Abstract
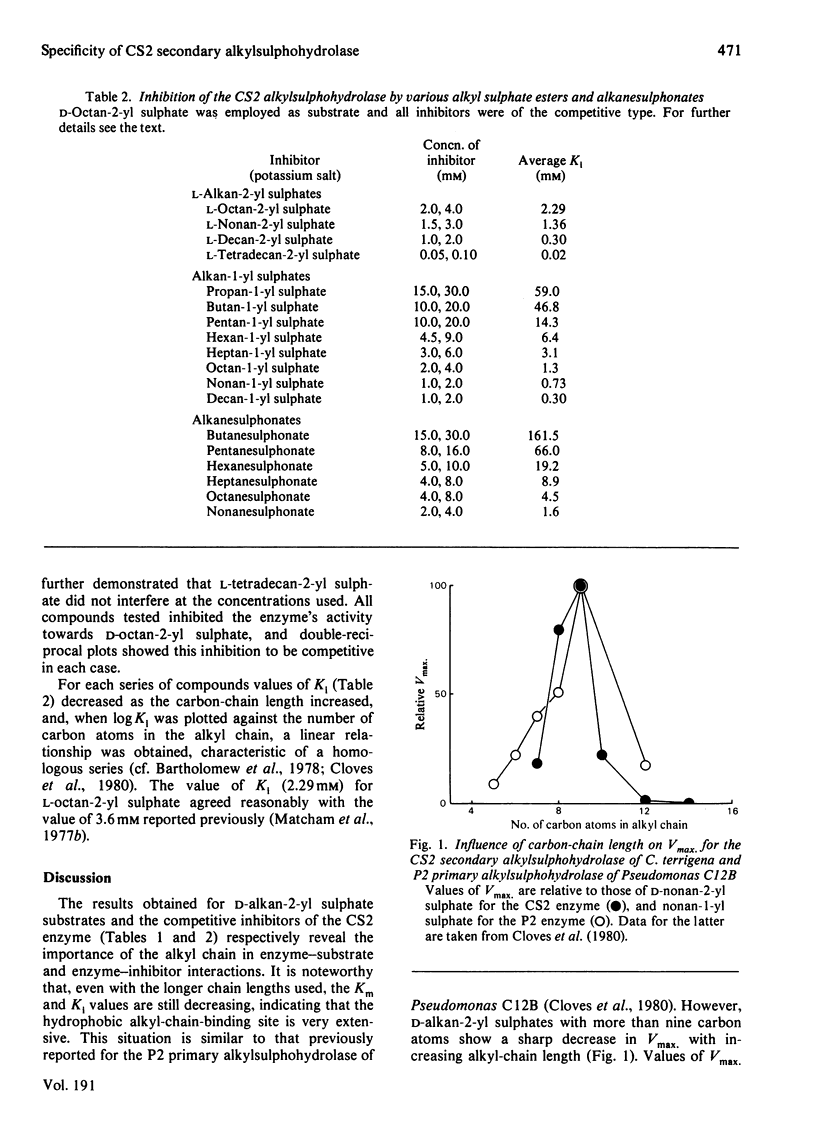
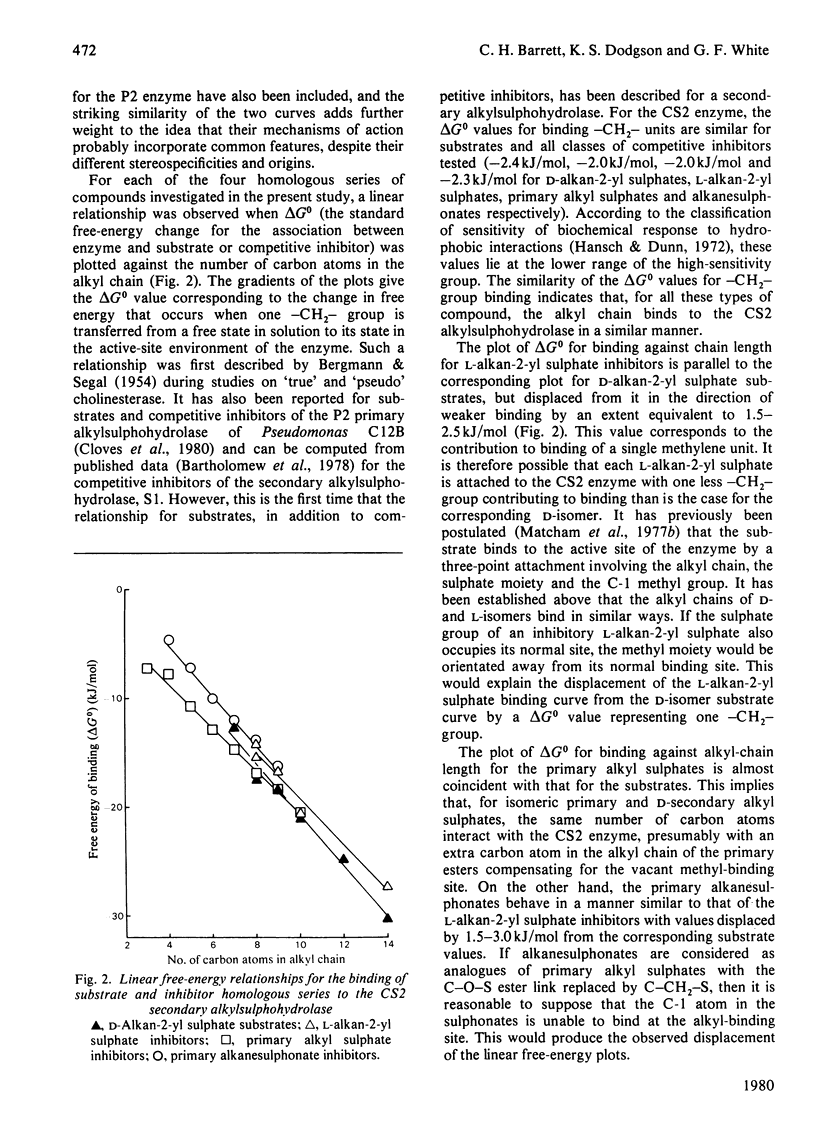
A series of d-alkan-2-yl sulphate esters (C7–C14) were prepared by sulphation of the resolved parent alcohols by a method that entails complete retention of configuration. These sulphate esters were tested as substrates for the stereospecific CS2 secondary alkylsulphohydrolase of Comamonas terrigena. Vmax. reached a maximum with the C9 compound, whereas logKm decreased linearly as the alkyl-chain length was increased from C7 to C14. A parallel series of l-alkan-2-yl sulphates was also prepared, and these esters, together with homologous series of primary alkyl sulphates and primary alkanesulphonates, were shown to be competitive inhibitors of the CS2 enzyme. For each series of compounds, logKi values decreased linearly with increasing alkyl-chain length. Plots of chain length against the standard free energy of binding (ΔG0) of substrate and inhibitors to the CS2 enzyme showed that the standard free energy of association of a –CH2– group with the enzyme was 2.0–2.4kJ/mol for all classes of compound studied, indicating an important contribution from hydrophobic interactions to the overall binding. Plots for d-alkan-2-yl sulphate substrates and primary alkyl sulphate inhibitors were nearly coincident, suggesting that the overall interaction between a primary ester and the enzyme is the same as that between the isomeric secondary substrate and the enzyme. Plots for l-alkan-2-yl sulphate and alkanesulphonate inhibitors were very similar to each other, but were displaced by 1.5–3.0kJ/mol from that for substrate binding. This indicates that the binding of any one of these particular inhibitors involves one carbon atom fewer than the number involved in binding a substrate of the same chain length. These observations are discussed in terms of a three-point attachment of substrate to the enzyme involving the alkyl chain, sulphate group and the C-1 methyl group.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGMANN F., SEGAL R. The relationship of quaternary ammonium salts to the anionic sites of true and pseudo cholinesterase. Biochem J. 1954 Dec;58(4):692–698. doi: 10.1042/bj0580692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew B., Dodgson K. S., Gorham S. D. Purification and properties of the S1 secondary alkylsulphohydrolase of the detergent-degrading micro-organism, Pseudomonas C12B. Biochem J. 1978 Mar 1;169(3):659–667. doi: 10.1042/bj1690659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHERNIAK R., DAVIDSON E. A. SYNTHESIS OF ADENYLYL SULFATE AND ADENYLYL SULFATE 3'-PHOSPHATE. J Biol Chem. 1964 Sep;239:2986–2990. [PubMed] [Google Scholar]
- Cloves J. M., Dodgson K. S., White G. F., Fitzgerald J. W. Purification and properties of the P2 primary alkylsulphohydrolase of the detergent-degrading bacterium pseudomonas C12B. Biochem J. 1980 Jan 1;185(1):23–31. doi: 10.1042/bj1850023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodgson K. S., Fitzgerald J. W., Payne W. J. Chemically defined inducers of alkylsulphatases present in Pseudomonas C12B. Biochem J. 1974 Jan;138(1):53–62. doi: 10.1042/bj1380053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J. W. Secondary alkylsulphatases in a strain of Comamonas terrigena. Biochem J. 1975 Aug;149(2):477–480. doi: 10.1042/bj1490477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansch C., Dunn W. J., 3rd Linear relationships between lipophilic character and biological activity of drugs. J Pharm Sci. 1972 Jan;61(1):1–19. doi: 10.1002/jps.2600610102. [DOI] [PubMed] [Google Scholar]
- Lijmbach G. W., Brinkhuis E. Microbial degradation of secondary n-alkyl sulfates and secondary alkanols. Antonie Van Leeuwenhoek. 1973;39(3):415–423. doi: 10.1007/BF02578884. [DOI] [PubMed] [Google Scholar]
- Matcham G. W., Dodgson K. S. Preparation and characterization of substrates suitable for the study of stereospecific secondary alkylsulphohydrolases of detergent-degrading micro-organisms. Biochem J. 1977 Dec 1;167(3):717–722. doi: 10.1042/bj1670717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matcham G. W., Dodgson K. S. Purification, properties and cellular localization of the stereospecific CS2 secondary alkylsulphohydrolase of Comamonas terrigena. Biochem J. 1977 Dec 1;167(3):723–729. doi: 10.1042/bj1670723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J., Williams J. P., Mayberry W. R. Primary alcohol sulfatase in a Pseudomonas species. Appl Microbiol. 1965 Sep;13(5):698–701. doi: 10.1128/am.13.5.698-701.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D. J., Dodgson K. S., White G. F. Substrate specificity and other properties of the inducible S3 secondary alkylsulphohydrolase purified from the detergent-degrading bacterium Pseudomonas C12B. Biochem J. 1980 Apr 1;187(1):181–190. doi: 10.1042/bj1870181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. H., Tudball N. Studies on the enzymic degradation of L-serine O-sulphate by a rat liver preparation. Biochem J. 1967 Nov;105(2):467–472. doi: 10.1042/bj1050467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G. F., Lillis V., Shaw D. J. An improved procedure for the preparation of alkyl sulphate esters suitable for the study of secondary alkylsulphohydrolase enzymes. Biochem J. 1980 Apr 1;187(1):191–196. doi: 10.1042/bj1870191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. P., Mayberry W. R., Payne W. J. Metabolism of linear alcohols with various chain lengths by a Pseudomonas species. Appl Microbiol. 1966 Mar;14(2):156–160. doi: 10.1128/am.14.2.156-160.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


