Abstract
An enzyme able to catalyse the polymerization of deoxyribonucleotides in a template-independent manner was isolated from dry wheat germ. This activity is associated with a soluble protein which is homogeneous with respect to the molecular weight (approx. 500000) and, under denaturing conditions, dissociates into product of two size classes, 67000 and 45000 daltons respectively. The enzyme-catalysed polymerization can be primed by oligo- as well as poly-deoxyribonucleotides, and is highly efficient (234 nmol/h per mg of finally purified protein) when only one of the four deoxyribonucleoside 5'-triphosphates is present in the incubation mixture. An extension of the 3'-hydroxy termini of polydeoxyribonucleotide chains for approx. 40 nucleotide residues was achieved when non-denatured DNA and [3H]dTTP were used as the primer and substrate respectively. It is concluded that the enzyme isolated from wheat germ shares catalytic properties with the terminal deoxynucleotidyltransferase of mammalian thymus. Unlike that transferase, however, the plant enzyme prefers non-denatured to single-stranded DNA as primer and requires both Mg2+ and Mn2+ ions for maximal activity.
Full text
PDF


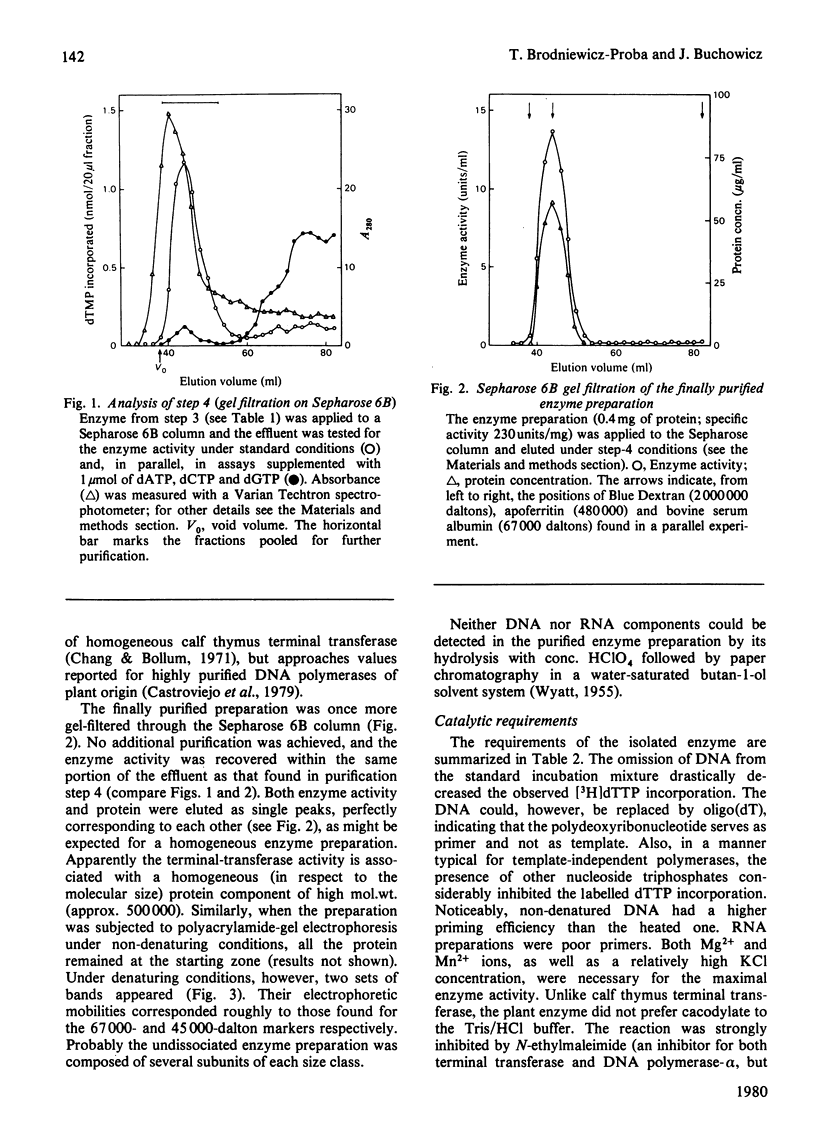

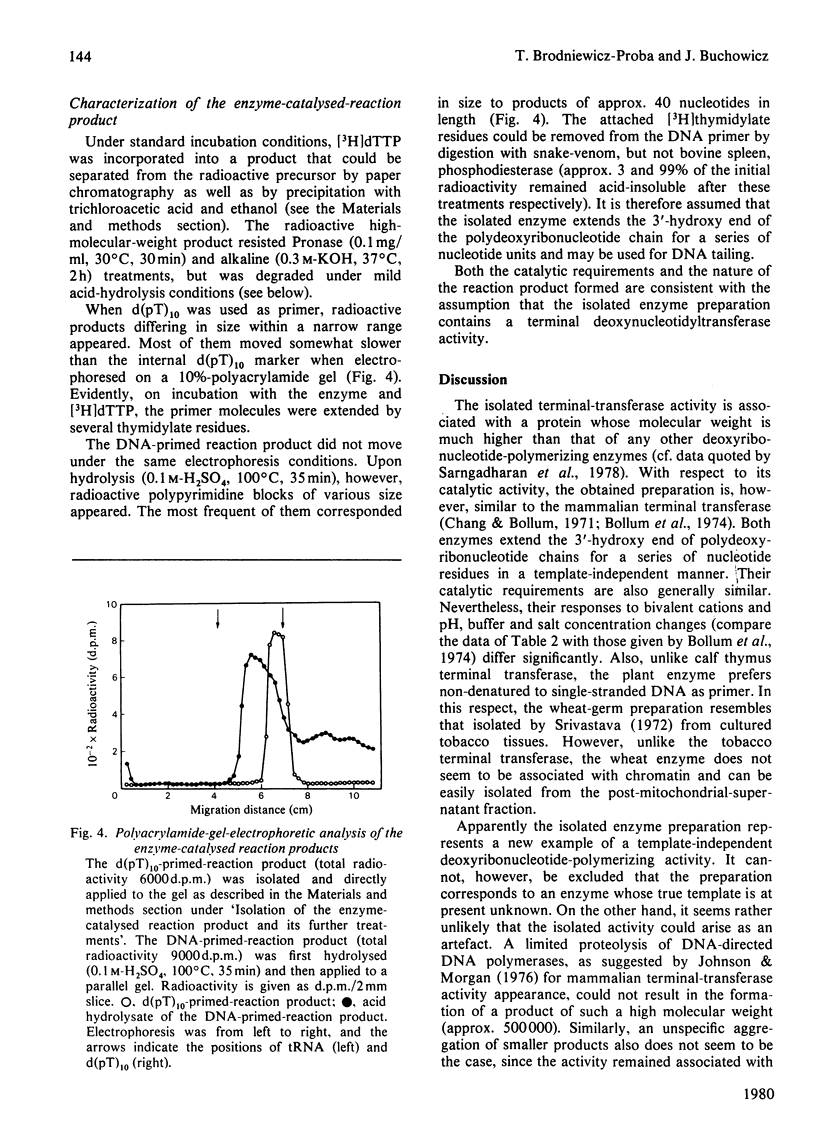

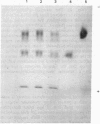
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bollum F. J., Chang L. M., Tsiapalis C. M., Dorson J. W. Nucleotide polymerizing enzymes from calf thymus gland. Methods Enzymol. 1974;29:70–81. doi: 10.1016/0076-6879(74)29010-4. [DOI] [PubMed] [Google Scholar]
- Brodniewicz-Proba T., Buchowicz J. Terminal deoxyribonucleotidyl transferase activity of germinating wheat embryo. FEBS Lett. 1976 Jun 1;65(2):183–186. doi: 10.1016/0014-5793(76)80475-9. [DOI] [PubMed] [Google Scholar]
- Castroviejo M., Tharaud D., Tarrago-Litvak L., Litvak S. Multiple deoxyribonucleic acid polymerases from quiescent wheat embryos. Purification and characterization of three enzymes from the soluble cytoplasm and one from purified mitochondria. Biochem J. 1979 Jul 1;181(1):183–191. doi: 10.1042/bj1810183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Deoxynucleotide-polymerizing enzymes of calf thymus gland. V. Homogeneous terminal deoxynucleotidyl transferase. J Biol Chem. 1971 Feb 25;246(4):909–916. [PubMed] [Google Scholar]
- Coleman M. S. A critical comparison of commonly used procedures for the assay of terminal deoxynucleotidyl transferase in crude tissue extracts. Nucleic Acids Res. 1977 Dec;4(12):4305–4312. doi: 10.1093/nar/4.12.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deibel M. R., Jr, Coleman M. S. Purification of a high molecular weight human terminal deoxynucleotidyl transferase. J Biol Chem. 1979 Sep 10;254(17):8634–8640. [PubMed] [Google Scholar]
- JOHNSTON F. B., STERN H. Mass isolation of viable wheat embryos. Nature. 1957 Jan 19;179(4551):160–161. doi: 10.1038/179160b0. [DOI] [PubMed] [Google Scholar]
- Johnson D., Morgan A. R. The isolation of a high molecular weight terminal deoxynucleotidyl transferase from calf thymus. Biochem Biophys Res Commun. 1976 Oct 4;72(3):840–849. doi: 10.1016/s0006-291x(76)80209-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazuś B., Brodniewicz-Proba T. RNA polymerases I and II in germinating wheat embryo. Acta Biochim Pol. 1976;23(2-3):261–267. [PubMed] [Google Scholar]
- Okamura S., Crane F., Messner H. A., Mak T. W. Purification of terminal deoxynucleotidyltransferase by oligonucleotide affinity chromatography. J Biol Chem. 1978 Jun 10;253(11):3765–3767. [PubMed] [Google Scholar]
- SHAPIRO H. S., CHARGAFF E. STUDIES ON THE NUCLEOTIDE ARRANGEMENT IN DEOXYRIBONUCLEIC ACIDS. VII. DIRECT ESTIMATION OF PYRIMIDINE NUCLEOTIDE RUNS. Biochim Biophys Acta. 1963 Sep 17;76:1–8. [PubMed] [Google Scholar]
- Sarngadharan M. G., Robert-Guroff M., Gallo R. C. DNA polymerases of normal and neoplastic mammalian cells. Biochim Biophys Acta. 1978 Dec 11;516(4):419–487. doi: 10.1016/0304-419x(78)90019-7. [DOI] [PubMed] [Google Scholar]
- Siddiqui F. A., Srivastava B. I. Terminal deoxyribonucleotidyl transferase from acute lymphoblastic leukemia cells and production of antisera. Biochim Biophys Acta. 1978 Jan 26;517(1):150–157. doi: 10.1016/0005-2787(78)90042-4. [DOI] [PubMed] [Google Scholar]
- Srivastava B. I. Association of therminal deoxynucleotidyl transferase activity with chromatin from plant tissue. Biochem Biophys Res Commun. 1972 Jul 25;48(2):270–273. doi: 10.1016/s0006-291x(72)80045-7. [DOI] [PubMed] [Google Scholar]