Abstract
Background
Fanconi syndrome is a disorder of the proximal tubule that leads to malabsorption of various electrolytes and substances and is a common consequence of drug-induced nephrotoxicity. However, cases of dietary supplement-induced Fanconi syndrome are rare, and detailed reports on the evaluation of renal histology in patients with this syndrome are lacking.
Case presentation
We present two cases of dietary supplement-induced Fanconi syndrome that was confirmed by kidney biopsy. Based on their medical history and laboratory and histological findings, both cases were diagnosed as acute proximal tubular injury caused by ingestion of a lipid-lowering dietary supplement containing beni-koji leading to Fanconi syndrome. After discontinuation of the dietary supplement and correction of dehydration and electrolyte imbalance, renal function completely recovered in one case but progressed to chronic kidney disease in the other.
Conclusions
Clinicians should consider dietary supplement-induced Fanconi syndrome as a differential diagnosis in patients who become ill while taking a dietary supplement. Kidney biopsy is useful for diagnosing acute tubular injury with Fanconi syndrome and investigating its pathogenesis. Patients who have developed dietary supplement-induced Fanconi syndrome require long-term monitoring to detect and prevent progression to chronic kidney disease.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-024-03903-5.
Keywords: Fanconi syndrome, Proximal tubular injury, Dietary supplement, Beni-koji
Background
Dietary supplements are easily accessible and likely to be used without proper guidance or monitoring by healthcare professionals. This raises concerns about potential interactions, contraindications, and unfavorable outcomes. Between 2004 and 2021, 79,071 adverse events related to use of dietary supplements were reported to the Center for Food Safety and Applied Nutrition in the US [1]. Fanconi syndrome is caused by an abnormality of proximal tubule epithelial cells (PTECs) that leads to malabsorption of various electrolytes and substances [2] and is a common consequence of drug-induced nephrotoxicity [3]. However, cases of dietary supplement-induced Fanconi syndrome are rare, and detailed reports on the evaluation of renal histology in patients with this syndrome are lacking. In this report, we describe two rare biopsy-proven cases of acute proximal tubular injury attributable to a dietary supplement containing beni-koji, made from rice fermented with Monascus purpureus, a reddish-purple species of mold that is expected to reduce blood cholesterol [4].
Case 1
A 49-year-old woman was referred to our hospital with a 13-day history of anorexia, nausea, and bubbling urine. She had taken a dietary supplement containing beni-koji for dyslipidemia for the previous 1.5 years but had stopped taking it 10 days before admission. She had also been prescribed bilastine for pollinosis. During her hospital stay, she had a temperature of 36.5ºC, a blood pressure of 128/81 mmHg, a pulse rate of 76 beats per minute, and oxygen saturation of 100% on room air. She appeared slightly unwell, but findings on physical examination were normal. Blood studies showed renal dysfunction, hypokalemia, hypophosphatemia, hypouricemia, and metabolic acidosis. Urinalysis showed proteinuria, glycosuria, aminoaciduria, increased excretion of potassium, and increased phosphorus and uric acid levels, which were not explained by plasma concentrations. She also had high levels of urinary β2-microglobulin and N-acetyl-beta-D-glucosaminidase. We performed a drug-induced lymphocyte stimulation test on her serum, which was positive for the dietary supplement. Table 1 and Supplementary Table 1 show the clinical data. Percutaneous kidney biopsy was performed on the second day after admission. Light microscopy showed considerable tubular changes, with approximately 50% of the tubular epithelial cells showing simplification, vacuolization, or shedding, indicating acute tubular necrosis (Fig. 1A-c). Immunohistochemical staining for megalin [5], a proximal tubule marker, was positive, indicating injury to the tubular epithelium (Fig. 2a), confirming that the proximal tubules were the central lesion in this disease. Lymphocyte infiltration in the interstitium and tubules was unremarkable, and scattered interstitial fibrosis was observed. The glomerular structure appeared normal, and immunofluorescence staining for immunoglobulins and complement factors was negative in the glomeruli and tubular basement membrane. Electron microscopy did not reveal any glomerular abnormalities. Considering the patient’s medical history and her laboratory and pathological findings, we made a diagnosis of acute proximal tubular injury caused by the dietary supplement leading to Fanconi syndrome. Her general condition and clinical laboratory data improved dramatically after discontinuation of the dietary supplement, administration of intravenous fluids, and oral electrolyte correction. She was discharged 14 days after admission. Her renal function, electrolyte abnormalities, and laboratory findings in urine completely recovered to within the normal ranges by 22 weeks after onset (Supplementary Fig. 1).
Table 1.
Patient characteristics and laboratory data on admission
| Variable | Case 1 | Case 2 |
|---|---|---|
| Demographics and comorbidity | ||
| Age, y | 49 | 68 |
| Sex | Female | Female |
| Comorbidity | Dyslipidemia | Dyslipidemia |
| Serum | ||
| White blood cells, /µL | 5,500 | 11,000 |
| Hemoglobin, g/dL | 12.7 | 13.8 |
| Platelets×104/µL | 31.7 | 40.2 |
| TP, g/dL | 6.8 | 7.7 |
| Albumin, g/dL | 4.0 | 4.1 |
| BUN, mg/dL | 5.0 | 31.8 |
| Creatinine, mg/dL | 1.5 | 3.0 |
| eGFR, mL/min/1.73 m2 | 35.2 | 12.9 |
| Sodium, mEq/L | 137 | 137 |
| Potassium, mEq/L | 2.2 | 2.4 |
| Chloride, mEq/L | 104 | 108 |
| Bicarbonate, mEq/L | 16.7 | 13.6 |
| Anion gap, mEq/L | 11.8 | 19.6 |
| Calcium, mg/dL | 8.9 | 9.1 |
| Phosphorus, mg/dL | 1.2 | 2.4 |
| Uric acid, mg/dL | 1.4 | 2.4 |
| Glucose, mg/dL | 155 | 114 |
| LDL-C, mg/dL | 97 | 153 |
| M-protein | (-) | NA |
| ANA, index | < 40 | 160 |
| SS-A, U/mL | < 1.0 | 162.8 |
| SS-B, U/mL | 3.8 | 16.2 |
| MPO-ANCA, U/mL | < 1.0 | < 1.0 |
| PR3-ANCA, U/mL | < 1.0 | < 1.0 |
| DLST for dietary supplement | (+) | NA |
| Urine | ||
| Urine output, mL/day | 1,100 | 1,400 |
| pH | 6.0 | 5.5 |
| Protein | (2+) | (2+) |
| Protein, g/g creatinine | 2.09 | 2.41 |
| Protein, g/day | 0.86 | 1.53 |
| Albumin, g/g creatinine | 1.03 | NA |
| Glucose | (4+) | (3+) |
| Occult blood/HF | 1–4 | 10–19 |
| Leukocytes | (-) | (1+) |
| Eosinophils | (-) | (-) |
| Bacteria | (2+) | (-) |
| β2-MG, μg/L | 50,870 | 50,857 |
| NAG, IU/L | 28.9 | 46.0 |
| FENa, % | 0.6 | 0.6 |
| FEK, % | 19.9 | 64.2 |
| FEPi, % | 27.2 | NA |
| FEUA, % | 51.2 | NA |
| Aminoaciduria | Supplementary Table 1 | |
Abbreviations: ANA antinuclear antibody, ANCA antineutrophil cytoplasmic antibody, BUN blood urea nitrogen, DLST drug-induced lymphocyte stimulation test, eGFR estimated glomerular filtration rate, FEK fractional excretion of potassium, FENa fractional excretion of sodium, FEPi fractional excretion of phosphorus, FEUA fractional excretion of uric acid, HF high-power field, LDL-C low-density lipoprotein cholesterol, MG microglobulin, MPO myeloperoxidase, NA not applicable, NAG N-acetyl-beta-D-glucosaminidase, PR3 proteinase 3, TP total protein
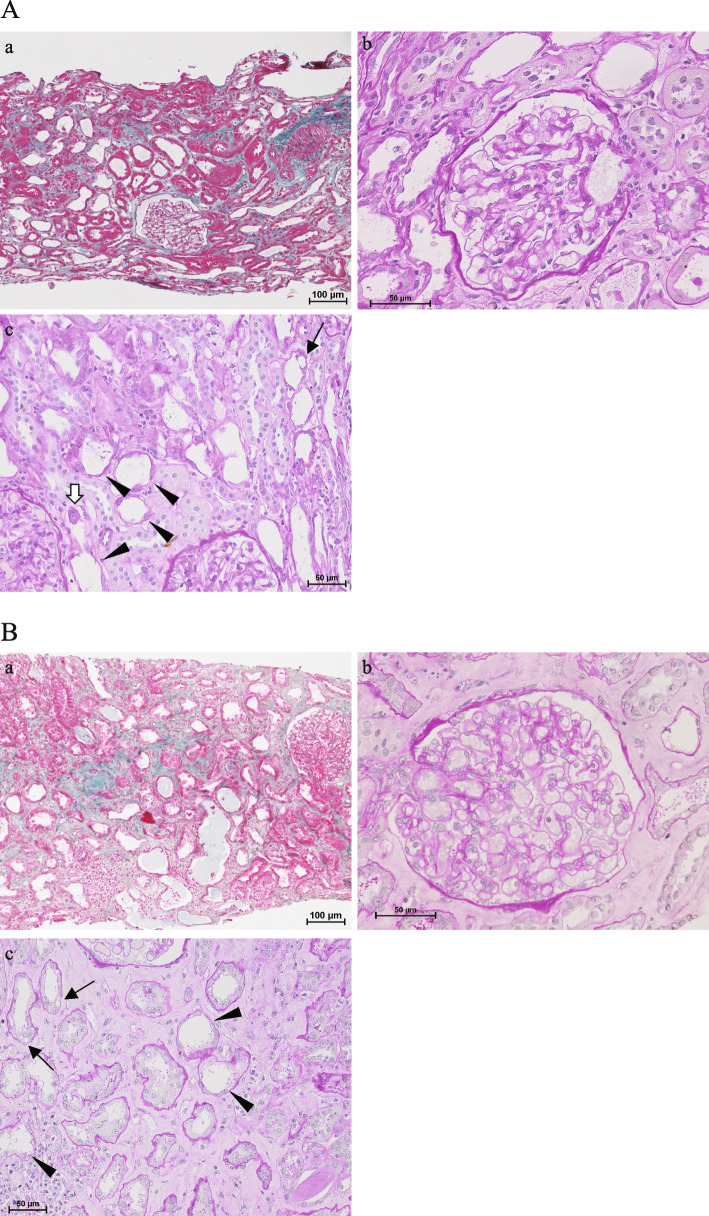
Fig. 1.
A Light microscopic findings on kidney biopsy in case 1. (a) Low-power views show diffuse attenuation of the tubular epithelium and scattered infiltration of inflammatory cells (Elastica–Masson trichrome stain). (b) There were no pathological changes in the glomeruli (periodic acid–Schiff stain). (c) Loss of brush borders, coarse vacuolar degeneration (arrows), flattened cytoplasm, cell detachment (arrowheads), and mitoses were observed in tubular epithelial cells (periodic acid–Schiff stain). B Light microscopic findings on kidney biopsy in case 2. (a) Low-power views show diffuse attenuation of the tubular epithelium, scattered infiltration of inflammatory cells, and focal interstitial fibrosis (Elastica–Masson trichrome stain). (b) There were no pathological changes in the glomeruli (periodic acid–Schiff stain). (c) Loss of brush borders, coarse vacuolar degeneration (arrows), flattened cytoplasm, and cell detachment (arrowheads) were observed in tubular epithelial cells (periodic acid–Schiff stain)
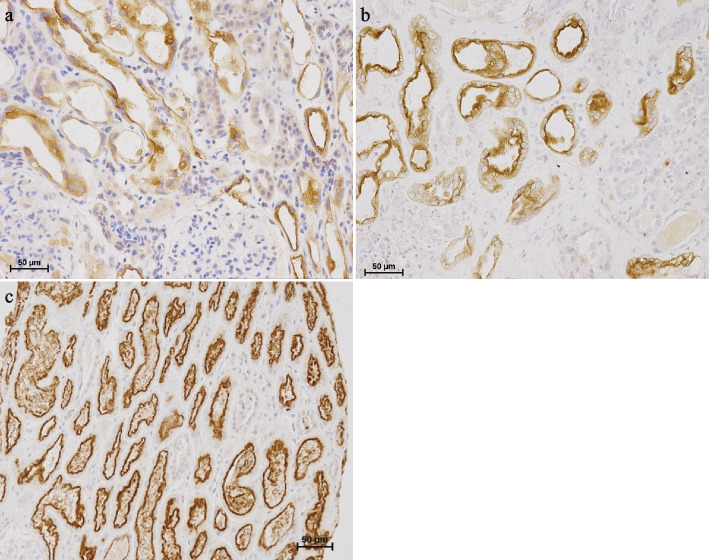
Fig. 2.
Immunohistochemistry of megalin on kidney biopsy. a, b Megalin was positive on the injured tubular epithelium in cases 1 and 2, respectively. c Megalin was strongly positive in the luminal region of the proximal tubule in normal renal tissue from a patient diagnosed with orthostatic proteinuria
Case 2
A 68-year-old woman was referred to our hospital complaining of anorexia and palpitations that had been present for 10 days. She had a history of dyslipidemia, for which she had taken a dietary supplement containing beni-koji for 7 months, but had stopped taking it 1 month before admission because of constipation. She was not on any medications except for the dietary supplement. During hospitalization, she had a temperature of 36.6ºC, blood pressure of 116/73 mmHg, pulse rate of 156 beats per minute, and oxygen saturation of 98% on room air. She appeared slightly unwell, but findings on physical examination were normal. Blood studies showed renal dysfunction, hypokalemia, hypophosphatemia, hypouricemia, and metabolic acidosis. Urinalysis revealed proteinuria, glycosuria, hematuria, aminoaciduria, and increased excretion of potassium, which was not explained by plasma concentrations. She also had high levels of urinary β2-microglobulin and N-acetyl-beta-D-glucosaminidase. Table 1 and Supplementary Table 1 summarize the clinical data. A 12-lead electrocardiogram showed a heart rate of 94 with no irregular rhythm. A consultation with an ophthalmologist did not support a diagnosis of Sjögren’s syndrome or uveitis. Percutaneous kidney biopsy was performed on day 4 after admission. Light microscopy showed considerable tubular changes, with approximately 25% of the tubular epithelial cells showing simplification, vacuolization, or shedding, indicating acute tubular necrosis (Fig. 1B-c). The result of immunostaining for megalin was similar to that in case 1 (Fig. 2b). Lymphocyte infiltration in the interstitium and tubules was unremarkable, and fibrosis was observed in approximately 40% of the interstitium. The glomerular structure appeared normal, and immunofluorescence staining for immunoglobulins and complement factors was negative in the glomeruli and tubular basement membrane. No glomerular abnormalities were found on electron microscopy. In view of her medical history and laboratory and pathological findings, we made a diagnosis of acute proximal tubular injury caused by the dietary supplement leading to Fanconi syndrome. Her general condition and clinical laboratory data improved dramatically after she discontinued the dietary supplement and received intravenous fluids with oral electrolyte correction. She was discharged on day 13 after admission. The electrolyte imbalance and abnormal laboratory findings in urine had recovered by 26 weeks after onset, although slight renal dysfunction remained (Supplementary Fig. 2).
Discussion and conclusions
Both the women in this report were taking a dietary beni-koji supplement and presented to our hospital with clinical findings consistent with Fanconi syndrome. The important clinical teaching points in this report are that dietary supplements can cause Fanconi syndrome and that kidney biopsy is useful for diagnosing acute tubular injury with Fanconi syndrome and investigating its pathogenesis.
In Fanconi syndrome, generalized proximal tubule dysfunction occurs without primary glomerular involvement. It is characterized by variable degrees of phosphate, glucose, amino acid, and bicarbonate wasting and is classified as genetic or acquired. Fanconi syndrome causes loss of water and electrolytes, leading to thirst, fatigue, weakness, and polyuria. General measures used to address this condition include avoidance of dehydration and replacement of lost electrolytes, including potassium, phosphate, and bicarbonate. Any medication that causes this condition should be avoided [2]. The patients described in this report discontinued the supplement and received intravenous treatment to correct dehydration and electrolyte imbalance.
Drug-induced nephrotoxicity is the most common cause of acquired Fanconi syndrome [6]. Cisplatin, ifosfamide, tenofovir, sodium valproate, and aminoglycoside antibiotics are the agents most frequently implicated [6–8]. These agents are taken up by PTECs from the bloodstream or glomerular filtrates via various transporters or receptors expressed on cell surface membranes. Therefore, intracellular drug concentrations in PTECs can become elevated, which likely accounts for the prevalence of toxicity in these cells [6]. However, cases of Fanconi syndrome caused by dietary supplements are quite rare. Kidney damage caused by a dietary supplement containing beni-koji has recently become a serious issue in Japan, and the relevant ministries and agencies are conducting intensive searches to identify possible causative agents, including puberulic acid [4, 9, 10]. A literature search using the terms “dietary supplement” and “Fanconi syndrome” identified only 11 reported cases (Table 2) [10–15], in 10 of which slight renal dysfunction remained despite appropriate treatment. In the present cases, renal function completely recovered in Case 1but progressed to chronic kidney disease in Case 2, similar to previous reports. Both cases require careful monitoring for long-term renal prognosis. Many patients do not admit their use of dietary supplements to their physicians. Therefore, medical history-taking, including about dietary supplements, is essential for early diagnosis of dietary supplement-induced Fanconi syndrome.
Table 2.
Reported cases of dietary supplement-induced Fanconi syndrome
| Age (years)/sex | Underlying condition | Duration of supplement intake | Urine protein | DLST | Kidney biopsy | Steroid therapy | Full recovery of kidney function | Reference |
|---|---|---|---|---|---|---|---|---|
| 73/F | Dyslipidemia | 8 months | 2.47 g/g creatinine | NA | ATIN | Yes | No | Murata et al. (2024) [10] |
| 53/F | Dyslipidemia | 8 months | 4.25 g/g creatinine | NA | ATIN | Yes | No | Murata et al. (2024) [10] |
| 55/F | Dyslipidemia | 9 months | 0.65 g/g creatinine | + | ATIN | Yes | No | Murata et al. (2024) [10] |
| 66/F | Hashimoto’s disease | 14 months | 1.04 g/g creatinine | NA |
MGA ATI |
No | No | Takeuchi et al. (2024) [11] |
| 54/M | Dyslipidemia, constipation | 24 months | 0.25 g/g creatinine | NA |
Normal glomeruli ATI |
No | No | Takeuchi et al. (2024) [11] |
| 62/M | Reflux esophagitis | NA | 1.63 g/day | NA |
MGA ATI |
No | No | Oda et al. (2024) [12] |
| 58/F | Dyslipidemia | 6 weeks | 1.06 g/day | NA |
Normal glomeruli ATIN |
Yes | Yes | Maiguma et al. (2024) [13] |
| 47/F | Dyslipidemia | 7 months | 1.38 g/day | NA | ATIN | Yes | No | Miyazaki et al. (2024) [14] |
| 49/F | No | 2 weeks | 0.70 g/g creatinine | NA |
Normal glomeruli ATIN |
Yes | No | Chikasue et al. (2024) [15] |
| 55/M | Intestinal obstruction | 6 months | 7.1 g/g creatinine | NA | No | No | No | Chikasue et al. (2024) [15] |
| 60/F | No | 7 months | 2.87 g/g creatinine | NA | No | No | No | Chikasue et al. (2024) [15] |
| 49/F | Dyslipidemia, pollinosis | 1.5 years | 0.86 g/day | + |
Normal glomeruli ATI Megalin positive in injured tubular epithelium |
No | Yes | Present case 1 |
| 68/F | Dyslipidemia | 7 months | 1.53 g/day | NA |
Normal glomeruli ATI Megalin positive in injured tubular epithelium |
No | No | Present case 2 |
Abbreviations: ATI acute tubular injury, ATIN acute tubulointerstitial nephritis, DLST drug-induced lymphocyte stimulation test, MGA minor glomerular abnormality, NA not applicable
These cases demonstrated that biopsy is useful for diagnosis of acute tubular injury with Fanconi syndrome and investigating its pathogenesis. Generally, this syndrome is diagnosed by physical and laboratory examinations, but kidney biopsy is often needed to diagnose or confirm the underlying disease. Cisplatin-induced nephrotoxicity, which is accompanied by the development of Fanconi syndrome, is characterized by swelling and vacuolation of the tubular epithelium, dilation of the tubules, sloughing of the epithelium in the tubular lumen, loss of normal histologic architecture, and multifocal areas of degeneration and necrosis of PTECs [16, 17]. Nine of the previously reported cases underwent kidney biopsy and were diagnosed with acute tubular injury or acute tubulointerstitial nephritis. However, none of the reports on these cases included details on the pathology of the tubular compartment by immunostaining (Table 2). In our two cases, with immunostaining for megalin, kidney biopsy revealed acute proximal tubular injury that was morphologically similar to cisplatin-induced nephrotoxicity. Considering that there was no overdose of the supplement in either of our two cases, a high-dose effect was unlikely. Although the duration of treatment was longer in case 1, the histological findings indicated more advanced chronic lesions in case 2 (Fig. 1B-a), suggesting the possibility of differences in host responsiveness. Case 1 had a positive drug-induced lymphocyte stimulation test, so an allergic mechanism could not be ruled out. However, inflammatory findings in the renal histology were minimal. Both patients recovered without use of steroids, indicating a direct toxicological action on PTECs. Unknown components of the dietary supplement could be absorbed by specific transporters or endocytic receptors localized in PTECs and lead to cell injury and Fanconi syndrome.
In conclusion, dietary supplements can cause Fanconi syndrome. Therefore, comprehensive medical history-taking, including about the use of dietary supplements, is necessary, and dietary supplement-induced Fanconi syndrome should be considered as a differential diagnosis in patients taking dietary supplements who complain of feeling unwell. Kidney biopsy is useful for diagnosing acute tubular injury with Fanconi syndrome and investigating its pathogenesis. Patients who have developed dietary supplement-induced Fanconi syndrome require long-term monitoring to detect and prevent progression to chronic kidney disease. However, further reports should be accumulated to identify the exact mechanism involved.
Supplementary Information
Supplementary Material 1. Supplementary Figure 1. Clinical course in case 1. Abbreviations: FEUA, fractional excretion of uric acid; MG, microglobulin; TRP, tubular reabsorption of phosphate.
Supplementary Material 2. Supplementary Figure 2. Clinical course in case 2. Abbreviations: MG, microglobulin; NA, not applicable.
Acknowledgements
Not applicable.
Abbreviation
- PTECs
Proximal tubule epithelial cells
Authors’ contributions
MH, MH, YY, KK, MY, MY, MS, HK, AO, HH, YI, and NI were involved in the clinical care of the patient, managed the literature searches, and wrote the first draft of the manuscript. SY, AS and SG helped in drafting the manuscript. All authors have read and approved the final version of the manuscript.
Funding
No funding was received for this study.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li W, Wertheimer A. Narrative review: the FDA’s perfunctory approach of dietary supplement regulations giving rise to copious reports of adverse events. Innov Pharm. 2023;14(1):1–5. [DOI] [PMC free article] [PubMed]
- 2.Keefe P, Bokhari SRA. Fanconi syndrome. In: StatPearls. Treasure Island: StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC.; 2024. [PubMed]
- 3.Kashoor I, Batlle D. Proximal renal tubular acidosis with and without Fanconi syndrome. Kidney Res Clin Pract. 2019;38(3):267–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka S, Masumoto N, Makino T, Matsushima Y, Morikawa T, Ito M. Novel compounds isolated from health food products containing beni-koji (red yeast rice) with adverse event reports. J Nat Med. 2024;78(4):845–8. [DOI] [PubMed] [Google Scholar]
- 5.Tanuma A, Sato H, Takeda T, Hosojima M, Obayashi H, Hama H, et al. Functional characterization of a novel missense CLCN5 mutation causing alterations in proximal tubular endocytic machinery in Dent’s disease. Nephron Physiol. 2007;107(4):p87-97. [DOI] [PubMed] [Google Scholar]
- 6.Hall AM, Bass P, Unwin RJ. Drug-induced renal Fanconi syndrome. QJM. 2014;107(4):261–9. [DOI] [PubMed] [Google Scholar]
- 7.Verhelst D, Monge M, Meynard JL, Fouqueray B, Mougenot B, Girard PM, et al. Fanconi syndrome and renal failure induced by tenofovir: a first case report. Am J Kidney Dis. 2002;40(6):1331–3. [DOI] [PubMed] [Google Scholar]
- 8.Ghiculescu RA, Kubler PA. Aminoglycoside-associated Fanconi syndrome. Am J Kidney Dis. 2006;48(6):e89-93. [DOI] [PubMed] [Google Scholar]
- 9.Murata Y, Hemmi S, Akiya Y, Miyasato K, Kobayashi H, Maruyama T, et al. Certain red yeast rice supplements in Japan cause acute tubulointerstitial injury. Kidney Int Rep. 2024;9(9):2824–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Summary of Minister Takemi’s press conference. Ministry of Health, Labour and Welfare. 2024. Available from: https://www.mhlw.go.jp/stf/kaiken/daijin/0000194708_00690.html.
- 11.Takeuchi K, Kawamura S, Wada Y, Sakamoto E, Kuno H, Sakurabayashi S, et al. Renal impairment of proximal tubular injury caused by red yeast rice supplement: report of 2 cases. Case Rep Nephrol Dial. 2024;14(1):128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oda K, Murata T, Tanaka F, Oda H, Tsujimoto K, Fukumori A, et al. A case of acute kidney injury and Fanconi syndrome while taking multiple supplements, including Red Yeast Rice Cholesterol Help(®). CEN Case Rep. 2024. [DOI] [PubMed]
- 13.Maiguma M, Kihara M, Hamaguchi M, Kobayashi T, Yamada K, Takagi M, et al. Successful treatment of acute tubulointerstitial nephritis probably due to Benikoji CholesteHelp(®), a supplement containing red yeast rice. CEN Case Rep. 2024. [DOI] [PubMed]
- 14.Miyazaki R, Takahashi Y, Kawamura T, Ueda H, Tsuboi N, Yokoo T. Acute kidney tubular injury after ingestion of red yeast rice supplement. Clin Kidney J. 2024;17(6):sfae151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chikasue A, Taguchi K, Iwatani R, Kimura K, Okuda S, Uesugi N, et al. Three cases of red yeast rice-containing supplement-induced acute kidney injury and Fanconi syndrome. Am J Kidney Dis. 2024. [DOI] [PubMed]
- 16.Vickers AE, Rose K, Fisher R, Saulnier M, Sahota P, Bentley P. Kidney slices of human and rat to characterize cisplatin-induced injury on cellular pathways and morphology. Toxicol Pathol. 2004;32(5):577–90. [DOI] [PubMed] [Google Scholar]
- 17.Jennette JC, Vivette DDA. Heptinstall’s pathology of the kidney, 8th ed. Wolters Kluwer; Lippincott Williams & Wilkins; 2024.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1. Supplementary Figure 1. Clinical course in case 1. Abbreviations: FEUA, fractional excretion of uric acid; MG, microglobulin; TRP, tubular reabsorption of phosphate.
Supplementary Material 2. Supplementary Figure 2. Clinical course in case 2. Abbreviations: MG, microglobulin; NA, not applicable.
Data Availability Statement
No datasets were generated or analysed during the current study.