Abstract
1. The distribution and properties of superoxide dismutase were examined in mammalian semen, and the enzyme was used to investigate the role of superoxides in metal-ion-catalysed lipid-peroxidation reactions in spermatozoa. 2. Superoxide dismutase activity was detected in seminal plasma and spermatozoa from all species studied, exceptionally high activity being found in donkey semen. The enzyme is easily solubilized from spermatozoa, as 85-90% of the total activity is released by cold shock, a relatively mild form of cellular damage. 3. Purification and characterization of the enzyme from supernatant fractions prepared from cold-shocked boar spermatozoa showed it to be cyanide-sensitive, to have a mol.wt. of 31 000, a pI of 5.9 and to contain 1.85 g-atoms of copper and 1.91 g-atoms of zinc per mol of protein. However, extensive sonication of spermatozoa released a small amount of a cyanide-insensitive enzyme, presumably a mangano superoxide dismutase, from the mitochondrial matrix. 4. The presence of superoxide dismutase in spermatozoa, either intracellularly or extracellularly, did not inhibit ascorbate/Fe2+-catalysed lipid-peroxidation reactions, suggesting that superoxides are not essential intermediates in this system.
Full text
PDF


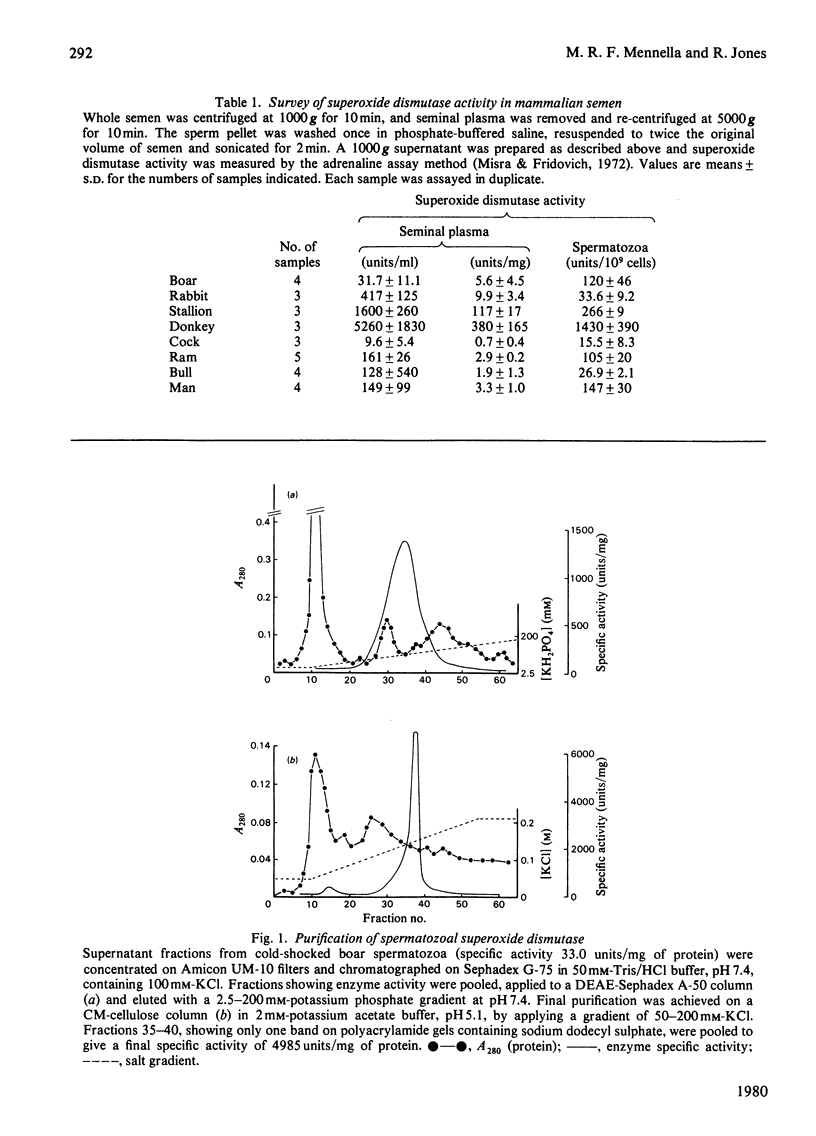

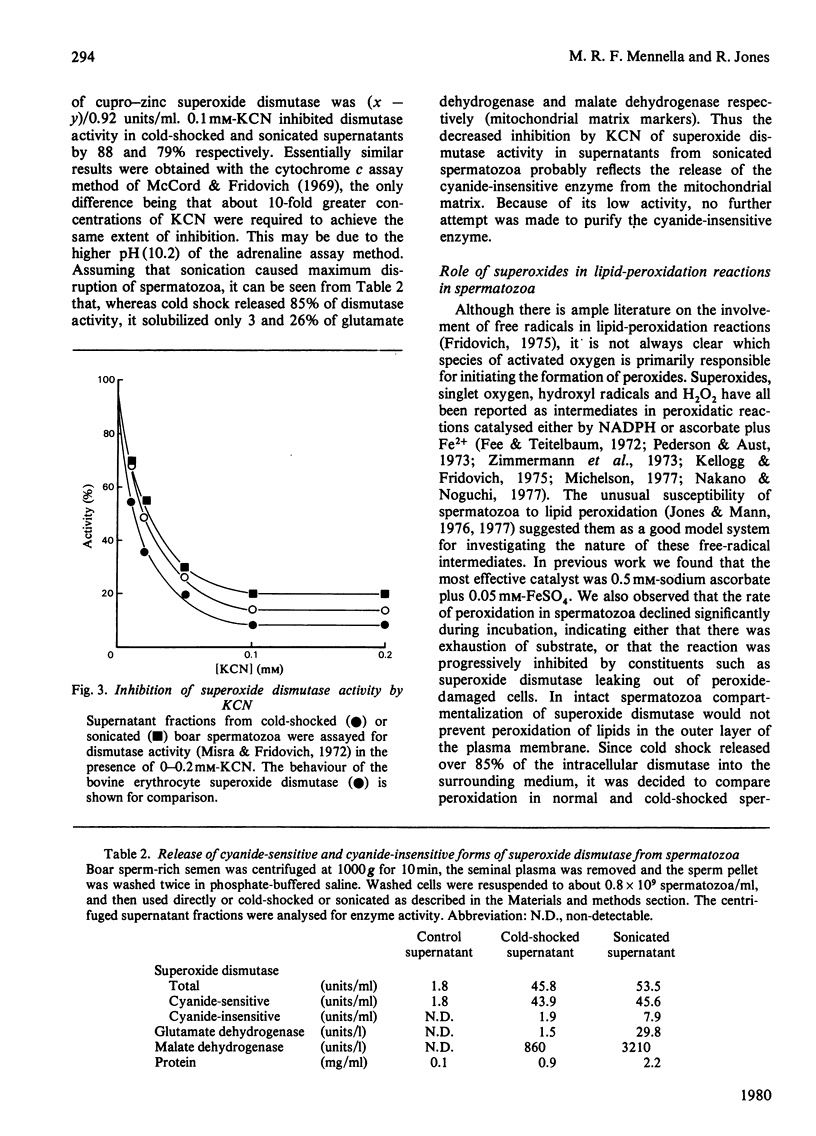
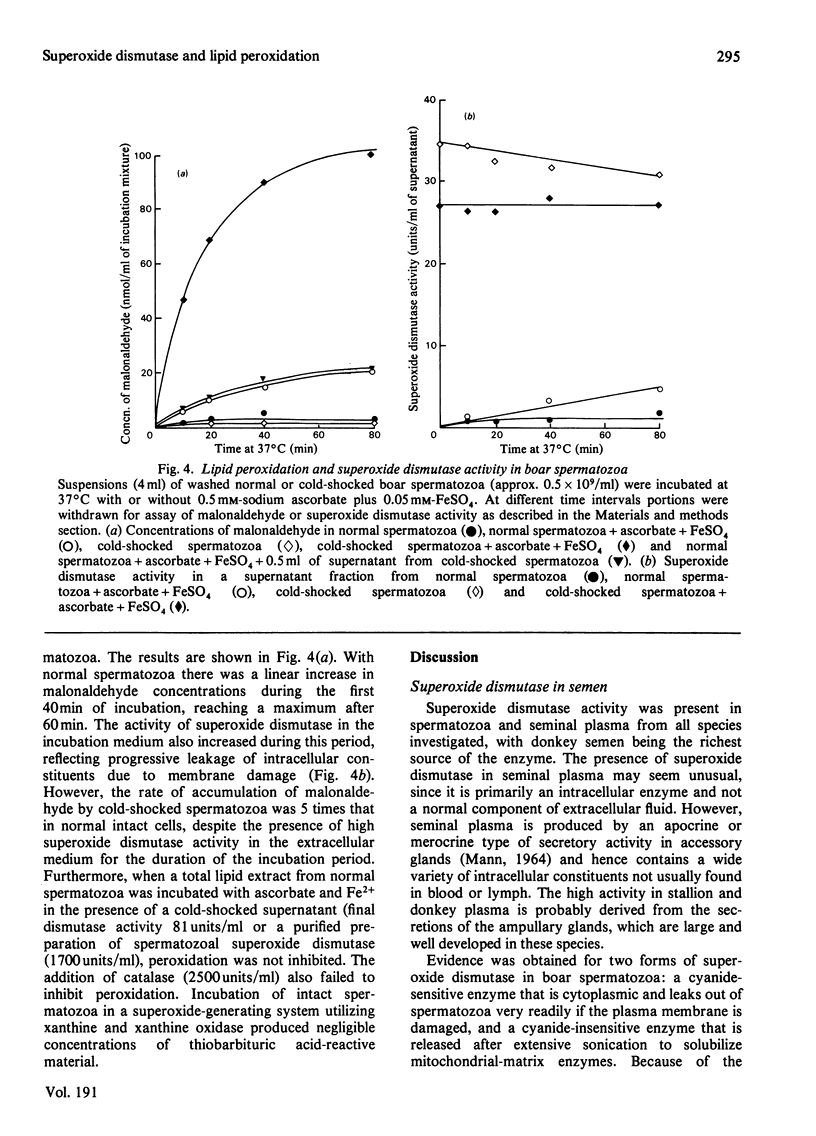


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu-Erreish G., Magnes L., Li T. K. Isolation and properties of superoxide dismutase from ram spermatozoa and erythrocytes. Biol Reprod. 1978 May;18(4):554–560. doi: 10.1095/biolreprod18.4.554. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Barber A. A., Bernheim F. Lipid peroxidation: its measurement, occurrence, and significance in animal tissues. Adv Gerontol Res. 1967;2:355–403. [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fee J. A. Studies on the reconstitution of bovine erythrocyte superoxide dismutase. IV. Preparation and some properties of the enzyme in which Co(II) is substituted for Zn(II). J Biol Chem. 1973 Jun 25;248(12):4229–4234. [PubMed] [Google Scholar]
- Fee J. A., Teitelbaum H. D. Evidence that superoxide dismutase plays a role in protecting red blood cells against peroxidative hemolysis. Biochem Biophys Res Commun. 1972 Oct 6;49(1):150–158. doi: 10.1016/0006-291x(72)90022-8. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- GERSCHMAN R., GILBERT D. L., NYE S. W., DWYER P., FENN W. O. Oxygen poisoning and x-irradiation: a mechanism in common. Science. 1954 May 7;119(3097):623–626. doi: 10.1126/science.119.3097.623. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Richmond R., Halliwell B. Inhibition of the iron-catalysed formation of hydroxyl radicals from superoxide and of lipid peroxidation by desferrioxamine. Biochem J. 1979 Nov 15;184(2):469–472. doi: 10.1042/bj1840469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R., Mann T. Damage to ram spermatozoa by peroxidation of endogenous phospholipids. J Reprod Fertil. 1977 Jul;50(2):261–268. doi: 10.1530/jrf.0.0500261. [DOI] [PubMed] [Google Scholar]
- Jones R., Mann T. Lipid peroxides in spermatozoa; formation, rôle of plasmalogen, and physiological significance. Proc R Soc Lond B Biol Sci. 1976 Jun 30;193(1113):317–333. doi: 10.1098/rspb.1976.0050. [DOI] [PubMed] [Google Scholar]
- Jones R., Mann T., Sherins R. Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil Steril. 1979 May;31(5):531–537. doi: 10.1016/s0015-0282(16)43999-3. [DOI] [PubMed] [Google Scholar]
- Kellogg E. W., 3rd, Fridovich I. Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J Biol Chem. 1975 Nov 25;250(22):8812–8817. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Li T. K. The glutathione and thiol content of mammalian spermatozoa and seminal plasma. Biol Reprod. 1975 Jun;12(5):641–646. doi: 10.1095/biolreprod12.5.641. [DOI] [PubMed] [Google Scholar]
- MANN T., LUTWAK-MANN C. Biochemical changes underlying the phenomenon of cold-shock in spermatozoa. Arch Sci Biol (Bologna) 1955 Nov-Dec;39(6):578–588. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972 May 25;247(10):3170–3175. [PubMed] [Google Scholar]
- Pederson T. C., Aust S. D. The role of superoxide and singlet oxygen in lipid peroxidation promoted by xanthine oxidase. Biochem Biophys Res Commun. 1973 Jun 8;52(3):1071–1078. doi: 10.1016/0006-291x(73)91047-4. [DOI] [PubMed] [Google Scholar]
- Rotilio G., Bray R. C., Fielden E. M. A pulse radiolysis study of superoxide dismutase. Biochim Biophys Acta. 1972 May 12;268(2):605–609. doi: 10.1016/0005-2744(72)90359-2. [DOI] [PubMed] [Google Scholar]
- Tyler D. D. Polarographic assay and intracellular distribution of superoxide dismutase in rat liver. Biochem J. 1975 Jun;147(3):493–504. doi: 10.1042/bj1470493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALES R. G., WHITE I. G., LAMOND D. R. The spermicidal activity of hydrogen peroxide in vitro and in vivo. J Endocrinol. 1959 May;18(3):236–244. doi: 10.1677/joe.0.0180236. [DOI] [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Superoxide dismutase. Organelle specificity. J Biol Chem. 1973 May 25;248(10):3582–3592. [PubMed] [Google Scholar]
- Zimmermann R., Flohé L., Weser U., Hartmann H. J. Inhibition of lipid peroxidation in isolated inner membrane of rat liver mitochondria by superoxide dismutase. FEBS Lett. 1973 Jan 15;29(2):117–120. doi: 10.1016/0014-5793(73)80539-3. [DOI] [PubMed] [Google Scholar]



