of cell fusion. J Cell Sci. 1977 Dec;28:167–177. doi: 10.1242/jcs.28.1.167. [DOI] [PubMed] [Google Scholar]
- Kim J., Hama K., Miyake Y., Okada Y. Transformation of intramembrane particles of HVJ (Sendai virus) envelopes from an invisible to visible form on aging of virions. Virology. 1979 Jun;95(2):523–535. doi: 10.1016/0042-6822(79)90506-3. [DOI] [PubMed] [Google Scholar]
- Knutton S., Bächi T. The role of cell swelling and haemolysis in Sendai virus-induced cell fusion and in the diffusion of incorporated viral antigens. J Cell Sci. 1980 Apr;42:153–167. doi: 10.1242/jcs.42.1.153. [DOI] [PubMed] [Google Scholar]
- Knutton S. Changes in viral envelope structure preceding infection. Nature. 1976 Dec 16;264(5587):672–673. doi: 10.1038/264672a0. [DOI] [PubMed] [Google Scholar]
- Knutton S., Jackson D., Graham J. M., Micklem K. J., Pasternak C. A. Microvilli and cell swelling. Nature. 1976 Jul 1;262(5563):52–54. doi: 10.1038/262052a0. [DOI] [PubMed] [Google Scholar]
- Kobayashi N. Hemolytic activity of rubella virus. Virology. 1978 Sep;89(2):610–612. doi: 10.1016/0042-6822(78)90202-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maeda T., Asano A., Okada Y., Ohnishi S. I. Transmembrane phospholipid motions induced by F glycoprotein in hemagglutinating virus of Japan. J Virol. 1977 Jan;21(1):232–241. doi: 10.1128/jvi.21.1.232-241.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., Asano A., Oki K., Okada Y., Onishi S. A spin-label study on fusion of red blood cells induced by hemagglutinating virus of Japan. Biochemistry. 1975 Aug 26;14(17):3736–3741. doi: 10.1021/bi00688a003. [DOI] [PubMed] [Google Scholar]
- Micklem K. J., Pasternak C. A. Surface components involved in virally mediated membrane changes. Biochem J. 1977 Feb 15;162(2):405–410. doi: 10.1042/bj1620405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath A. R., Vernon S. K., Hartzell R. W., Wiener F. P., Rubin B. A. Haemolysis by Sendai virus: involvement of a virus protein component. J Gen Virol. 1973 Apr;19(1):21–36. doi: 10.1099/0022-1317-19-1-21. [DOI] [PubMed] [Google Scholar]
- Okada Y., Murayama F. Requirement of calcium ions for the cell fusion reaction of animal cells by HVJ. Exp Cell Res. 1966 Nov-Dec;44(2):527–551. doi: 10.1016/0014-4827(66)90458-7. [DOI] [PubMed] [Google Scholar]
- Pasternak C. A., Micklem K. J. Permeability changes during cell fusion. J Membr Biol. 1973;14(3):293–303. doi: 10.1007/BF01868082. [DOI] [PubMed] [Google Scholar]
- Pasternak C. A., Micklem K. J. The biochemistry of virus-induced cell fusion. Changes in membrane integrity. Biochem J. 1974 Jun;140(3):405–411. doi: 10.1042/bj1400405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak C. A., Micklem K. J. Virally mediated membrane changes: inverse effects on transport and diffusion. Biochem J. 1974 Dec;144(3):593–595. doi: 10.1042/bj1440593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk S. J., Ahkong Q. F., Botham G. M., Vos J., Lucy J. A. Membrane proteins in human erythrocytes during cell fusion induced by oleoylglycerol. Biochem J. 1978 Oct 15;176(1):159–167. doi: 10.1042/bj1760159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson C. J. The uptake and retention of chromium by cells. Transplantation. 1976 Jun;21(6):526–529. [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Isida N. The smallest protein of Sendi virus: its candidate function of binding nucleocaspsid to envelope. Virology. 1975 Oct;67(2):427–437. [PubMed] [Google Scholar]
- Shimizu Y. K., Shimizu K., Ishida N., Homma M. On the study of Sendai virus hemolysis. II. Morphological study of envelop fusion and hemolysis. Virology. 1976 May;71(1):48–60. doi: 10.1016/0042-6822(76)90093-3. [DOI] [PubMed] [Google Scholar]
- Simpson I., Rose B., Loewenstein W. R. Size limit of molecules permeating the junctional membrane channels. Science. 1977 Jan 21;195(4275):294–296. doi: 10.1126/science.831276. [DOI] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Volsky D. J., Loyter A. An efficient method for reassembly of fusogenic Sendai virus envelopes after solubilization of intact virions with Triton X-100. FEBS Lett. 1978 Aug 15;92(2):190–194. doi: 10.1016/0014-5793(78)80751-0. [DOI] [PubMed] [Google Scholar]
- Volsky D. J., Loyter A. Role of Ca++ in virus-induced membrane fusion. Ca++ accumulation and ultrastructural changes induced by Sendai virus in chicken erythrocytes. J Cell Biol. 1978 Aug;78(2):465–479. doi: 10.1083/jcb.78.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz K. W. Transfer of phospholipids between membranes. Biochim Biophys Acta. 1974 Sep 16;344(2):95–117. doi: 10.1016/0304-4157(74)90001-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Inoue K. Interaction of paramyxoviruses with concanavalin A-modified erythrocyte membranes. Virology. 1978 Jan;84(1):203–206. doi: 10.1016/0042-6822(78)90234-9. [DOI] [PubMed] [Google Scholar]