Abstract
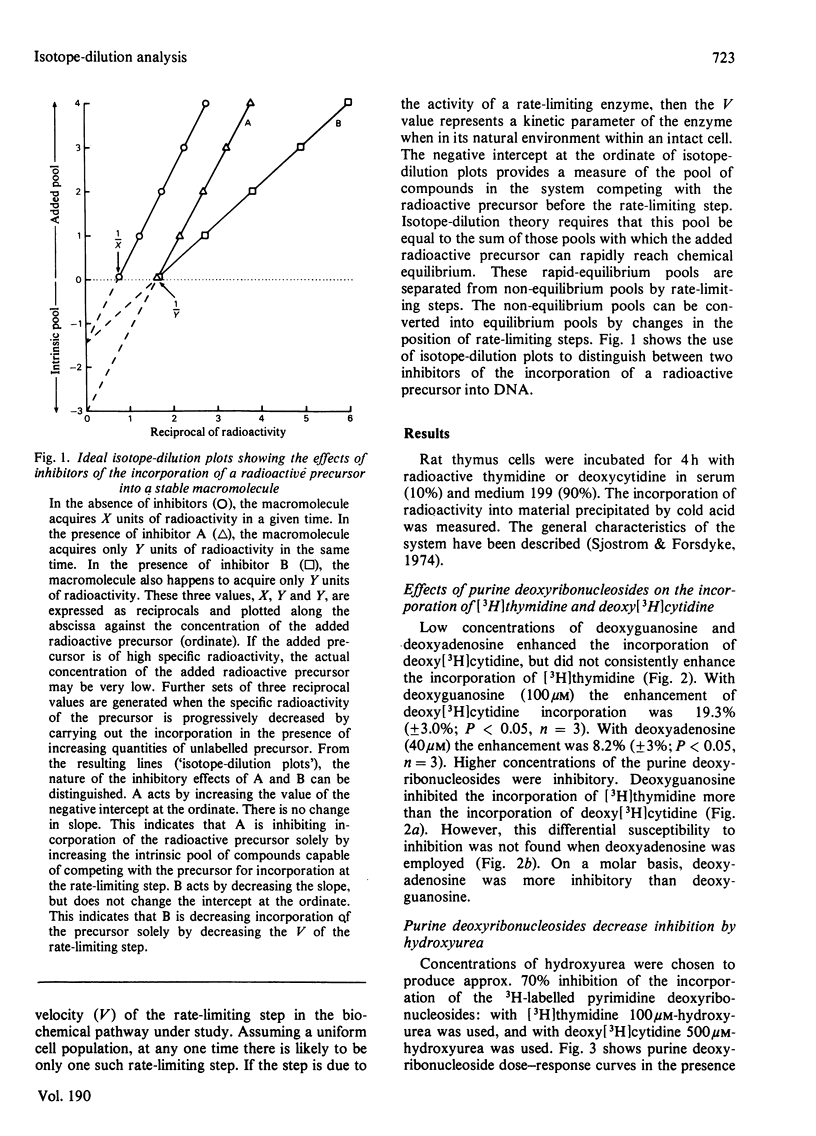
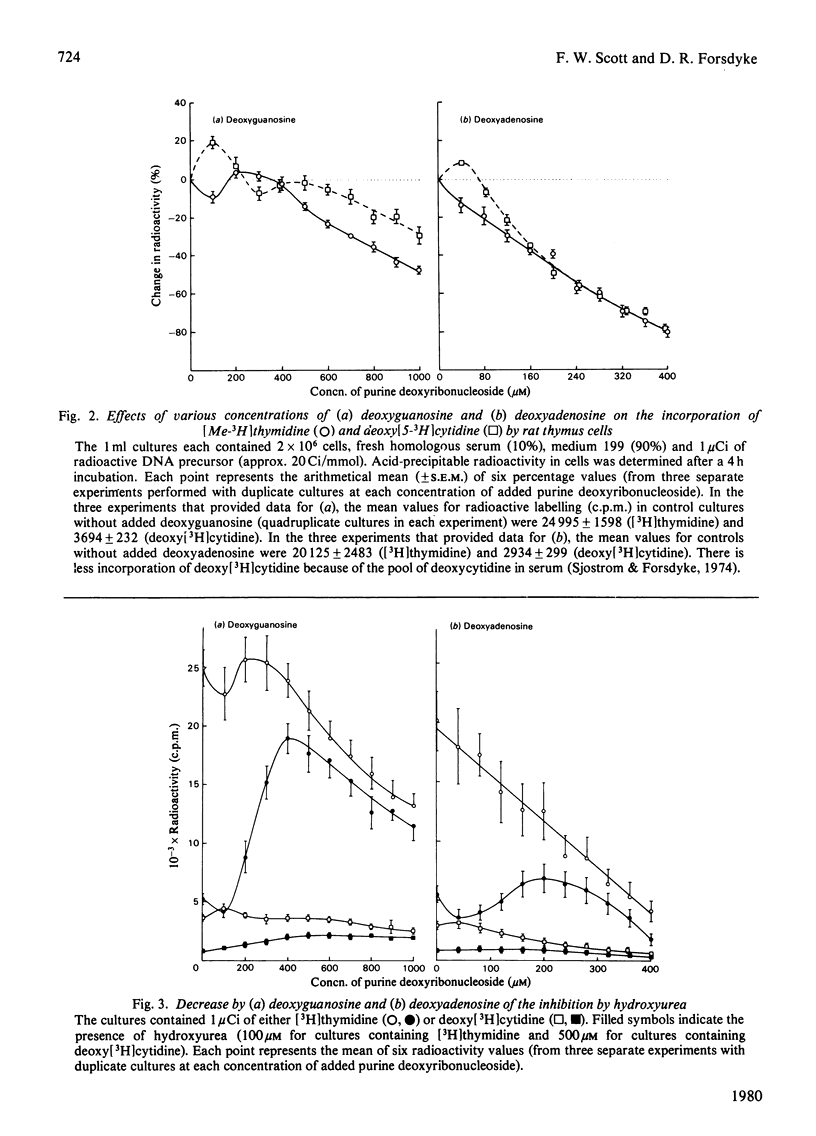
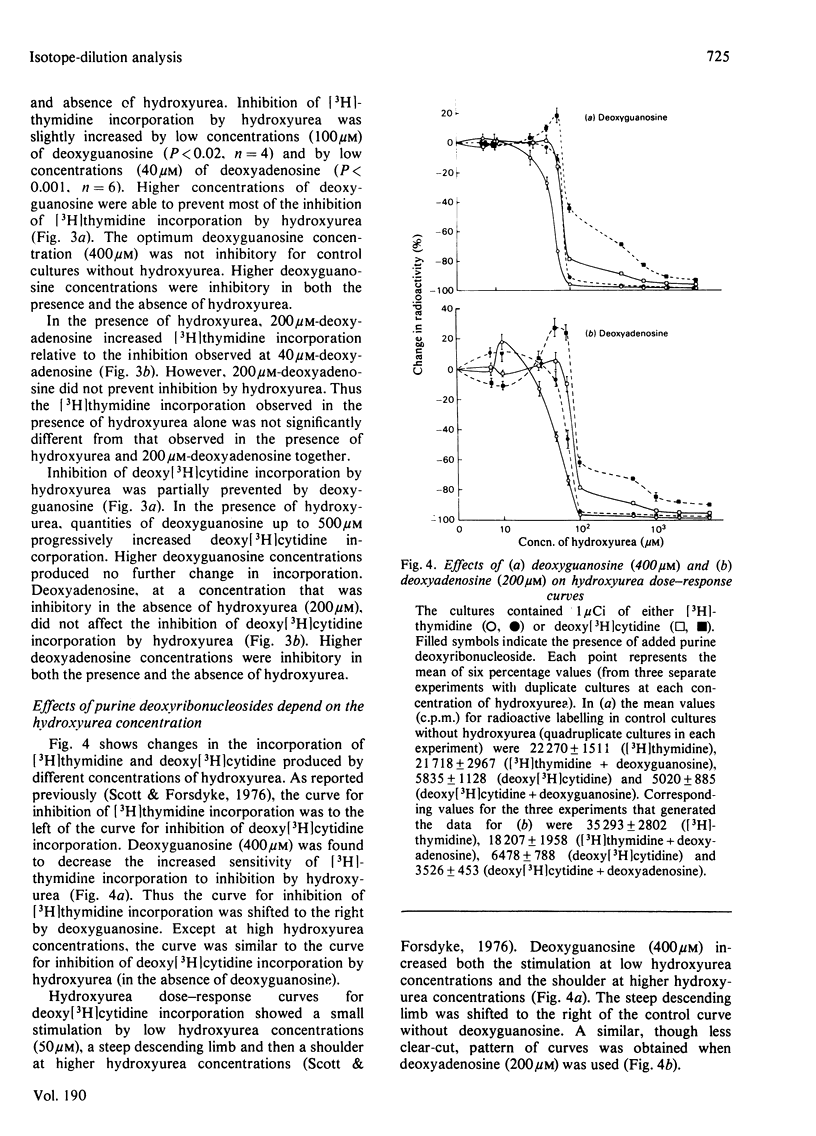
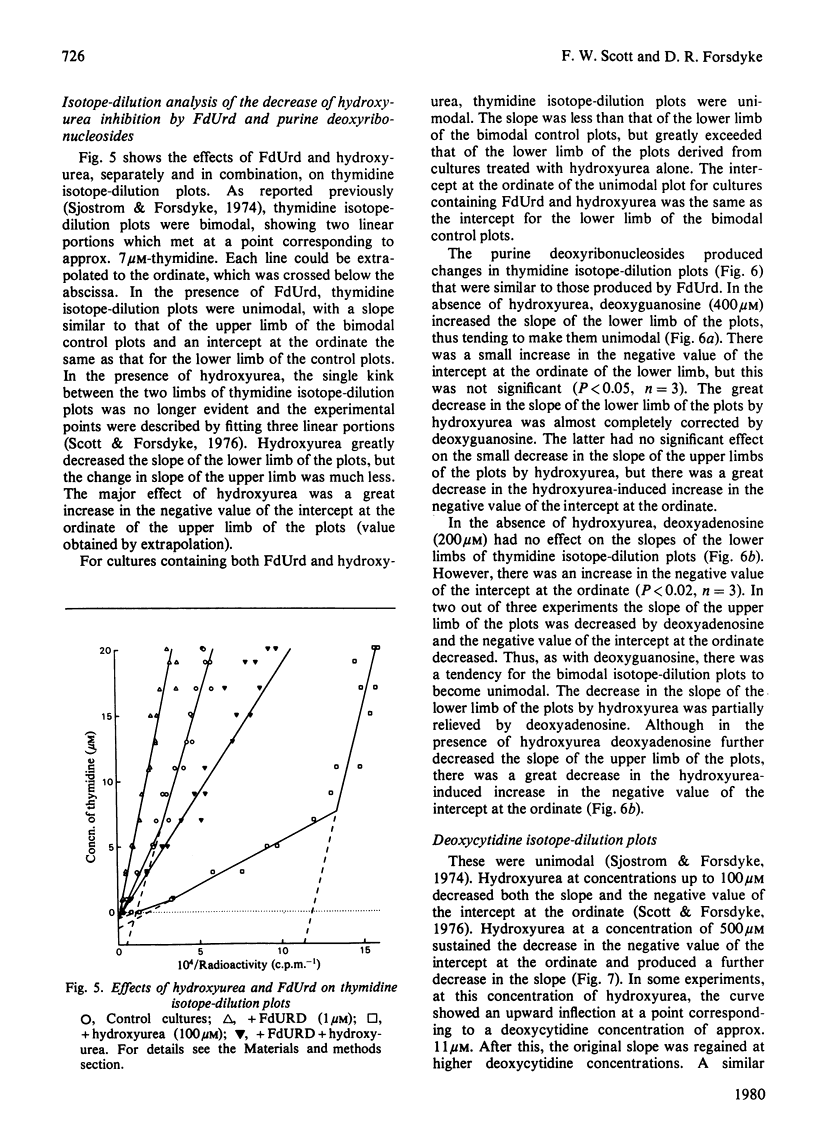
It is presumed that the dGTP and dATP needed for replicative DNA synthesis can be formed by way of either `salvage' pathways or biosynthesis de novo. This was examined by adding hydroxyurea to cultures of rat thymus cells to inhibit ribonucleoside diphosphate reductase, a key enzyme of the `de novo' pathway. Most of the inhibition of the incorporation of [Me-3H]thymidine and deoxy[5-3H]cytidine by low concentrations of hydroxyurea (100–500μm) was prevented by substrates of the salvage pathway (400μm-deoxyguanosine and, to a lesser extent, 200μm-deoxyadenosine). However, isotope-dilution studies indicated that the purine deoxyribonucleosides prevented inhibition by decreasing pyrimidine deoxyribonucleotide competitor pools. Evidence was obtained that a hydroxyurea-induced increase in the thymidine-competitor pool (probably dTTP) was prevented to an equal extent by deoxyguanosine and by the inhibitor of thymidylate synthase, deoxy-5-fluorouridine. These compounds had almost identical effects on hydroxyurea dose–response curves and on thymidine isotope-dilution plots. The evidence suggests that exogenous purine deoxyribonucleosides cannot prevent the inhibition by hydroxyurea of thymus-cell DNA synthesis. This could mean that, with respect to the metabolism of purine deoxyribonucleotides, ribonucleoside diphosphate reductase is tightly coupled to DNA polymerase in a multienzyme complex. The complex would not permit entry of exogenous metabolic intermediates into the `de novo' pathway, but would still be subject to the regulatory effects of these intermediates. Thus dGTP and dATP formed from exogenous purine deoxyribonucleosides by salvage pathways might deplete pyrimidine deoxyribonucleotide competitor pools by inhibiting relatively hydroxyurea-insensitive activities of ribonucleoside diphosphate reductase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. L., Berryman S., Thomson A. Deoxyribonucleoside triphosphate pools in synchronized and drug-inhibited L 929 cells. Biochim Biophys Acta. 1971 Jul 29;240(4):455–462. doi: 10.1016/0005-2787(71)90702-7. [DOI] [PubMed] [Google Scholar]
- Adams R. L., Lindsay J. G. Hydroxyurea reversal of inhibition and use as a cell-synchronizing agent. J Biol Chem. 1967 Mar 25;242(6):1314–1317. [PubMed] [Google Scholar]
- Brutlag D., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. 36. A proofreading function for the 3' leads to 5' exonuclease activity in deoxyribonucleic acid polymerases. J Biol Chem. 1972 Jan 10;247(1):241–248. [PubMed] [Google Scholar]
- Carson D. A., Kaye J., Seegmiller J. E. Differential sensitivity of human leukemic T cell lines and B cell lines to growth inhibition by deoxyadenosine. J Immunol. 1978 Nov;121(5):1726–1731. [PubMed] [Google Scholar]
- Carson D. A., Kaye J., Seegmiller J. E. Lymphospecific toxicity in adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency: possible role of nucleoside kinase(s). Proc Natl Acad Sci U S A. 1977 Dec;74(12):5677–5681. doi: 10.1073/pnas.74.12.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham J. P., Ives D. H. Deoxycytidine kinase. I. Distribution in normal and neoplastic tissues and interrelationships of deoxycytidine and 1-beta-D-arabinofuranosylcytosine phosphorylation. Mol Pharmacol. 1969 Jul;5(4):358–375. [PubMed] [Google Scholar]
- Forsdyke D. R. Application of the isotope-dilution principle to the analysis of factors affecting the incorporation of (3H) uridine and (3H) cytidine into cultured lymphocytes. Evaluation of pools in serum and culture media. Biochem J. 1971 Dec;125(3):721–732. doi: 10.1042/bj1250721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawtrey A. O., Scott-Burden T., Robertson G. Inhibition of glycoprotein and glycolipid synthesis in hamster embryo cells by cytosine arabinoside and hydroxyurea. Nature. 1974 Nov 1;252(5478):58–60. doi: 10.1038/252058a0. [DOI] [PubMed] [Google Scholar]
- Jackson R. C. The regulation of thymidylate biosynthesis in Novikoff hepatoma cells and the effects of amethopterin, 5-fluorodeoxyuridine, and 3-deazauridine. J Biol Chem. 1978 Oct 25;253(20):7440–7446. [PubMed] [Google Scholar]
- Kizer D. E., Holman L. Purification and properties of thymidine kinase from regenerating rat liver. Biochim Biophys Acta. 1974 May 20;350(1):193–200. doi: 10.1016/0005-2744(74)90217-4. [DOI] [PubMed] [Google Scholar]
- MORGAN J. F., MORTON H. J., PARKER R. C. Nutrition of animal cells in tissue culture; initial studies on a synthetic medium. Proc Soc Exp Biol Med. 1950 Jan;73(1):1–8. doi: 10.3181/00379727-73-17557. [DOI] [PubMed] [Google Scholar]
- Marz R., Wohlhueter R. M., Plagemann P. G. Relationship between thymidine transport and phosphorylation in Novikoff rat hepatoma cells as analyzed by a rapid sampling technique. J Supramol Struct. 1977;6(3):433–440. doi: 10.1002/jss.400060316. [DOI] [PubMed] [Google Scholar]
- Mathews C. K., North T. W., Prem veer Reddy G. Multienzyme complexes in DNA precursor biosynthesis. Adv Enzyme Regul. 1978;17:133–156. doi: 10.1016/0065-2571(79)90011-6. [DOI] [PubMed] [Google Scholar]
- Mitchell B. S., Mejias E., Daddona P. E., Kelley W. N. Purinogenic immunodeficiency diseases: selective toxicity of deoxyribonucleosides for T cells. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5011–5014. doi: 10.1073/pnas.75.10.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch-Petersen B., Tyrsted G. Induction of thymidine kinases in phytohaemagglutinin-stimulated human lymphocytes. Biochim Biophys Acta. 1977 Oct 4;478(3):364–375. doi: 10.1016/0005-2787(77)90152-6. [DOI] [PubMed] [Google Scholar]
- Nawata H., Kamiya T. Two molecular forms of thymidine kinase in the cytosol of regenerating rat liver. J Biochem. 1975 Dec;78(6):1215–1224. doi: 10.1093/oxfordjournals.jbchem.a131019. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Erbe J. Intracellular conversions of deoxyribonucleosides by Novikoff rat hepatoma cells and effects of hydroxyurea. J Cell Physiol. 1974 Jun;83(3):321–336. doi: 10.1002/jcp.1040830302. [DOI] [PubMed] [Google Scholar]
- Scott F. W., Forsdyke D. R. Isotope-dilution studies of the effects of 5-fluorodeoxyuridine and hydroxyurea on the incorporation of deoxycytidine and thymidine by cultured thymus cells. Can J Biochem. 1976 Mar;54(3):238–248. doi: 10.1139/o76-037. [DOI] [PubMed] [Google Scholar]
- Scott F. W., Forsdyke D. R. The rate of deoxyribonucleic acid synthesis by cultured Chinese-hamster ovary cells. An application of isotope-dilution analysis. Biochem J. 1978 Mar 15;170(3):545–549. doi: 10.1042/bj1700545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom D. A., Forsdyke D. R. Isotope-dilution analysis of rate-limiting steps and pools affecting the incorporation of thymidine and deoxycytidine into cultured thymus cells. Biochem J. 1974 Feb;138(2):253–262. doi: 10.1042/bj1380253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog L., Nordenskjöld B. Effects of hydroxyurea and 1-beta-D-arabinofuranosyl-cytosine on deoxyribonucleotide pools in mouse embryo cells. Eur J Biochem. 1971 Mar 1;19(1):81–89. doi: 10.1111/j.1432-1033.1971.tb01290.x. [DOI] [PubMed] [Google Scholar]
- Snyder F. F., Henderson J. F. Alternative pathways of deoxyadenosine and adenosine metabolism. J Biol Chem. 1973 Aug 25;248(16):5899–5904. [PubMed] [Google Scholar]
- Tattersall M. H., Ganeshaguru K., Hoffbrand A. V. The effect of external deoxyribonucleosides on deoxyribonucleoside triphosphate concentrations in human lymphocytes. Biochem Pharmacol. 1975 Aug 15;24(16):1495–1498. doi: 10.1016/0006-2952(75)90025-8. [DOI] [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Yarbro J. W. Further studies on the mechanism of action of hydroxyurea. Cancer Res. 1968 Jun;28(6):1082–1087. [PubMed] [Google Scholar]
- Young C. W., Schochetman G., Hodas S., Balis M. E. Inhibition of DNA synthesis by hydroxyurea: structure-activity relationships. Cancer Res. 1967 Mar;27(3):535–540. [PubMed] [Google Scholar]


