Abstract
Glioblastoma (GBM) is the most aggressive primary brain tumor in adults, with a universally lethal prognosis despite maximal standard therapies. Here, we present a consensus treatment protocol based on the metabolic requirements of GBM cells for the two major fermentable fuels: glucose and glutamine. Glucose is a source of carbon and ATP synthesis for tumor growth through glycolysis, while glutamine provides nitrogen, carbon, and ATP synthesis through glutaminolysis. As no tumor can grow without anabolic substrates or energy, the simultaneous targeting of glycolysis and glutaminolysis is expected to reduce the proliferation of most if not all GBM cells. Ketogenic metabolic therapy (KMT) leverages diet-drug combinations that inhibit glycolysis, glutaminolysis, and growth signaling while shifting energy metabolism to therapeutic ketosis. The glucose-ketone index (GKI) is a standardized biomarker for assessing biological compliance, ideally via real-time monitoring. KMT aims to increase substrate competition and normalize the tumor microenvironment through GKI-adjusted ketogenic diets, calorie restriction, and fasting, while also targeting glycolytic and glutaminolytic flux using specific metabolic inhibitors. Non-fermentable fuels, such as ketone bodies, fatty acids, or lactate, are comparatively less efficient in supporting the long-term bioenergetic and biosynthetic demands of cancer cell proliferation. The proposed strategy may be implemented as a synergistic metabolic priming baseline in GBM as well as other tumors driven by glycolysis and glutaminolysis, regardless of their residual mitochondrial function. Suggested best practices are provided to guide future KMT research in metabolic oncology, offering a shared, evidence-driven framework for observational and interventional studies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-024-03775-4.
Keywords: Cancer, Glioblastoma, Metabolism, Research design, Warburg Effect, Glutaminolysis, Precision medicine
Background
Standard of care for brain cancer management
Glioblastoma (GBM), the most common and aggressive primary brain tumor in adults, has one of the highest mortality rates of all cancers. Despite the advent of multimodality in neuro-oncology and emergence of novel therapies, long-term survival remains poor for most high-grade brain tumors [1–4]. In fact, median overall survival (mOS) for GBM is only marginally better today than it was in 1926: 14–21 months versus 8–14 months, respectively [5, 6]. More importantly, incremental improvements in mOS or progression-free survival (PFS) should not be confused with long-term survival, which remains less than 0.8% at 10 years from diagnosis [7, 8]. None of the current cytotoxic, molecularly targeted, or immune-based therapies have translated into robust improvements in long-term survival at the population level [9–11]. When deciding on palliative care, oncologists and patients may have a different understanding of therapeutic goals, and patients may not understand that the proposed treatments are “unlikely to be curative”, leading to inaccurate expectations [12, 13]. If therapeutic success is defined as long-term survival, it becomes clear that no major advancements have been made in GBM therapy despite a century of cancer research [14].
The current standard of care (SOC) involves maximal safe surgical resection, radiotherapy, and temozolomide chemotherapy, with an average mOS across clinical trials of 15.6 months (compared to 10.1 with surgery alone in historical cohorts), reaching a 5-year relative survival rate of less than 10% [15, 16]. A small improvement in mOS is observed in younger patients and high-grade gliomas with specific isocitrate dehydrogenase (IDH) mutations [17, 18]. The degree of surgical debulking is considered one of the most important prognostic factors, which could explain the survival differences between SOC (which includes debulking) and biopsy alone (without debulking) in best supportive care [19–21]. Elective treatments such as FDA-approved Tumor-Treating Fields (TTF) or novel immune-based therapies are occasionally offered after SOC for a modest increase in PFS and mOS [22, 23]. Unfortunately, despite providing desirable benefits in the form of transient tumor control and short-term survival, SOC does not yield meaningful improvements in long-term survival in comparison with post-surgical “best supportive care,” defined as symptom management (edema, nausea, pain, and malnutrition) [24, 25]. For recurrent GBM, consensus guidelines such as the NCCN encourage participation in clinical trials due to dissatisfactory treatment outcomes [26, 27]; unfortunately, clinical trials with various therapies, alone or in combination, have not yet achieved a significant extension of survival [28]. Therefore, patients should be informed of the expected benefits and adverse effects of existing therapeutic approaches to assist with informed consent and shared decision-making [9, 29]. Considering the dismal prognosis despite maximal SOC, novel clinical research frameworks are urgently needed to drive improvements in quality of life and long-term survival.
Cancer as a mitochondrial metabolic disease: an emerging therapeutic paradigm
To address these challenges, we propose research guidelines for the management of GBM based on the understanding of cancer as a mitochondrial metabolic disease [30, 31].
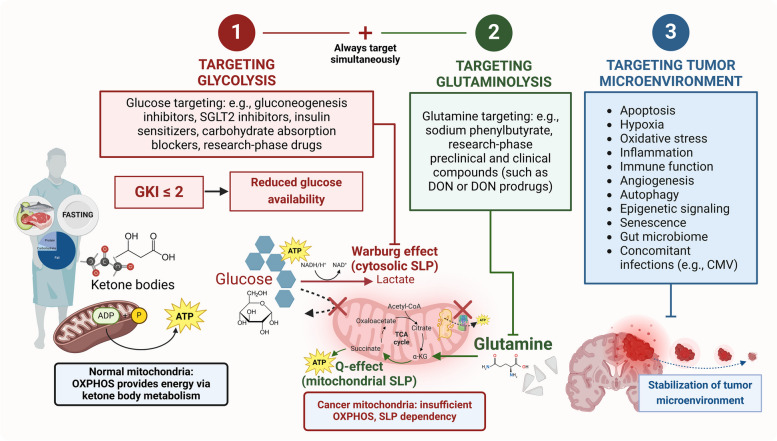
Two major biochemical processes exist to generate energy in eukaryotic animal cells: substrate-level phosphorylation (SLP), also known as fermentation, and mitochondrial oxidative phosphorylation (OXPHOS), via electron transport chain-induced chemiosmosis. Non-tumoral cells are metabolically flexible: in the presence of oxygen, OXPHOS is sufficient to supply most of the energy requirements in a highly efficient and regulated system, relying on SLP only under certain physiological conditions [32]. Conversely, SLP can produce energy in the cytosol (e.g., Embden-Meyerhof-Parnas glycolytic pathway) and in the mitochondria (e.g., succinate-CoA ligase reaction in the TCA cycle), independent of OXPHOS [33, 34]. Cancer cells, including GBM, are largely dependent on increased SLP flux of glucose and glutamine through the glycolysis and glutaminolysis pathways, regardless of the presence of oxygen [33, 35–38]. In this protocol, we favor a functional definition of SLP dependency as the comparatively limited capacity of malignant cells to sustain long-term proliferation when forced to use OXPHOS-exclusive metabolism (e.g., deprivation of glucose and glutamine, the two primary SLP fuels, at the substrate, transport, or utilization level). Insufficient or “dysfunctional” OXPHOS in cancer cells, as compared to normal cells, is hypothesized to arise from the well-documented and universal abnormalities in the number, structure, dynamics, and collective functional efficiency of the mitochondrial population [39–45].
To our knowledge, there are no models of cancer that retain aggressive and limitless replicative capacity in the simultaneous absence of glycolysis and glutaminolysis, despite substitution with non-fermentable OXPHOS fuels (e.g., ketone bodies, fatty acids, pyruvate, lactate), as recapitulated by essential nutrient constraints in cell culture [46–48]. Similarly, neither basic nor clinical research to date supports the notion that tumors with certain mutations (e.g., BRAF V600E) can effectively metabolize fatty acids or ketone bodies to maintain constant growth after effective dual targeting of glucose and glutamine, even if they may do so over short experimental endpoints as long as SLP flux is maintained [49–52]. While it is possible that insights from in vitro mechanistic studies do not fully translate to the in vivo condition [53–55], we hypothesize that the minimal bioenergetic requirements for cell viability (ATP sufficiency) may be applicable across model systems, even if heterogeneity in fuel utilization may arise once energy constraints have been met. Therefore, historical controversies regarding the role of OXPHOS in cancer may have originated from imprecise definitions; as stated by Otto Warburg himself, “we have here a perfect example of a dispute about words” [56, 57].
To avoid these issues, we identify “respiratory insufficiency” or “insufficient OXPHOS” as the therapeutically exploitable fact that cancer cells, unlike normal cells, appear unable to proliferate exclusively via OXPHOS when SLP is absent, not by the relative degree of mitochondrial function they may still retain [58–60]. Residual OXPHOS is a quantifiable category but, from a purely utilitarian point of view, it may not be able to support long-term proliferation in the absence of sufficient SLP flux, representing a targetable difference between non-tumoral and tumoral cells [61]. The proposed metabolic dependencies are summarized in Fig. 1. In this model, oxidative fuel utilization becomes functionally constrained by baseline SLP requirements and absolute OXPHOS efficiency, not substrate uptake or labeling, accounting for the relative metabolic heterogeneity across tumors (for example, in ketolytic activity) [62–65]. From a translational perspective, attaining a sufficient level of nutrient stress in vivo will likely require whole-body physiological adaptations (recapitulating fasting metabolism) as well as pharmacological interventions (metabolic inhibitors), reducing the effective ATP/biosynthetic output of the glycolytic and glutaminolytic pathways even if the input metabolites are still present in the tumor microenvironment. In preclinical models, dietary interventions that induce or “mimic” fasting have been tested to protect normal cells and potentiate the anti-tumoral effects of such metabolic inhibitors [66–69], but most clinical trials to date involved differential stress sensitization to conventional chemoradiotherapy rather than diet-drug combinations directed exclusively at cancer metabolism [70, 71].
Fig. 1.
Simplified diagram of normal and cancer cell metabolism, with special emphasis on ATP synthesis (SLP and OXPHOS). All living cells must meet their ATP demands. Normal cells, including growth-regulated proliferating cells, generate the majority of ATP through the multi-step, ultrastructure-dependent process of OXPHOS. Cancer cells exhibit abnormalities in mitochondrial structure, function and/or number, as well as increased biosynthetic and redox demands, leading to a comparatively reduced efficiency of OXPHOS and compensatory upregulation of cytosolic and mitochondrial SLP. Cytosolic SLP is driven by glycolytic flux but is not synonymous with the Warburg effect (aerobic lactic acid fermentation). Oxidative metabolites can feed into the TCA cycle through catabolic pathways (glycolysis, glutaminolysis, lactate oxidation, β-oxidation, ketolysis), contributing to both SLP and OXPHOS; the total ATP yield is determined by nutrient availability and transport, as well as pathway flux, integrity, and efficiency. Cell division can be constrained by biosynthesis in the excess (assuming sufficient ATP), but energy is limiting for survival under nutrient depletion. The goal of KMT is to synergize with other therapies by targeting SLP flux in cancer cells and upregulating OXPHOS in normal cells, increasing metabolic stress and whole-body ecological competition
Regrettably, standard GBM therapeutics are not designed to take advantage of the metabolic vulnerabilities of cancer cells; instead, they focus on DNA repair mechanisms. In fact, as an unintended consequence of non-specific cell damage, radiotherapy has been shown to induce detrimental metabolic changes and inflammation in the tumor microenvironment, impacting the phenotype of recurrence, which should be weighed against the desirable short-term cytotoxic or immune-potentiation effects [72–75]. In a similar fashion, temozolomide may increase systemic inflammation and tumor-driver mutations [76, 77]. Both brain-directed radiotherapy and systemic antineoplastic therapy can result in neurological complications (including brain tissue necrosis, brain atrophy, and neurocognitive impairment), which should be prevented if long-term survival is expected [78]. Furthermore, as part of supportive therapy, patients with brain cancer often receive corticosteroids (e.g., dexamethasone) to reduce vasogenic edema [27, 79]. The injudicious use of corticosteroids has been questioned due to correlations with reduced survival via dysregulated glucose metabolism, increased insulin signaling and immune suppression [80–88]. Current recommendations specify that “the lowest dose of steroids should be used for the shortest time possible,” in contrast with the “traditional, often uncritical use of steroids” [80], but this advice has yet to be widely adopted [89–91]. Finally, bevacizumab, a second-line anti-angiogenic therapy, may harbor unwanted adverse effects by facilitating distal tumor invasion through the neural parenchyma and perivascular network, without offering improvements to long-term survival [92–94].
While conventional chemoradiotherapies in GBM are well-intentioned, not addressing the unique characteristics of cancer metabolism may hinder their long-term effectiveness. Given the emphasis on patient autonomy in contemporary medical ethics, we advocate for well-informed patients to actively participate in their disease management, fostering supportive follow-up care to explore suitable clinical trials and complementary therapies [95–98]. Therefore, to reach a broader patient population, novel evidence-based treatments must be developed, tested, and accepted into standard clinical guidelines. In pursuit of this goal, accumulating evidence suggests that targeting glycolysis and glutaminolysis while transitioning the patient’s whole-body physiology into therapeutic ketosis could be an effective and translationally viable antineoplastic strategy [35]. Winter and colleagues coined the term “Ketogenic Metabolic Therapy” (KMT) to describe the systemic metabolic changes induced by very low carbohydrate (ketogenic) diets, calorie restriction, and/or fasting [99].
In the current framework, KMT is redefined and expanded as an “umbrella” term that includes long-term dietary, physical activity, and lifestyle modifications (requiring objective, measurable biological outcomes), combined with pharmacological targeting of glycolysis, glutaminolysis, and the tumor microenvironment. KMT is increasingly recognized as an emerging therapeutic approach for a broad range of cancers, while also improving quality of life [99–114].
Very low-carbohydrate, moderate-protein, high-fat ketogenic diets (KDs) induce a metabolic state of increased glycolytic substrate competition for cancer cells while also elevating non-fermentable ketone bodies to serve as an alternative energy source in normal cells [63, 99, 115–117]. In this context, KDs, calorie restriction, and fasting are anti-angiogenic, anti-inflammatory, and anti-invasive and can facilitate cancer cell death through multiple mechanisms [118–126]. Additionally, ketone body metabolism will enhance the ΔG′ATP hydrolysis in normal cells, thus awarding normal cells a bioenergetic advantage over tumor cells [127, 128]. A reduction in the rate of SLP flux will also lower the acidity in the tumor microenvironment, subsequently reducing inflammation and potentially limiting distant metastases [129]. Activities associated with cancer cell proliferation, such as biomass synthesis, are also inhibited by restricting the rate of glucose and glutamine fermentation [130, 131].
Dietary KMT has been found to interact synergistically with other drugs, procedures, and specific molecular tumor characteristics such as the IDH1-R132H mutation [132–134]. Gain-of-function IDH mutations can induce the production of 2-hydroxyglutarate (2-HG), an “oncometabolite” with aberrant epigenetic and immunosuppressive effects [135]. At the same time, accumulation of 2-HG may inhibit SLP flux, limiting the biomass and energy synthesis required for tumor growth [136–139]. From a metabolic perspective, in the specific case of high-grade glioma, IDH1-R132H could be viewed as a “therapeutic” mutation. In light of the inconsistent clinical outcomes with IDH inhibitors in high-grade gliomas so far [140, 141], we and others have proposed that “instead of shutting down mutant IDH enzymes, exploiting the selective vulnerabilities caused by them might be another attractive and promising strategy” [142].
It is important to mention, however, that dietary changes alone are unlikely to control tumor progression in most patients. While rigorous calorically restricted KDs and fasting may be effective in targeting glycolysis, insulin, and growth signaling, they do not adequately inhibit glutaminolysis [143–147]. Consequently, it will be essential to design and test KMT protocols with drugs that also inhibit glutaminolysis at the substrate, enzyme, and/or transport level. Current perspectives on leveraging cancer metabolism are mixed and often contradictory, although most agree on the need for combinatorial approaches [70, 148]. We propose that the best possibility of effective metabolic therapies will involve the simultaneous targeting of glucose and glutamine (specifically, SLP flux) after whole-body adaptation to therapeutic ketosis, leading to a normalization of the tumor microenvironment and enhancement of OXPHOS function and adaptive capacity in normal cells [129, 132, 143].
It should be noted that most early clinical trials explored additivity with SOC of either dietary modification alone (e.g., KDs, caloric restriction, amino acid depletion, fasting-mimicking protocols) [149], or single pathway metabolic inhibition (e.g., systemic glucose or insulin regulation via metformin or SGLT2 inhibitors; glycolysis inhibitors such as 2-Deoxy-D-glucose; glutaminolysis inhibitors such as CB-839 or DON prodrugs) [70].
In preclinical models, KDs in monotherapy induce predominantly favorable survival-prolonging effects across syngeneic and xenogeneic models, with variability in outcomes attributable to methodological differences (timing of intervention, tumor localization, diet composition, and degree of caloric restriction) [150]. Experimental factors such as failure to consistently reduce glycemia/insulin (despite increases in ketonemia), diet initiation (before or after tumor implantation), composition (ketogenic ratio), and palatability, as well as ad libitum or restricted feeding, could account for diverging results even when using identical tumor models [151, 152].
For high-grade glioma therapy, a cumulative total of 187 patients have been treated in more than 13 clinical studies thus far [153], demonstrating feasibility, safety, and tolerability, as well as improvements in quality of life and self-management [154, 155]. Additionally, more than 60 ongoing clinical trials are testing KDs in combination with standard, immune-based, and other targeted approaches (such as PI3K inhibitors), in GBM and other solid malignancies [156]. Unfortunately, there are no established “therapeutic targets” for clinical implementation beyond achieving a minimal state of ketosis (usually at a very modest ≥ 0.3 mM capillary βHB) and, if possible, sporadic but not sustained improvements in glycemia or insulin signaling; these have not been considered primary endpoints in any published study so far. If we conceptualize the KD as a bona-fide systemic “drug” intervention to reduce glycolytic flux, we lack data describing the area under the curve (AUC) of different ranges of glycemia and the anti-tumor effects across time. We suggest that future clinical trials should be designed to reach surrogate biomarkers of biological efficacy (such as real-time monitoring and stratification based on glycemia and ketonemia ranges, or chronic insulin suppression), rather than relying on self-reported dietary adherence. Conversely, there has been extensive preclinical development of pharmacological inhibitors aimed at nearly all metabolic pathways identified as upregulated or aberrant in cancer, subsequently added to various SOC regimens upon reaching clinical testing (without dietary intervention) [157]. Canonical pathways include glycolysis and glutaminolysis, but also other amino acids (methionine, arginine, tyrosine), the electron transport chain, fatty acid oxidation, lactate transport, mutant IDH enzymes, the kynurenine pathway, and even ketolysis. We have limited our proposal to mechanisms related to ATP synthesis, with the intention of establishing a clear therapeutic prioritization (SLP > OXPHOS). The goal of this framework is to formalize and build upon previous studies by constructing rational combinatory diet-drug approaches.
We acknowledge that the pleiotropic effects of dietary KMT may be equally mediated through decreases in growth signaling (insulin/IGF-1, AMPK, PI3K/AKT/mTOR axis), immune responses, post-translational epigenetic modification, gut microbiome, and/or regulation of the systemic hormonal milieu, rather than direct suppression of ATP-generating pathways [158, 159]. It is also possible that cancer cells exhibit increased sensitivity to SLP targeting due to biosynthetic or redox requirements (NAD+/NADH, NADP+/NADPH) [130, 160]. However, we argue that bioenergetics are interconnected with all the above, with major relevance for cell viability under metabolic stress, while intra/extracellular growth factors and biosynthesis may be determining of maximal proliferation (assuming baseline viability, and thus ATP sufficiency). Accordingly, it can be expected that healthy cell populations will display unique vulnerability thresholds to combined diet-drug metabolic pressure, carrying a risk of toxicity (e.g., rapidly proliferating immune and epithelial cells are more sensitive to pharmacological inhibition of glutamine) [161]. While we hypothesize that neoplastic cells are comparatively more susceptible to metabolic stress due to SLP dependency, mutational burden, and dysregulated growth itself, we aim to minimize off-target effects by following the press-pulse therapeutic principle, where drugs with a narrow therapeutic index (such as cytotoxic agents or metabolic inhibitors) are carefully dose-escalated and applied intermittently on a “metabolic priming” dietary KMT baseline [132].
Purpose and rationale
Building upon this knowledge, we offer a framework for future research on KMT with additional pharmacological targeting of glycolysis and glutaminolysis as a minimally toxic therapeutic strategy for GBM management. The resulting shift to fat-derived ketone body metabolism allows for the relative reduction of glucose and glutamine-driven SLP flux while maintaining normal cell function by upregulating oxidative metabolism and increasing competitive evolutionary pressure [105, 145, 162]. The proposed drugs and strategies are intended to further restrict biosynthetic and bioenergetic pathways in tumor tissues. We have constructed this proposal by synthetizing the expert opinion of researchers and clinicians involved in previous preclinical and translational KMT research. Importantly, while this approach was developed primarily for GBM, the mechanistic basis should be applicable to all malignant cancers exhibiting SLP dependency on glucose and glutamine, as defined above [35, 115]. In this case, GBM was selected due to poor SOC outcomes and ethical considerations, as well as the potential benefit of therapeutic ketosis to seizure management and intracranial edema, rather than intrinsic bioenergetic characteristics [163].
It is important to acknowledge that forthcoming clinical research on cancer metabolism will likely involve combined testing with standard chemoradiotherapeutics as well as novel targeted and immune-based treatments, as the natural consequence of the incremental “one drug-one target” model [164, 165]. Under this research paradigm, current SOC serves as the gold standard, while KMT is tested as a secondary, adjuvant therapy. In this scenario, the utility of KMT is being demonstrated to enhance the anti-tumor effects of radiotherapy, chemotherapy, and targeted approaches (e.g., VEGF and immune checkpoint inhibitors) across different cancer models, via reductions in tumor nutrient utilization, hypoxia, inflammation, invasion, and angiogenesis, as well as regulation of pathways mediating tumor growth such as mTOR, insulin-PI3K, AMPK-PGC-1α, autophagy, epigenetic signaling, immune recognition, and multiple other pleiotropic mechanisms [68, 126, 158, 166–171]. In this way, changes in metabolism are being shown to mimic or potentiate the action of pharmaceutical agents, often without additional toxicity.
In the proposed framework, KMT is positioned as an evolutionarily advantageous prerequisite “metabolic priming” baseline upon which other cytotoxic therapies are introduced to assess potential synergy, additivity, or antagonism, rationalizing research priorities. It is an implicit assumption that clinical studies exploring precision nutrition or single metabolic inhibitors as adjuncts with SOC will be carried out in parallel, particularly for tumors where SOC offers a well-established track record of survival benefit; in cases where SOC may be deemed insufficient (as determined by the patient), a conceptual reframing of KMT at the foundational level may provide an ethical opportunity to explore the effectiveness of standalone diet-drug metabolic targeting.
A growing body of evidence suggests that well-formulated KDs can slow tumor progression, but most published reports to date have lacked a robust, modifiable protocol for clinical implementation and data collection. There is a lack of consensus for optimal KD therapy in cancer, leading to a heterogeneity of methodological approaches and lapses in effective monitoring [153]. Poor standardization has led to difficulties with inter-study comparability, as not all protocols described as “ketogenic” will offer therapeutic benefits in cancer-specific settings [172, 173]. A general, isocaloric/eucaloric, ad libitum KD is not synonymous with dietary KMT. The application of KDs in cancer should be nuanced and must fulfill a set of measurable biological criteria, with each patient exhibiting an individualized response over time. It is therefore essential to record data systematically (ideally, in real time), correlating cumulative physiological changes with anti-tumor effects. As such, the glucose-ketone index (GKI) was developed as a unifying biomarker for assessing “biological” compliance and outcomes in brain cancer [174]. Rather than relying on self-reported dietary compliance, any evaluation of clinical efficacy should be correlated with measurements of blood glucose and blood ketones (which can then be used to derive the GKI), as well other objective biological measurements (e.g., insulin, metabolic imaging, metabolomics), allowing for inter-study comparisons and external validity under different methodologies [102, 134, 145, 175].
It should be noted that a single dietary intervention is unlikely to affect all patients equally despite standardization efforts, with population-level genetic variability across endocrine and metabolic phenotypes [176, 177]. We recognize that real-world, large-scale clinical implementation of KMT will carry inherent heterogeneity that cannot (and perhaps should not) be avoided, granting patients and clinicians the freedom to adapt to specific and changing needs. However, it is necessary to develop initial best practices for KMT to serve as an evidence-based reference point without sacrificing therapeutic efficacy, addressing challenges raised in previous reports and being mindful of resource constraints for clinical research in smaller, financially constrained institutions.
Ketone body metabolism in cancer: why therapeutic ketosis?
Russell Wilder at the Mayo Clinic formally developed the KD as a treatment for pediatric epilepsy in the 1920s, although various forms of very low carbohydrate diets and fasting have been used empirically for seizure control, diabetes, obesity, and other diseases since antiquity [178]. Prescription of KDs for epilepsy declined with the advent of new anticonvulsants but continues to be a cornerstone in the treatment of drug-resistant epilepsy as well as inborn errors of carbohydrate metabolism [179–181]. Recently, KDs experienced a major resurgence in clinical applications, particularly for insulin resistance, obesity, and neuroprogressive disorders [158, 182, 183], while ketogenically compensated glucose modulation as a cancer therapy has been described more than 80 years ago in a case series by Brünings [184].
Achieving stable therapeutic ketosis requires adjustments to the macronutrient composition of the diet. The KD is defined as a dietary pattern that is very low in carbohydrates (typically less than 20 g/day, which depletes liver glycogen and initiates ketogenesis), adequate in high-quality protein (sufficient for muscle maintenance, without excessive contribution to endogenous glucose production), and variable in fat, depending on whether it is intended to be hypocaloric (loss of adipose tissue), eucaloric (weight maintenance), or hypercaloric (recovery of adipose tissue). Restricted consumption of carbohydrates elicits a physiological metabolic adaptation favoring fat-derived fuels over glucose, resulting in the endogenous production of water-soluble metabolites collectively known as ketone bodies: acetoacetate, beta-hydroxybutyrate (βHB), and acetone [128, 185].
Acetoacetate and βHB are synthesized predominantly in the liver and exported into the bloodstream, serving as a “glucose substitute” for energy and biosynthesis in mitochondrially healthy cells [186]. Acetone is a breakdown product of acetoacetate that is released in breath and urine [187]. Acetoacetate and βHB are readily oxidized by all major organs, except for the liver, which relies on fatty acid oxidation and gluconeogenic substrates under glycogen depletion [188, 189]. After ketogenic adaptation, ketone bodies can supply more than 50% of the energy requirements of the human body, and over 70% of the brain’s energy needs [190–192]. From an endocrine perspective, dietary carbohydrate restriction reduces plasma glucose excursions, bolus insulin spikes, and basal insulin levels, removing insulin’s suppression of key enzymes controlling ketogenesis [193, 194]. Moreover, glucagon secretion decreases over time, further reducing basal hepatic glucose output (glycogenolysis and gluconeogenesis), glucose availability, and basal insulin [195]. A more in-depth discussion regarding physiological requirements for exogenous carbohydrates and endogenous glucose production, as well as metabolic acidosis, is offered in Additional File 1: Appendix 1.
Standardizing KDs for biological efficacy
Different versions of the KD have been described in both scientific and lay texts, often including conflicting advice, especially for cancer management. This has led to widespread confusion in the public sphere and obstacles for clinical implementation. In the following sections, we summarize ketogenic procedures that have been tested for GBM. These practical definitions may help in choosing the intervention that best suits a particular need, with most seeking as much flexibility as possible without compromising therapeutic efficacy. It is important to remember that efforts to improve diet adherence, which are vital for patient accrual, are still bound by the GKI or other objective metrics of metabolic and tumor responses.
The GKI is the ratio of glucose to βHB, the two metabolites of interest in dietary KMT [174]. Glucose and ketones are assessed by capillary blood sampling using specialized handheld glucometers or extrapolated from interstitial fluid measurements using real-time wearable monitors. Steady-state GKI levels are used to estimate the degree of therapeutic ketosis and other biological processes, such as insulin signaling, growth-promoting pathways, and systemic inflammation, which are not readily accessible for repeated or real-time sampling, and are generally correlated with persistent decreases in glycemia and increases in ketonemia (resulting in a decreased GKI) [102]. In future clinical trials, it will be essential to capture the AUC and variability of glycemia and ketonemia over extended study intervals to establish statistical correlations with therapeutic efficacy, as short-term metabolic changes are not expected to induce sufficient competitive metabolic pressure. To facilitate longitudinal tracking, an updated version of the GKI tracking tool is provided in Additional File 2: GKI tracking spreadsheet.
The baseline dietary strategy is to follow a macronutrient distribution that facilitates ketogenic adaptation, preserves lean body mass (LBM), and maintains an adequate micronutrient balance, while keeping sustained daily GKI values below 2.0, ideally near 1.0 or below (Fig. 2). In clinical studies, averaged weekly, monthly, and yearly values should be collected for a data-driven appraisal of efficacy [134]. Continuous, uninterrupted maintenance of therapeutic GKI ranges may be preferable to occasional, short-term, or cyclical strategies [145]. It is important to note that dietary KMT is defined by a gradual, sustained, whole-body metabolic and endocrine adaptation in fuel partitioning. Absolute blood glucose levels should be consistently below 90 mg/dl or 5 mM; this is an arbitrary, statistically derived cut-off that has been associated with improved survival but does not define a known biological constraint [85, 102, 196, 197]. Preclinical and clinical evidence suggests that patients should aim for the lowest, physiologically safe and sustainable glucose and insulin levels [198–202], where the proxy indicating effective insulin suppression is via elevated blood ketone levels throughout the day, especially during the evening pre-prandial time [203, 204]. Patients with cancer can present with normal to low glycemia (and consequently low fasting insulin) due to tumor hypermetabolism, concealing metabolic dysregulation; it is therefore valuable to measure glycemia, ketonemia, and insulin secretion during the feeding period (e.g., before dinner). A morning fasted reading can be misleading, as healthy populations (and even type 2 diabetics) can present with low levels of nutritional ketosis after the overnight fast [205, 206].
Fig. 2.
Illustrative diagram of blood glucose, βHB, and GKI during different phases of dietary KMT. Note that the suggested glucose and ketone levels are representative of inter-individual and intra-individual variability, not prescriptive. In this example, after initiating a GKI-adjusted KD, glycemia is maintained below 5 mM and ketonemia above 1–2 mM. The proposed therapeutic zone has been achieved once glucose levels are less than two-fold ketone levels (e.g., 5 mM glucose, 2.5 mM βHB, GKI ≤ 2), and optimal when glucose levels are equal or lower than ketone levels (e.g., 4 mM glucose, 4 mM βHB, GKI ≤ 1). Absolute glucose levels should be at their physiological minimum. Dietary, stress, or therapy-induced excursions (e.g., corticosteroids) should be minimized. Exercise-induced gluconeogenesis is expected and offset via skeletal muscle demand. As a long-term therapeutic strategy, dietary KMT may continue as long as there is evidence of persistent disease or risk of recurrence. Real-time GKI tracking is recommended in research settings to avoid ambiguity regarding biological outcomes
Classic KD: ketogenic ratios, macronutrients, diet adherence
The classic KD developed by Wilder is still prescribed in the epilepsy field. In adults, the patient’s energy needs are initially calculated using standard formulas in a 3:1 to 4:1 ratio of fat grams to combined carbohydrate-plus-protein grams. The main benefit of this approach is that both carbohydrate and protein are kept very low (together, < 10% of total calories), making it easier to reach higher levels of ketosis. There is consensus on how to maintain this diet, several medical foods are available, contraindications are clearly defined, and potential side effects can be proactively monitored and addressed [207–209]. The classic KD may be too rigid for broad clinical application and adherence across heterogeneous cancer populations, but it serves as a well-documented reference template from which to extrapolate introductory practical guidance (e.g., ketogenic recipes and cookbooks), long-term patient monitoring and diet troubleshooting [208].
Recently, macronutrient distributions have been adapted for classic KDs, as they are more intuitive than diet ratios. The macronutrient distribution (% energy) of the classic KD is commonly defined as 88–90% fat, 6–8% protein, and 4% carbohydrate. It should be noted that the daily energy intake will determine the absolute quantity of macronutrients (grams), making flexible distributions less suitable for higher caloric expenditures. For example, a KD consisting of 10% carbohydrate for a total caloric intake of 2500 kcal/day equals to approximately 60 g/day, which may be incompatible with therapeutic ketosis for most patients. At the physiological level, reaching sufficient liver ketogenesis typically involves a carbohydrate intake below 20–50 g/day, depending on the metabolic fitness of the individual [210]. Consequently, the maximum threshold of carbohydrate intake that still allows for the desired degree of ketosis and glycemic control will need to be individually titrated, followed by protein for muscle maintenance, and fat for the desired caloric density. An automated calculator based on the Mifflin-St Jeor equation is provided in Additional File 2: GKI tracking spreadsheet; it is important to note that predictive equations can underestimate the energy requirements of patients with cancer, which are ultimately dictated by the desired weight evolution over time [211, 212].
Diet adherence to classic high-ratio KDs can be perceived as challenging in free-living adults [213]. However, highly motivated patients with an adequate understanding of the scientific rationale have been able to maintain strict compliance over prolonged periods [214, 215]. Patients with cancer may require more protein to preserve LBM, especially if the diet is calorically restricted [216, 217]. In this regard, KDs with adequate protein and micronutrient content (such as paleolithic KDs), which induce a lower than baseline, stable GKI, could improve feasibility and long-term compliance [134, 203, 218, 219].
Calorically restricted KD (KD-R): reaching GKI targets while preserving muscle mass
The classic KD was originally intended to be eucaloric or unrestricted (“ad libitum”) to allow for the appropriate maturation of pediatric patients with epilepsy, and while therapeutic benefits have been reported in preclinical cancer models in both unrestricted and calorically restricted amounts [150, 220], most clinical studies focused on eucaloric feeding to promote weight maintenance [114, 155, 221]. A failure to reduce proliferation could be a consequence of persistently elevated glucose availability, endocrine, or growth-promoting signaling due to energy surplus, despite shifting to a ketogenic state [222, 223].
In contrast, KDs consumed in calorically restricted amounts, resulting in a gradual, deliberate reduction of fat mass (with preserved muscle mass), could produce better cumulative, steady-state GKI values, in tandem with the underlying metabolic and signaling effects, such as insulin suppression [102, 134, 224, 225]. Calorie restriction (independent of macronutrient composition) increases metabolic pressure on tumor cells by modulating nutrient-sensing pathways [226–228]. Similarly, reduced energy intake makes it easier to adapt to the higher overall fat intake despite enhanced satiety [229].
The KD-R protocol should be personalized in duration, periodicity, and degree, while being monitored to ensure mild calorie restriction does not increase the risk of malnutrition. After setting a carbohydrate limit to induce ketogenesis and calculating protein needs to preserve muscle mass, the energy density of the diet will be adjusted by total fat intake. It may be necessary to exclude calorie restriction in malnourished or underweight patients (as a rule, BMI < 18). In practice, patients with lower body fat percentages can follow KD-R in a cyclical fashion, introducing a return to previous isocaloric conditions or a slight caloric surplus when weight recovery is required; these intervals should still be GKI-adjusted, that is, adhering to ketogenic ratios and aiming for the lowest possible GKI.
In all cases, excessive LBM loss should be avoided. A classic KD-R with a high ketogenic ratio is typically too low in protein for long-term muscle maintenance in adults. Emerging evidence suggests that a well-formulated, protein sufficient KD may exert global anti-cachexic effects by decreasing pro-inflammatory cytokines and metabolites (inducing a protein-sparing metabolic shift) [230–234], with further anti-catabolic effects mediated by ketone bodies [235–238]. Therefore, protein intake should be modified for sufficiency, monitoring the impact on GKI, glucose variability, and ketogenesis. Adequate protein intake has either neutral or minor effects on ketogenesis and insulin signaling, as well as hepatic/renal gluconeogenesis [239–241]. Total protein intake can be started at the minimum recommended daily intake of 0.8 g/kg of body weight (for a sedentary individual in isocaloric conditions), and then increased progressively based on factors related to protein needs, such as age, physical activity, or health status [242–244].
It is important to emphasize that dietary amino acids cannot be restricted for clinically relevant glutamine depletion, as glutamine levels remain relatively stable through de novo synthesis regardless of diet composition [245, 246]. Physical activity coupled with a low-carbohydrate diet as well as prolonged fasting are potential non-pharmacological strategies to achieve transient or chronic reduction in plasma glutamine, respectively [247–250].
Supplementation of medium-chain triglycerides (MCTs) and exogenous ketone bodies
MCTs (particularly C8 caprylic acid) are a type of dietary fat that can be supplemented to potentiate liver ketogenesis [251]. MCTs bypass normal fat digestion and diffuse across the intestinal membrane into the hepatic capillary bed, where they are readily converted into ketones. Mild gastrointestinal side effects may arise during the initial weeks of supplementation, with tolerance improving through gradual dose escalation [252–254]. KDs with supplemental MCTs typically pre-specify a set daily intake (e.g., 2–8 tbsp, or 10–30% of total daily calories in the form of MCTs), which is intended to improve ketonemia but also to lower other sources of fat, simplifying trial design and improving adherence [222, 255–257]. A possible drawback of a diet enriched in purified MCTs, as opposed to naturally occurring high-fat foods, is that they are comparatively devoid of micronutrients, particularly liposoluble vitamins. For this reason, overall food choices should emphasize micronutrient density [258–261], especially if the baseline KD is composed exclusively of medical foods that may be missing essential nutrients, or if significant amounts of dietary supplements such as MCTs are needed to achieve specific biological outcomes (e.g., GKI stability or cachexia prevention).
Analogously, exogenous ketone bodies (e.g., ketone esters or ketone salts) are a novel dietary formulation that can be taken orally to temporarily enhance circulating βHB levels [262]. Beyond their bioenergetic role, ketone bodies act as pleiotropic signaling molecules with potential antineoplastic benefits on their own [100, 263–268]. It is unclear, however, whether short-term decreases in the GKI value via supplemental MCTs or exogenous ketones, without a global metabolic transition to therapeutic ketosis by chronic KDs and/or fasting (increased oxidative efficiency of fat-derived metabolites), would retain protective effects against SLP inhibition in normal cells [269]. Elevated blood glucose and ketone levels are typically not found during the natural physiology of calorie restriction or fasting [270]. In the context of dietary KMT, exogenous ketones can also have a measurable impact on glycemic regulation [271–275]. Therefore, supplementation of ketone bodies could be considered to further enhance the therapeutic efficacy of KD/KD-R, particularly under circumstances of reduced compliance, or to reach the higher levels hypothesized to mitigate cancer cachexia [230, 235, 236].
In conclusion, supplementation of MCTs and exogenous ketones can be viewed as a valuable tool to empower patients to modulate their ketonemia, ketogenesis, and gluconeogenesis, without being an absolute requirement. Boosting ketone levels while following a GKI-adjusted KD may be especially useful during the initial adaptation to fasting, lower limits of euglycemia, radiotherapy, hyperbaric medicine, and conventional and adjuvant drug therapies [103, 276–280].
Flexible protocols and quantifiable criteria of compliance
Beyond the classic KD, several dietary regimens to achieve various degrees of ketosis have been described in clinical studies, including the modified Atkins diet (MAD) (60–65% fat, 25–35% protein, 5–10% carbohydrate) [281]; high-protein KDs (60% fat, 35% protein, 5% carbohydrates) [282]; paleolithic KDs (based on animal fat, meat, and offal with a 2:1 fat:protein ratio) [218, 283]; Mediterranean KDs (< 15% carbohydrates, based on green vegetables, olive oil, fish, and meat) [284, 285]; general, non-otherwise specified KDs (70–80% fat, 10–20% protein, < 10% carbohydrate) [286]; plant-based, low carbohydrate diets (generally not sufficiently ketogenic) [287]; as well as other targeted and cyclical variations, with or without calorie restriction [288]. Intermittent or prolonged water-only fasting can be included regardless of diet composition [145, 289, 290]. The primary differences are in the maximum limit of carbohydrate and protein, the timing of feeding, and intermittent calorie restriction, as well as the underlying food selection to accommodate personal dietary preferences. Nevertheless, diet flexibility should be contingent upon the patient’s individual physiological response. Biological, measurable, and quantifiable effects (not subjective biases or beliefs) will ultimately determine the suitability of the chosen foods. If glycemia/ketonemia, and, by extension, the sustained GKI values are not in the prespecified target zone, the selected diet may not be appropriate for the patient.
Given the flexibility in implementation and interpersonal variability, any prospective KD protocol for cancer therapy should favor unbiased compliance biomarkers (e.g., longitudinal, steady-state GKI), as well as periodic blood markers and surrogate endpoints (e.g., comprehensive metabolic panel, tumor biomarkers, anatomic-metabolic imaging, metabolomics). Critical benchmarks, laboratory tests, and troubleshooting for dietary KMT are presented in Table 1. Any KD protocol, whatever the practical food selection may be, should fulfill the following criteria:
Table 1.
Key criteria for GKI-adjusted KD/KD-R implementation and troubleshooting
| Implementation criteria | Troubleshooting |
|---|---|
| GKI ≤ 2.0, ideally ≤ 1.0, with absolute glucose levels < 90 mg/dl (5 mM) | A knowledgeable dietitian should be available to assist patients in optimizing biological changes (e.g., maintaining deep ketosis), beyond ensuring diet compliance. Reduce total carbohydrate intake to less than 5% of total calories, replace carbohydrate with fat sources. Review food tracking to detect hidden carbohydrate sources or ingredients that may impact glycemia even if present in small amounts that only minimally change the food nutrition label. Pay attention to hidden carbohydrates in dietary supplements and excipients, as well as cephalic insulin secretagogues due to sweet taste stimulus, such as “keto-friendly” zero-calorie sweeteners (e.g., xylitol, erythritol, allulose, stevia). If necessary and feasible, reduce total caloric intake (KD-R), e.g., reduce calories by 5% increments each week until GKI goals are met. Implement intermittent fasting (e.g., 16:8 or 20:4), alternate-day fasting (ADF), fasting-mimicking diet (FMD) or prolonged water-only fasting. High fiber KDs can hinder therapeutic GKI targets. An excess of dietary fiber can reduce stomach acidity, thereby decreasing cholecystokinin stimulated bile synthesis and excretion into the small intestine, which could lead to reduced essential fatty acid and fat-soluble vitamin uptake [291]. Real-time monitoring via CGM/CKM coupled with event logging can detect sources of variability, such as cortisol, sleep disturbances or pharmacological interference (e.g., corticosteroids). Medium chain triglycerides (MCTs) are more ketogenic than long-chain fatty acids and can be incorporated into meals to increase ketosis. Ketone supplements can provide a temporary boost in ketonemia but are not a substitute for nutritional ketosis. Consider the impact of physical activity, stress management and circadian rhythms. To facilitate GKI targets, evaluate the suitability of gluconeogenesis and glycolysis-targeting drugs (Additional File 5: Table S2) |
| Preserve LBM, recover fat stores if gradually depleted. Weight (fat) loss during KMT should be controlled and therapeutic, improving general and metabolic health | Optimize protein quality and quantity, especially during chronic calorie restriction or higher levels of physical activity. Protein intake in patients with cancer and normal kidney function is typically set above 1 g/kg/day and, if possible, over 1.5 g/kg/day [216]. Impact on GKI of higher protein targets should be monitored. It is important to account for the protein content in natural food sources; for instance, fattier cuts of meat tend to have slightly lower protein compared to lean cuts. Total fat intake will be variable to meet satiety and/or protocol (e.g., caloric restriction). Cyclical caloric surplus (from fat and protein, not carbohydrate), while maintaining ketosis, can mitigate unintentional weight loss. Advanced disease driving tumor-host pro-catabolic effects and intensive treatment can aggravate pathological weight loss (muscle wasting) that must be differentiated from dietary KMT |
| Adequate micronutrient intake, avoid initial symptoms of keto-adaptation (“keto flu”) and potential long-term side effects. Baseline assessment, blood panel analysis, and monitoring of health status | Metabolic adaptation can be facilitated by a gradual decrease in carbohydrate intake (e.g., change meal composition and frequency in stages until all meals conform to ketogenic ratios) [292, 293]. Assess micronutrient status if food choices are not sufficient to reach the recommended levels of essential nutrients: supplement as needed. Assess electrolyte balance: supplement as needed. Ensure adequate hydration. Consider supplementation to prevent both common and rare side effects, such as constipation (fiber and non-digestible carbohydrates, when necessary), hypocarnitinemia (L-carnitine) or nephrolithiasis (e.g., potassium citrate) [294–296]. Ensure adequate protein intake, as long-term side effects can develop due to excessive protein restriction. Monitoring should be adjusted to the demands of SOC and KMT interventions. Suggested laboratory testing (not prescriptive nor exhaustive): hepatic/renal function (including cystatin C), hemogram, glucose and insulin homeostasis (e.g., HbA1c, fructosamine, HOMA-IR, IGF-1 and binding proteins, c-peptide), hormone testing (e.g., vitamin D, thyroid, glucagon, osteocalcin), inflammation markers and lipid panel, including triglycerides, LDL particle size, apoB100/apoA-I ratio and lipoprotein (a) (traditional cholesterol surrogate markers might not be applicable for patients following KDs) [297–300]. Oral glucose tolerance tests (OGTT) with glucose, insulin and c-peptide sampling can be informative to define the insulin-response phenotype in healthy individuals but may not be advisable in the context of sustained dietary KMT and active cancer [195, 301] |
Allow for a sustained GKI of 2.0 or below, ideally 1.0 or below. This involves the lowest physiologically achievable absolute glucose levels (ideally less than 5 mM or 90 mg/dl), minimal glycemic variability (difference between the highest and lowest glucose level), as well as reduced insulin signaling and the related growth-promoting and energy-sensing pathways (e.g., PI3K, mTOR). Glucose and βHB are expected to fluctuate depending on carbohydrate and calorie restriction, as well as fasting duration, protein intake, drug therapies, hormonal balance, emotional stress, circadian rhythms, and nutritional status (vitamin and mineral sufficiency). Reaching the proposed GKI targets implicitly translates into lowering carbohydrate to < 20–50 g/day, regardless of ketogenic ratios or macronutrient percentages, unless concurring with a high level of physical activity [302]. Technologies such as continuous glucose monitoring (CGM) and continuous ketone monitoring (CKM) should be leveraged during the learning phase as the patient explores the impact of different foods on GKI variability [303–305]; it should be noted that CKM sensors are currently available as non-medical devices [306], while clinical testing of dual glucose-ketone monitoring systems is underway [306–308].
Allow for a sustained GKI of 2.0 or below, ideally 1.0 or below. This involves the lowest physiologically achievable absolute glucose levels (ideally less than 5 mM or 90 mg/dl), minimal glycemic variability (difference between the highest and lowest glucose level), as well as reduced insulin signaling and the related growth-promoting and energy-sensing pathways (e.g., PI3K, mTOR). Glucose and βHB are expected to fluctuate depending on carbohydrate and calorie restriction, as well as fasting duration, protein intake, drug therapies, hormonal balance, emotional stress, circadian rhythms, and nutritional status (vitamin and mineral sufficiency). Reaching the proposed GKI targets implicitly translates into lowering carbohydrate to < 20–50 g/day, regardless of ketogenic ratios or macronutrient percentages, unless concurring with a high level of physical activity [302]. Technologies such as continuous glucose monitoring (CGM) and continuous ketone monitoring (CKM) should be leveraged during the learning phase as the patient explores the impact of different foods on GKI variability [303–305]; it should be noted that CKM sensors are currently available as non-medical devices [306], while clinical testing of dual glucose-ketone monitoring systems is underway [306–308].
Patients are often faced with uncertainty regarding “optimal” GKI targets that would be safe and physiologically attainable, depending on their evolving disease status and concomitant therapies. Two empirical GKI baselines can be determined to serve as idiosyncratic biological reference points. Once completing the initial ketogenic adaptation via dietary modification, a fasting GKI baseline can be measured after at least 72 h of water-only fasting (e.g., days 4 to 7 of a 5–7-day water-only fast), which produces GKI values unaffected by dietary inputs [145]. A zero carbohydrate, paleolithic KD with intermittent fasting (e.g., one meal per day) can provide a second baseline that is representative of the lowest GKI variability during minimal dietary inputs (fat and protein only, in a compressed feeding window) [134, 218, 283, 309]. The influence of preexisting conditions, such as insulin resistance, can be captured with a repeated measures design. All subsequent diet adjustments can be compared to these two benchmarks. During study planning and subsequent data analysis, diet flexibility should not compromise GKI targets: “biological” compliance outweighs self-reported or perceived “dietary” compliance.
Adequate protein intake to maintain LBM without disrupting GKI, starting at 0.8 g/kg of body weight and typically settling between 1.2 and 1.5 g/kg for most individuals [310]. Higher initial targets are justified in certain patient demographics (e.g., older age), preexisting comorbidities or anticipated negative impacts of the cancer diagnosis (e.g., loss of appetite during active cancer treatment, or limited physical activity due to cancer fatigue) [311]. Protein quality should be a focus to ensure adequate amino acid ratios without forcing protein overconsumption [312].
Changes in LBM should be monitored on a regular basis. Patients at borderline low weight or with insufficient LBM may alternate between KD-R and GKI-adjusted eucaloric/surplus intervals to preserve and rebuild muscle tissue. Although the systemic metabolic alterations induced by tumor-derived factors secreted directly by GBM cells are still under study, functional impairment leading to undernutrition and side effects of treatment may contribute to progressive loss of skeletal muscle [313–316]. Importantly, irreversible or accelerated cachexia has not been reported in clinical trials examining KDs across several cancer subtypes (despite variable reductions in fat mass), but underweight patients were often excluded a priori, and most studies were designed to prevent weight loss by minimizing calorie restriction [221, 231, 232, 317]. It will be important to examine the impact of well-formulated KDs on cancer-related cachexia in the clinic, ensuring adequate nutrition and protein sufficiency while managing its multifactorial origins, such as systemic inflammation and endocrine dysregulation, which may be difficult to capture in preclinical models [236, 318–321]. Off-label and research-phase anti-catabolic agents, anti-inflammatory drugs, and appetite regulators can synergize with exercise and nutrition therapy to prevent muscle wasting [322, 323].
Adequate micronutrient and vitamin intake. It is preferable to obtain all dietary elements from nutrient-dense foods (e.g., eggs, beef, oily fish, offal) [324]. If the included foods cannot maintain adequate levels of certain essential nutrients or minerals, specific multivitamin and mineral supplementation is warranted [325]. Monitor for secondary hypocarnitinemia and supplement if needed [326]. Macronutrient and micronutrient tracking can be simplified using diet-tracking software [327, 328].
Lessons learned from clinical research evaluating KDs for GBM
Large-scale clinical integration of precision nutrition for cancer management still poses a challenge, with no consensus on best practices [290, 329]. Consequently, patients tend to freely choose their dietary plan [330, 331]. KMT is a potential biomarker-driven metabolic therapy to lower glycolytic SLP, insulin, and oncogenic signaling below baseline, while also stabilizing the tumor microenvironment, contingent upon biological compliance and impacts from other therapies [332, 333]. Additional File 3: Table S1 provides relevant examples of realistically achievable glucose and βHB values that have been reported in studies examining KDs in high-grade brain tumors; additional cancer subtypes have been discussed in [290, 334–336].
Concerns have been raised about the feasibility of reaching and sustaining the hypothetical therapeutic window of KMT, suggesting that “dietary-induced hypoglycemia as a treatment for brain tumors may be simplistic” [222]. While a high degree of personal motivation, specific domain knowledge, and (typically) the assistance of a KD-trained professional is indeed critical for strict diet adherence, more research is needed to establish causal links between quantifiable metabolic changes and therapeutic outcomes, as well as synergistic pharmacological interventions to enhance efficacy [337]. Unfortunately, most GBM studies investigating KDs have not consistently tracked glycemia/ketonemia or other biochemical parameters across time (e.g., serial metabolic imaging or metabolomic profiling), thus patient stratification based on total cumulative exposure to different ranges cannot be performed [153]. A minority of studies documented daily glucose and ketone readings at different non-standardized endpoints but did not report raw data. To embody the goals of precision nutrition, future clinical studies will have to measure, report, and analyze biological responses separately for each patient, regardless of outcome, avoiding group averages [338, 339].
Common pitfalls in clinical research methodology
Clinical studies evaluating KMT may fail due to early oversights in experimental design that can be mitigated with the right knowledge and preparation. Table 2 summarizes recommended and alternative methods for dietary KMT implementation.
Trials often lack ongoing communication and support to retain participants and reduce non-compliance. Recent technologies such as smartphone monitoring applications, telemedicine, and real-time biofeedback (e.g., CGM/CKM or multi-metabolite sensors) may alleviate this issue. Frequent communication with a dietitian/nutritionist trained in KMT as well as a “research kitchen” may improve adherence (e.g., NCT03451799 and NCT03535701). Tracking and optimization of the desired biological markers should be emphasized over self-reported dietary compliance during nutritional counseling [340].
It is exceptionally difficult to gain Institutional Review Board (IRB) approval for KMT trials without concurrent chemoradiotherapeutics, even if their contribution to the long-term management of GBM remains limited [7]. Considering the inadvertent consequences on tumor metabolism, it will be important to design GBM trials with at least one KMT intervention arm in which, after surgical debulking, carefully selected components of SOC (e.g., conventional fractionated radiotherapy) will be tentatively delayed for a clinically acceptable period until an interim evaluation of response. Based on predefined outcomes (partial remission or stable disease), SOC would be delayed again until a subsequent evaluation or disease progression. In this paradigm, KMT refers to both dietary and pharmacological targeting of tumor metabolism, as defined in the protocol below, not a generic KD as monotherapy.
Results from several GBM trials indicate that chemoradiotherapy can be safely delayed for up to 6 weeks after surgery; in some trials, delaying chemoradiotherapy has been paradoxically associated with improved outcomes [341–345]. It is not inconceivable, however, that delaying chemoradiotherapy may have a negative impact on PFS or mOS, despite dubious influence on long-term survival [346–348]. Consequently, well-informed GBM patients should be given the choice to enroll into any prospective group after evaluating the abovementioned survival data (e.g., dietary and pharmacological KMT, or in combination with dose-adjusted temozolomide and/or radiotherapy). Alternatively, patients that are unable or unwilling to undergo some or all aspects of SOC could be offered enrollment in diet-drug KMT trials. In a similar way, the active monitoring period in low-grade gliomas confers an ethical opportunity for the evaluation of non-toxic therapeutic strategies such as dietary KMT, following the recent example of dual inhibitors of mutant IDH1/2 enzymes, which have been tested specifically to “delay the potential long-term toxic effects” of adjuvant chemoradiotherapy [349]. If relative disease stability is achieved despite tumor persistence, repeated surgical debulking could be considered to reduce tumor load [134, 350].
IRB approval for KMT as monotherapy or KMT with only partial SOC will likely demand frequent metabolic and/or anatomic imaging to ensure safety and ongoing tumor evaluation, with a modifiable treatment plan. Accordingly, in a fixed trial design, no GBM patient would be deprived of the potential benefit of chemoradiotherapy, which would be offered to all patients who request it (see Additional File 4: Figure S1). Clinical evaluation of KMT is ideally suited for adaptive trial designs, such as platform trials with response-adaptive randomization, given that it combines a metabolic priming baseline with additional, elective, synergistic press-pulse therapies that require a flexible implementation, compared to a common control group [351, 352]. A core set of interventions in the form of biomarker-driven dietary and pharmacological KMT could be included in the shared master protocol, but subsequent trial arms would need to be adjusted with experimental or salvage therapies based on pre-defined outcomes during each interim analysis.
Eligibility and exclusion criteria should consider the functional demands of the interventions to maximize sample size without compromising efficacy. Eligibility considerations include disease status, side effects or sequalae from prior therapies, comorbidities, performance status, organ function, and contraception and pregnancy testing; conversely, exclusion criteria must include the absolute contraindications of KDs, such as rare inborn errors of metabolism [353].
While well-controlled dietary studies where prepared food is provided to all participants are ideal in terms of diet adherence [209], offering patients to self-select their experimental group and foster self-efficacy may be advantageous in studies where therapeutic outcomes are linked to active participation [354, 355]. Similarly, in a recent KD trial originally planned with two diet arms, patients reported explicit disinterest to participate in the control diet arm (i.e., low-fat treatment) [114]. Therefore, elucidating biological mechanisms and maximal theoretical efficacy of any prospective diet-drug combination may benefit from pilot studies designed in non-randomized, “ideal” scenarios (e.g., self-selected patients with high functional status), proceeding to randomization in the general population after the most promising interventions and biomarker thresholds have been identified.
Most feasibility and tolerability trials have not aimed for the lowest sustained GKI, instead focusing on diet flexibility to ensure better adherence. Compliance is a major challenge, and the diet needs to be as easy to follow and palatable as possible (e.g., prepared meals, medical foods, enteral feeding), but simplicity should not outweigh biological efficacy even at the conceptual phase. Motivated, well-informed patients should understand that objective biomarkers of compliance (such as cumulative time in specific GKI ranges) may influence therapeutic outcomes [102]. Informed consent should be obtained not due to expected side effects, which are preventable or manageable, but to make patients consciously aware of the importance of active participation. Therefore, patients should be instructed to pursue the lowest possible GKI, beyond the trial’s basic requirements. Moreover, correlations with outcomes should be stratified according to biological readouts rather than dietary compliance.
Patients are seldom encouraged to reinforce GKI targets after the intervention period, which is generally no longer than 1–3 months due to budgetary constraints. Studies that are limited in time may fail to produce robust results given that achieving stable therapeutic ketosis often encompasses several weeks, and it is unknown whether long-term maintenance impacts the risk of recurrence. If a trend towards improved PFS or mOS is detected, it will be important to weigh the influence of KMT and SOC, which also requires extended follow-up. Ultimately, GKI-adjusted KD/KD-R should be considered a long-term strategy rather than a limited intervention.
Randomized trials assign patients to the KD intervention while maintaining “usual” (preferred) diet in the control group; a higher demand is consequently placed on the intervention group, especially given the overwhelming physical, emotional, and financial burdens that accompany a cancer diagnosis [356, 357]. Sufficient guidance and understanding of the scientific rationale are therefore essential for patient accrual, compliance, and optimization of therapeutic outcomes. As dietary KMT has been associated with improvements in quality of life and self-efficacy across a broad range of cancers [197, 221, 358–361], it will become important to develop insurance models and healthcare policies that facilitate access and minimize out-of-pocket costs [362].
KMT is often tested in smaller, single-center, investigator-initiated trials. Given that researchers proposing such trials may feel it would be unethical to exclude any potential participants, patients unable to keep GKI or predefined surrogate markers in specific ranges could be used as internal controls. Contemporaneous external controls (from the post-Stupp era) are also a consistent source of comparative survival data [23, 363, 364]. Understandably, dietary KMT studies of sufficient length cannot be easily double-blinded or placebo-controlled.
Individual case reports are highly heterogeneous and lack statistical power, even though they may be a more appropriate methodology for personalized medicine [365]. Case reports should be written following systematic reporting guidelines, such as the CARE guidelines [366, 367]. In contrast, larger clinical trials will require a multi-disciplinary team capable of tracking and supporting each patient individually. GKI allows for quantitative comparisons across different cohorts and types of cancer, but the dietary and/or pharmacological interventions to achieve GKI targets should be personalized.
Table 2.
Recommended and alternative methods for GKI-adjusted KD/KD-R implementation
| Recommended methods | Alternative methods |
|---|---|
| Instruct patients to measure blood glucose and βHB at least twice daily: after the overnight fast (morning), and 1–2 h prior to the last meal (evening). Real-time CGM/CKM is the preferred method for data collection. In future clinical research, it will be essential to track glucose/ketones continuously in order to stratify patients according to time spent in discrete GKI ranges. CGM/CKM can be validated via finger-prick sampling, reducing testing burden. If only CGM is available, it can be coupled with capillary ketone monitoring due to lower general variability in ketonemia. Pre-specified GKI targets should be considered primary trial endpoints | Measure GKI just once daily (ideally in the pre-prandial evening period, after the overnight fast, or prior to the first meal if practicing intermittent fasting, maintaining consistency across measurements). Urinary ketones are not accurate for GKI calculation but can inform sufficient carbohydrate restriction during diet initiation (first 2 weeks) [332]. Testing burden can be reduced after attaining diet stability (i.e., food selection remains unchanged), unless in a clinical trial. For long-term diet maintenance, glucose levels can be inversely correlated with ketosis: if carbohydrates are sufficiently restricted, maintaining a stable lower range of euglycemia (e.g., ≈ 60 mg/dl) is likely accompanied by higher ketonemia |
| KD/KD-R should be assigned depending on initial weight and estimated fat mass. Patients can incorporate fasting in a cyclical manner, as dictated by their adipose tissue reserves. For example, patients with sufficient fat mass can implement a 3–7-day water-only fast or fasting mimicking diets (FMDs) every 1–2 months [368]. Obese/overweight patients can extend fasting beyond 7 days under medical supervision. An average, temporary weight loss of ≈ 3 kg can be expected after a 3-day fast, increasing to ≈ 8 kg after 20 days [369]. A significant fraction of this weight comprises glycogen-associated water storage and intestinal contents, which are quickly recovered [370, 371]. Under proper implementation, LBM reduction is minimal, and most fat mass is subsequently regained [371, 372]. Gradual adipose tissue recovery is feasible if the caloric density of a low carbohydrate diet is sufficient [373, 374]. Fasting should not be limited or discouraged unless there is a risk of cancer cachexia, but each fasting period should be planned and supervised. Inexpensive and non-invasive methods such as bio-electrical impedance can be used to track approximate changes in fat and LBM over time within the same individual | Underweight patients should not practice prolonged fasting without alternating cycles of weight recovery. Loss of LBM should be avoided. A trained dietitian should advise patients on implementing a small caloric surplus with resistance/strength training to recover muscle and fat mass after fasting or KD-R intervals, while still adhering to GKI targets. If body fat stores are too low, it may be preferable to avoid fasting and focus on GKI while maintaining an isocaloric diet. Ongoing changes in average weight (over several weeks) will dictate whether the diet is effectively calorically restricted, eucaloric or hypercaloric, regardless of self-reported or estimated caloric intake. Safety and contraindications of water-only fasting are discussed in [375]; special attention should be given to electrolyte balance and refeeding. If the patient presents with low fat mass (but normal weight/BMI), a FMD may be considered to avoid protein breakdown while potentiating therapeutic GKI ranges [376] |
| In treatment-naïve GBM patients, dietary KMT can be initiated as a neoadjuvant strategy with the aim of reducing tumor growth rates. In the absence of life-threatening symptoms, after surgery, select elements of SOC that may be antagonistic could be preemptively scheduled but delayed until completing a standalone KMT period (including both dietary and pharmacological targeting, as defined in the treatment timeline), for a conservative maximum of 6 weeks. If sufficient radiologic responses or disease stability can be confirmed, radiotherapy and/or chemotherapy can be delayed again while intensifying KMT, for no more than 6 weeks, and reevaluated periodically as long as regression or stability are maintained. During SOC delay, it may be essential to perform sequential imaging to corroborate metabolic responses (see Additional File 4: Figure S1) | This “if/then” experimental design would be applicable to histologically and molecularly confirmed GBM (before or after debulking surgery), given that delaying chemoradiotherapy for up to 6 weeks has shown little to no impact on PFS and mOS (when no other treatment was given, representing a window of opportunity to institute KMT) [341–346]. Outside clinical trials, patients are encouraged to discuss survival data regarding SOC initiation with their treating physician [377]. It should be noted that the long-term survival of GBM with current SOC is less than 10% at 5 years, independent of timing or dosing schedule. Follow-up with imaging and bloodwork should be provided to all patients regardless of their desired treatment, preventing patient abandonment [378, 379] |
| Patients will be asked for informed consent after they receive education as to how dietary KMT will be administered as a therapy, including how non-compliance could have a detrimental impact on the expected benefits. Follow-up should be frequent enough to detect early trends in tumor progression. Researchers should have flexibility in trial design to react to this eventuality, intensifying SOC, metabolic inhibition, or microenvironment targeting | Even though strict adherence to the diet, biomarkers and treatment protocol is necessary, some flexibility should be offered in the timeline of diet-drug implementation. Patients that require second-line salvage therapies not previously defined in the trial design could be reported as individual cases in more heterogeneous cohorts |
Patient education and data collection
After enrollment, each participant should be provided with the following:
Description and informed consent for the proposed therapies. Dietary and pharmacological KMT could include a combination of GKI-adjusted KDs (with or without caloric restriction), fasting, and drug/adjuvant therapies (e.g., metabolic inhibitors, drug repurposing, investigational compounds, hyperbaric medicine, hyperthermia, photo/sonodynamic approaches), with elective and protractible 6-week delay of chemotherapy and/or radiotherapy prior to image-based reevaluation. Dietary KMT implementation requires some level of scientific literacy and active participation. Well-informed patients will be ultimately responsible for dietary compliance, and for requesting support if they are unable to meet biomarker targets. Patient education is key to fostering motivation and biological efficacy. Working with a dietitian/nutritionist knowledgeable in the initiation and maintenance of KMT is extremely helpful.
Tools to monitor blood glucose and ketones. Patients should be counseled on how to use finger-prick glucose/ketone meters, initially measuring at least twice a day to capture data variability. GKI tracking allows patients to actively engage in the treatment process, which could improve compliance. Researchers can expand this testing schedule; for example, 1–2 h post-meal when new foods or changes in portion size are introduced. Patients should also have leeway to reduce the testing burden after the end of the trial, eventually measuring only a few days per week if food selection remains unchanged. Depending on the trial budget, real-time CGM ± CKM is preferred, delivering more robust data collection and biofeedback [304, 305]. Longitudinal tracking should be emphasized to allow for correlations with long-term outcomes. Urine acetone strips (urinary ketones) and breath acetone analyzers are often poorly correlated with blood ketone levels and thus discouraged in research settings; however, they can be useful for outpatient self-tracking, when verified by gold-standard testing methods [204, 380, 381].
Preapproved food lists, meal templates, sample meal plans, and recipes to streamline macronutrient tracking. Patients and caregivers will be expected to keep food records, adhere to templates, or use diet tracking software, especially during the diet transition phase [327]. A photographic diary may help with logging and data sharing, with the added benefit of time-stamping the feeding schedule. In the future, image-based food recognition algorithms could reduce logging efforts [382, 383]. There may be circumstances, however, where short-term adherence to simplified lists of “allowed/excluded” foods could suffice, if GKI and biomarker targets are reached.
The healthcare staff should be prepared to answer general questions and help with diet implementation. Routine follow-up and troubleshooting sessions are recommended, particularly during the adaptation period (e.g., first appointment within 2 weeks to ensure biological endpoints are met). Compliance and motivation can be significantly improved when patients are held accountable via external monitoring, coaching, and remote care [384, 385]. In contrast, compliance may be compromised if disagreements exist between family members or external healthcare providers regarding the suitability of the treatment plan. Reaching consensus is encouraged, with examination of the scientific literature to resolve any questions regarding the rationale and expected outcomes of all proposed therapies, including SOC. General and disease-specific educational resources to implement long-term KD plans are available for both patients and clinicians [208, 311, 334, 386–390]. Given the wide access to low-carbohydrate recipe books for general audiences, it is important to reiterate that the suitability of the chosen plan should be determined by monitoring the lasting induction of the desired biological outcomes (e.g., sustained GKI or biomarker targets, such as insulin suppression), rather than any particular set of food recommendations, irrespective of the goal or medical condition they were originally designed for (e.g., weight loss, epilepsy, diabetes mellitus).
Appropriate psychological and emotional support, with individual or group counseling [391].
Key steps in the treatment timeline
All prospective participants should undergo a baseline evaluation before they are considered for KMT, including medical history, nutritional and anthropometric assessment, bloodwork, and anatomical/metabolic imaging; this is particularly relevant before pharmacological or systemic interventions, which may not be adequate for all patients. Psychological and neurocognitive health often suffers greatly after a GBM diagnosis, which can impact the ability of patients to follow treatments which require active participation [392]. Therefore, it is also important to assess if a proactive approach based on health ownership reduces morbidity and improves quality of life [393].
A chronological timeline is crucial for record-keeping and establishing associations between procedures and therapeutic outcomes or side effects. Figure 3 provides an overview of KMT for high-grade glioma and Table 3 summarizes key steps in the suggested clinical implementation of dietary and pharmacological KMT. The steps in this timeline are based on the “press-pulse” therapeutic strategy [132]. GKI-adjusted KD/KD-R and fasting are implemented as a metabolic “press” to restrict fermentable fuels, reduce inflammation, and normalize the tumor microenvironment, while drugs that simultaneously target glycolysis, glutaminolysis, and other cancer-associated pathways are defined as either “press” or “pulse” interventions, depending on pharmacodynamics and safety. Representative diet-drug combinations have been presented in case reports and pilot clinical trials, although most studies thus far emphasized feasibility and additivity with SOC, rather than integrating dietary and pharmacological KMT as a prerequisite, continuous, biomarker-driven “metabolic priming” baseline [103, 107, 109, 134, 279, 394–397].
Fig. 3.
Overview of KMT implementation in high-grade glioma research, including both dietary KMT (GKI-adjusted KD/KD-R and fasting, aimed at increasing chronic metabolic pressure on cancer cells while favoring OXPHOS metabolism in normal tissues), as well as pharmacological KMT (targeting of glycolysis and glutaminolysis in a press-pulse design, in addition to cancer-associated pathways to normalize the tumor microenvironment)
Table 3.
Recommended timeline for dietary and pharmacological KMT research in GBM
| Key steps in the clinical implementation of KMT | Overview |
|---|---|
| 1. Tumor diagnosis and shared decision-making | Brain tumor pathology is the gold standard for differential diagnosis. The preliminary diagnosis can be made via non-invasive techniques, as applicable (CT, MRI, PET; liquid biopsy can be informative in extra-neural tumors but has not been validated for GBM). If maximal surgical resection is feasible, histopathologic and molecular characterization can be performed after surgical debulking. Initial tissue biopsy in tumors suitable for later subtotal or complete resection is more valuable when it provides actionable therapeutic guidance; otherwise, final histopathological analysis can be obtained from surgical samples. Patients are encouraged to request detailed, written documentation regarding expected SOC outcomes (long-term survival and quality of life) to facilitate informed consent |
| 2. Blood panel analysis | Evaluation of basal health status will determine the best therapeutic approaches, as well as feasibility of KMT and chemoradiotherapy. It is advisable to formulate a well-defined schedule for tumor evaluation. In GBM, radiologic imaging is necessary due to lack of reliable blood-based biomarkers |
| 3. Metabolic stratification | Previously established bioenergetic phenotypes can be used as estimates to guide metabolic therapy. Glucose and glutamine are universally recognized as necessary for tumor growth, regardless of utilization pathway. In a research context, metabolite flux and substrate dependency of tumor samples can be assessed using in vitro/ex vivo bench top and in vivo metabolic imaging techniques. In the case of chemo/radiotherapy delay, clinical trials may require baseline and serial anatomic/metabolic imaging (e.g., every 3–6 weeks) to corroborate favorable responses |
| 4. Surgical debulking | The extent of surgical resection is one of the strongest prognostic factors in GBM. If presenting with life-threatening symptoms, surgery should be performed as soon as possible, ideally with intraoperative fluorescence mapping (e.g., 5-ALA or novel research-phase metabolic markers). In the absence of such symptoms, a short watchful waiting period with dietary KMT initiation (GKI-adjusted KD/fasting) could be considered prior to debulking, potentially facilitating better surgical delineation |
| 5. Initiate dietary KMT | KMT can be initiated before or after surgery. Self-reported dietary compliance and/or food records are not reliable sources of data in research settings. Instead, cumulative GKI ranges, or similar unbiased biological compliance markers, should be predefined as primary or surrogate study endpoints. Patients with good general health (as per clinical history, bloodwork, and stratification of disease) and sufficient body fat mass may initiate KMT with prolonged water-only fasting (≥ 3–5 days), which generally achieves GKI ≤ 2.0 during the fasting interval. Alternatively, it is possible to gradually transition to a GKI-adjusted KD/KD-R, which should be maintained as long as the tumor persists. Patients with sufficient body fat reserves may consider longer fasting periods (> 1–3 weeks), which are safe under medical supervision and will provide longer cumulative exposure to reduced GKI as well as autophagic effects |
| 6. Radiation therapy | For the clinical testing of combined dietary and pharmacological KMT, given the conflicting metabolic consequences of brain-directed radiation, a request to modify the timing of radiotherapy may be proposed by the investigators: late-stage adverse effects should be weighed against short-term anti-tumoral or synergistic benefits [11, 73, 75]. For IRB approval, radiotherapy could be conditionally delayed or used at low-dose regimens until signs of disease progression. During informed consent, patients should inquire about the absolute survival increase provided by radiotherapy along with the possible short- and long-term side effects. If the patient chooses a multimodal approach, dietary KMT may provide radiosensitizing potentiation, although some benefits may be blunted by radiation-induced destabilization of the tumor microenvironment and concomitant steroid administration. Untargeted radiation techniques should be avoided in all cases |
| 7. Drug treatments | Ideally, all drug treatments (especially metabolic inhibitors and chemotherapy) should be administered in a GKI ≤ 2.0 range. Corticosteroids should be used only when unavoidable, at the minimum effective dose, and frequently reassessed with the goal of de-escalation as soon as clinically feasible. After achieving a therapeutic GKI-adjusted baseline, glycolysis and glutaminolysis should be targeted simultaneously; various diet-drug combinations can be evaluated to this effect (e.g., targeting insulin signaling via repurposed drugs such as metformin or SGLT2 inhibitors, or direct metabolic inhibition using research-phase glucose and glutamine antagonists). Further stabilization of the tumor microenvironment or targeting of cancer-associated pathways, including immune-based therapies, can be initiated after the effective restriction of SLP metabolism while increasing whole-body adaptation to nutritional ketosis |
| 8. Physical activity | An individualized dose of physical activity that promotes long-term muscle maintenance and aerobic fitness is recommended. The type of exercise should be based on the patient’s training experience and current exercise capacity. Resistance training preserves muscle mass, increasing a major physiological glucose sink, further enhancing ketogenesis through decreased insulin secretion |
| 9. Stress management | Given that active participation and motivation is required for successful KMT implementation, patients should receive adequate psychological and emotional support to enhance quality of life, reduce non-compliance, and avoid stress-related therapy excursions |
| 10. Evaluation of outcomes and therapy adjustments | Radiologic imaging should be performed within 2–8 weeks of KMT initiation. Current guidelines recommend brain imaging every 2–4 months during the first 3 years from diagnosis [26]. More frequent reevaluation may be necessary in clinical trials of combined diet-drug KMT, especially if proposing changes to the SOC schedule. Treatment can be adjusted in the event of tumor progression. Repeated surgical debulking can be planned for slow growing tumors |
The suggested KMT framework is constructed in a modular fashion, with intrinsic flexibility during both routine clinical application and research design. It is not expected that all steps of the timeline, such as the testing of diverse diet-drug combinations, will be implemented by a single research institution. Understandably, the potential costs and resources will vary greatly depending on the number and complexity of the proposed interventions (e.g., from patient education and monitoring by a single dietitian, up to a multi-arm, multi-site platform clinical trial). As described in Fig. 4, the basic requirements of KMT can be adjusted to fit various clinical contexts. Even though adjustments to nutrition and over-the-counter supplementation are within the patient’s purview, the use of prescription medication involves the cooperation of a physician trained in metabolic oncology. Lack of familiarity, inertia of prescribing habits and fear of legal vulnerability can be reasons for not pursuing off-label use within standard practice [398–400]. Recently, educational resources and training programs have emerged to address these barriers [386, 401]. In research settings, any therapy beyond SOC must be presented in concordance with local deontological guidelines, with IRB-approval and informed consent. Outside research, dietary KMT (KD/KD-R and fasting) can be implemented freely by the patient, but active drug repurposing or compassionate use (as allowed by local regulations) requires a clear rationale and informed consent [402–404]. In the latter case, the primary goals should be safety, quality of life, and improved therapeutic outcomes, with emphasis on reporting the collected data to applicable regulating bodies (under certain compassionate use programs) as well as the broader scientific community [403, 405–407].
Fig. 4.
Prerequisites and potential experimental complexity of KMT. Any interested patient can initiate dietary KMT, ideally under the supervision of a trained dietitian. The resources and staff required for pharmacological KMT are dependent on the number of interventions and clinical settings (for example, a GKI-adjusted KD in addition to SOC, or research therapies such as glutaminolysis inhibition)
Tumor diagnosis and shared decision-making
It is preferable, if possible, to make the initial tentative diagnosis using non-invasive neuroimaging techniques (CT, MRI, PET) to avoid the risk of exacerbating tumor growth or iatrogenic cell dissemination through inflammatory oncotaxis [408–411]. Liquid biopsy can be informative for diagnosis and disease monitoring in extra-neural cancers but has not been sufficiently validated in GBM [412]. Initial tissue biopsy prior to surgery would be more applicable to cases where it can provide actionable information (that is, when histological, molecular, or metabolic characterization dictates subsequent therapies, beyond simple staging). In tumors suitable for maximal safe resection, histopathological and molecular analysis after maximal debulking would be preferred to fine-needle biopsy, serving as the gold-standard for differential diagnosis [413, 414]. Patients should be informed about the risk/benefit of contrast agents such as gadolinium and iodinated contrast media [415].
There has been increased emphasis on early cancer detection, including direct-to-consumer liquid biopsy and diagnostic imaging, but lacking consensus regarding dubious findings [416–418]. When facing benign or slow-growing tumors with conservative management [419, 420], patients may decide to request information about dietary KMT during active surveillance. It is important to mention that, despite mechanistic rationale and potential normalization of risk factors such as inflammation and metabolic syndrome [421–423], we are not aware of any longitudinal clinical trial exploring whether KDs and/or cyclical fasting could reduce the risk of high-grade transformation. Primary and tertiary cancer prevention have been longstanding targets for dietary modification [424, 425]. However, we recognize that proposing such interventional trials may be challenging due to lengthy follow-up periods, and thus encourage retrospective studies on patients that have chosen to follow long-term ketogenic lifestyles on their own [203].
After the most probable diagnosis has been determined, patients should be offered a follow-up consultation to explore treatment options and analyze expected outcomes. This step is applicable to all types of cancer. In ideal circumstances, an empathetic conversation should take place to fully inform the patient, as part of the shared decision-making process. Unfortunately, despite recent progress in patient-centered care, cancer treatments are still frequently delivered in a paternalistic framework, with poor communication about expected outcomes and reasonable alternative treatments [330, 426, 427]. To aid with informed consent, patients are encouraged to request written documentation about the estimated efficacy of conventional therapies, given that their high degree of protocolization allows for a relatively accurate calibration of expectations [428]. Ambiguous or ill-defined verbal descriptions of expected SOC outcomes are unacceptable. In our view, informed consent is only possible when the necessary time has been devoted to defining the long-term survival rates (not PFS or mOS) from applicable contemporary clinical trials that serve as the basis for SOC guidelines. This poses ethical considerations about the duty to inform and the “right not to know,” but also facilitates reaching consensus and allows the patient to judge the need for emerging/research therapies, off-label, or compassionate use, at different levels of the evidence-based pyramid [403, 404, 429, 430].
Blood panel analysis
It is important to analyze all relevant blood elements to establish a comparative baseline before KMT and surgery. As described in Table 1, suggested laboratory tests and monitoring frequency should be adjusted to the demands of the proposed interventions (e.g., diet-drug combinations). Biomarkers of general health, including hemogram, electrolytes, hepatic and renal function, inflammation, and vitamin status, should be within or near the normal range before initiating therapeutic measures that carry a risk of adverse effects. Key dietary nutrients to monitor on a carbohydrate-restricted diet include carnitine, thiamine, folate, pantothenic acid, calcium, phosphorus, iron, vitamin D, and trace minerals [431]. KDs, partial (“fasting-mimicking”), and/or water-only fasting can improve preexisting lifestyle-related blood panel abnormalities, particularly hyperinsulinemia, insulin resistance, and chronic inflammation [221, 368, 432].
GBM is generally not amenable for estimation of tumor burden using blood-based biomarkers due to the blood–brain barrier, which is only partially disrupted in most cases [433]. A small number of circulating proteins, extracellular vesicles, tumor cells, and DNA/RNA fragments in blood and CSF have been proposed for diagnosis and follow-up [412, 434–436]. However, until these methods are fully validated, imaging techniques remain the gold-standard for response and recurrence evaluation [437, 438]. In extra-neural cancers, a combination of traditional protein-based tumor markers, circulating tumor cells, DNA/RNA fragments, and imaging modalities (including ultrasound and infrared thermography) can be leveraged to track disease progression [439–441].
A transient elevation of circulating tumor markers during systemic therapy (a phenomenon known as “spiking”) could be misinterpreted as progressive disease [442–447]. It is therefore essential to correlate tumor markers with other clinical and radiological parameters, especially if the patient opts for active monitoring with standalone dietary and/or pharmacological KMT. Recently, liquid biopsy services have come to market (e.g., circulating tumor cells, including glial cells), which are sometimes requested independently without informing the treating physician; however, due to their novelty, therapeutic decisions should be made in conjunction with radiologic responses, clinical criteria, and additional laboratory testing [448, 449].
Metabolic stratification
During routine clinical practice, metabolic targeting can be informed by previous molecular and mechanistic characterizations of the tumor subtype: high uptake of glucose and glutamine is considered a common feature of high-grade gliomas, correlating with cell density and aggressiveness, especially in grade 3 and 4 tumors [450–452].
We recommend standardized metabolic imaging of glucose uptake (18F-FDG PET coupled with anatomic imaging) for all GBM patients, which may offer improved staging and delineation of surgical margins [453]. A fasting period (≥ 12 h) is advisable prior to the scan to facilitate 18-FDG transport into the tumor and lower insulin-mediated glucose uptake in the surrounding tissue [454, 455]. It is important to consider glycemia and ketonemia for standardized uptake value (SUV) normalization in patients that have already initiated a KD, as both can influence 18F-FDG uptake in normal brain [456, 457]. IRB approval of clinical trials proposing radiotherapy delay may necessitate a combination of anatomic and/or metabolic imaging at baseline and then sequentially every 3–6 weeks to corroborate pre-specified outcomes (e.g., stable disease). Other non-metabolizable glucose analogs and imaging modalities are being developed to circumvent the limitations of repeated PET radiation exposure [458].
The specific metabolic analysis of each patient’s tumor tissue is more relevant to research settings. Given the ongoing debate regarding the absolute degree of intratumoral metabolic heterogeneity, diagnostic tools have been developed to gain a more accurate picture of primary metabolic dependencies [58, 459, 460]. These include bench-top assays for the mapping of glycolytic, glutaminolytic and oxidative pathways, oxygen consumption and extracellular acidification rates (OCR/ECAR), mtDNA sequencing, ultrastructural characterization, novel PET tracers (e.g., glutamine, ketone bodies, oxygen sensors, amino acid metabolism, lipid synthesis, apoptosis), MRS/MRI imaging (e.g., glucose, glutamine, ATP synthesis, TCA cycle, ketone bodies), and NMR and LC/GC–MS metabolomics [61, 461, 462]. Metabolic assays can be performed non-invasively or in fresh tumor preparations and patient-derived organoids [463]. The translational value of metabolic stratification and the associated phenotypes has been further discussed in Additional File 1: Appendix 2.
The fundamental bioenergetic hierarchy of normal cells (OXPHOS > SLP) and cancer cells (OXPHOS < SLP) should not be forgotten when developing mitochondria-targeting drugs, such as electron transport chain inhibitors [63, 464–468]. Recent clinical efforts to target OXPHOS have been halted due to severe and arguably predictable toxicity, suggesting a very narrow therapeutic index [469]. In our view, low-dose mitochondrial targeting would be mechanistically sound only after the effective inhibition of SLP flux, as cancer cells have already adapted to fermentation as a compensatory and/or biosynthetic mechanism. From this perspective, cancer cells lacking SLP dependency may no longer display the primary hallmark of cancer (i.e., dysregulated cell growth). Better mechanistic insights into how restoring OXPHOS sufficiency regulates cell division could be gained from nuclear-cytoplasm transfer experiments and mitochondrial transplantation, rather than OXPHOS inhibition [470–472].
Surgical debulking
Surgical debulking should be performed promptly after diagnosis, while still prioritizing careful surgical planning to ensure maximal resection. In asymptomatic or slow-growing tumors, active surveillance provides an opportunity to implement dietary KMT as a neuroprotective intervention prior to surgery, which could reduce angiogenesis, inflammation, and edema [126, 221, 473], and thus potentially facilitate better surgical delineation when coupled with metabolic imaging and intraoperative markers [456, 474, 475]. A short active surveillance interval to allow for KMT initiation in suitable non-critical cases has not been explored in earlier GBM trials (Additional File 3: Table S1), likely due to IRB approval policies [112, 476]. The extent of surgical resection is one of the most important predictive factors for GBM survival [477]. To ensure complete resection of all contrast-enhancing areas, intraoperative fluorescence markers such as 5-aminolevulinic acid (5-ALA) or novel pH-sensitive agents can be considered for eligible patients [478–480]. Most recurrences are experienced locally or in proximity to the resection cavity of the first surgery [481, 482]. Patients initiating KMT after recurrence should evaluate the possibility of repeated surgical debulking, unless presenting with diffuse, multifocal, or deep infiltrative tumors; cytoreductive surgery for well-defined recurrent lesions extends survival and may facilitate salvage therapies [350, 483, 484].
Initiate dietary KMT
GKI-adjusted KD/KD-R and fasting can be administered in a neoadjuvant phase (in the peri-diagnostic period), uninterrupted, or resumed within 24–72 h of surgical debulking, depending on the patient’s condition [485, 486]. Mechanistically, these strategies have been studied to improve wound healing and reduce inflammatory markers [158, 487–492], alleviate pain [493, 494], and stimulate anti-tumor immunity [170, 495], thus inducing a favorable physiological environment for post-surgical recovery. Long-term adherence should be stratified according to cumulative biomarker ranges, such as real-time tracking of GKI, which can be predefined as primary or surrogate endpoints.
If KMT has been initiated prior to surgery during a watchful waiting period, patients with adequate body weight and good functional status may accelerate ketogenic adaptation through water-only fasting or fasting-mimicking diets (FMDs) [145, 368]. However, adjusting to an isocaloric KD or KD-R for 1 to 3 weeks before undergoing zero-calorie or partial fasting enables a more gradual metabolic transition. A well-formulated KD should proactively avoid common side effects, such as electrolyte imbalances or undesired LBM loss, to minimize negative impacts on quality of life. Initial weight loss during the transition to nutritional ketosis and fasting is mostly due to increased diuresis (water loss) and fat loss, not LBM [102, 496]. Adequate hydration and electrolyte supplementation (e.g., sodium, chloride, magnesium, and potassium) is recommended for both KDs and fasting [497, 498]. In clinical trials, successful implementation of KDs is often accompanied by a reduction or discontinuation of medication, particularly for chronic diseases associated with insulin resistance, such as type 2 diabetes, dyslipidemia, NAFLD, and hypertension [384, 499].
Based on changes in the metabolome, water-only fasting for periods over 72 h is likely required to fully transition into the fasted state in humans [371, 500, 501], although more research is needed to determine the appropriate timing for antineoplastic effects [502, 503]. Medically supervised water-only fasting for over 60 days has been shown safe and effective in obesity management [375], and fasting-mimicking protocols for up to 21 days have been implemented in large cohorts with normal baseline weight [369]. While sufficient body fat stores could allow for therapeutic fasting beyond 1–3 weeks in select patients, feasibility studies focused mostly on 5–7 days in GBM [145], as well as short-term and fasting-mimicking protocols in other malignancies [368, 504]. After the fast, the attending dietitian should instruct a slow and methodical refeeding (while still adhering to GKI targets) to prevent overfeeding, electrolyte imbalance, or reactive hyperglycemia [145, 311, 505].
If fasting is contraindicated due to risk of cachexia or preexisting health conditions, an isocaloric GKI-adjusted KD can be initiated instead [375, 506]. It is important to review sodium restriction, as low sodium diets have been shown to deplete magnesium and increase insulin resistance, thus promoting hyperglycemia [507, 508]. Similar to water-only fasting, a strict KD is expected to induce mild diuretic effects and improve glycemic control; accordingly, it is recommended to reevaluate existing prescriptions (e.g., antihypertensives, antidiabetics) and replenish electrolyte levels, especially prior to acute dietary changes. Asymptomatic hyperuricemia may also develop in a small subset of patients and should be monitored, resolving spontaneously in most cases [509–511].
The initial weight loss from adipose tissue is expected to continue slowly and controllably during KD-R. It is important to remember that gradual fat utilization associated with KD-R and fasting is therapeutic, whereas LBM loss associated with cachexia is pathogenic [236, 512]. Isocaloric feeding to maintain muscle mass should be favored over chronic calorie restriction in individuals at borderline low weight (e.g., BMI < 18, or as determined by the dietitian) [513]. Participants that cannot comply with the diet (e.g., impaired swallowing function) could receive nutrients in optimal balance via enteral feeding (as demonstrated in pediatric and adult patients with epilepsy), or, if enteral feeds are not possible, via parenteral ketogenic nutrition [256, 514–516].
Radiation therapy
In addition to neurosurgery, radiotherapy of growing sophistication has remained the cornerstone of GBM therapy [6, 517, 518]. The current SOC recommends postoperative radiation with target volume delineation, for a total dose of 60 Gy in 30 fractions [26]; temozolomide alone is typically only considered in elderly patients, especially if the tumor is MGMT-methylated [519, 520]. Given the conflicting effects on cancer metabolism described below, in the specific context of future research evaluating diet-drug KMT as the primary treatment modality, a proposal to modify the timing of radiotherapy could be requested by the investigators if biologically justified. Despite short-term cytotoxicity to cancer cells, ionizing radiation induces metabolic reprogramming in the tumor niche, negatively influences the phenotype of recurrence, and triggers secondary inflammatory responses in the peritumoral tissue [521–526], while also damaging normal brain parenchyma and blood vessels [527–529]. Even targeted modalities can cause delayed adverse effects that are seldom factored in the risk/benefit analysis given the poor overall prognosis [78, 530, 531]. Concerns have been raised about the potential off-target brain toxicity caused by conventional radiation protocols [532–536]. Consequently, it will be important to design clinical trials comparing the potential synergistic benefit of radiation-induced cytotoxicity, chemosensitization, and immune modulation, with the residual adverse effects on surviving tumor cells and their microenvironment [196, 537, 538]. To meet IRB requirements, radiotherapy could be conditionally and sequentially delayed for a clinically acceptable period based on interim response analysis or applied at low-dose regimens as a synergistic potentiation strategy (e.g., NCT01466686) [537–540].
In other types of cancer, KDs and fasting have been proposed as feasible and potentially effective radiotherapy adjuncts, acting as radiosensitizers while mitigating adverse effects [541–543]. It is worth reiterating that, in contemporary medical ethics, therapeutic decision-making is ultimately driven by the informed patient [330, 544–546]. Accordingly, brain-sparing modalities of radiation may be offered as auxiliary or salvage therapies if other approaches have failed or if they are actively requested by the participant [532, 533, 547].
Drug treatments
It is our view that any drug therapy will be most effective once the patient reaches a stable therapeutic GKI zone (for example, 2.0 or below, ideally 1.0 or below, with special attention to absolute glucose levels and insulin signaling, which should be at their physiological minimum). A combination of nutritionally balanced KDs, calorie restriction, and fasting will promote therapeutic ketosis, after which drug therapies can be initiated.
As corticosteroids decrease immune function and increase glycemia, independently associated with poor GBM survival, they should be used only when unavoidable, at the lowest dosage, for the shortest possible time [80, 81, 548]. Alternatives allowing for dose reduction include combinatory regimens of non-steroidal medications such as COX-2 inhibitors, fingolimod, acetazolamide, angiotensin II receptor antagonists, ACE inhibitors, or glyburide (which impacts insulin signaling) [549–552]; nutraceuticals such as boswellic acids [553]; as well as novel agents such as corticorelin acetate, vaptans and vascular endothelial growth factor (VEGF), or vascular endothelial protein tyrosine phosphatase (VE-PTP) antagonists [554]. The rationale for dexamethasone should be reevaluated upon edema reduction, rather than prescribed as an indefinite treatment [555]. Patients and caregivers are encouraged to inquire periodically about the clinical justification of the ongoing corticosteroid posology.
It is important to recognize that intensive SOC therapy (particularly, high-dose corticosteroids, and radiation) could lead to erratic glycemia or low ketonemia despite strict diet adherence [556, 557]. Based on previous reports, more intensive dietary changes, such as cyclical water-only fasting (≥ 3–5 days) and paleolithic KDs (≈ 0 g carbohydrates/day) with a narrow feeding window (e.g., intermittent fasting with one meal per day), may be required to reach a stable GKI during concomitant steroid and radiation therapy [108, 145, 196, 218, 558]. Such personalized dietary adjustments are compatible with trial planning and can be implemented at the discretion of the attending physician or dietitian (specific biomarker targets can be pursued as primary endpoints, but it may be impractical to predefine all possible methods to achieve them).
Metabolic imaging and previous characterizations of the tumor subtype can suggest a preliminary description of the primary metabolic dependencies [451, 559, 560]. After transitioning to a sustained GKI-adjusted KD/KD-R, pharmacological targeting of glycolysis and glutaminolysis should be implemented gradually, ensuring any off-target SLP inhibition in normal cells is buffered via ketone body metabolism. Baseline ketogenic adaptation is a “sine qua non” condition for the safe targeting of SLP fuels. This is not required for modulating other cancer-associated pathways, such as redox balance, immune response, or autophagy, but is recommended for its synergistic anti-proliferative, anti-inflammatory, and anti-angiogenic effects [68, 126, 166–170, 561].
Additional File 5: Table S2 and Additional File 6: Table S3 summarize repurposed drugs and novel research-phase chemicals for the targeting of SLP and tumor-associated pathways. While we provide general recommendations based on the press-pulse therapeutic principle, a multitude of clinically approved drugs have been proposed as candidates for GBM therapy [539, 562, 563]. Combinatory approaches, rather than single-pathway targeting, may be necessary for optimal results [394]. However, in efforts to isolate confounding variables and mitigate financial risk, only a small number of clinical trials have tested multi-drug additions to SOC, seldom with dietary metabolic priming [564–566]. The intellectual property landscape and lack of financial incentives to explore non-patentable combinatorial approaches is a significant challenge for the validation of promising preclinical observations [567, 568]. Improving drug bioavailability, blood–brain barrier transport and local delivery (e.g., intracranial drug reservoirs) are also critical factors [539, 569]. Understandably, even though off-label prescription is permissible in most countries on a case-by-case basis, the general use of any non-standard therapy will need be validated and incorporated into SOC through extensive clinical testing [567, 570]. Regardless of the clinical context, monitoring of adverse events and dose modification schedules must be in place for any tested pharmacological agent.
The timeline of drug administration is outlined in Fig. 5 and can be structured as follows:
Dietary KMT (GKI-adjusted KD/KD-R and fasting) reduces the glycolytic dependency of normal tissues and stimulates compensatory ketone body metabolism.
If the tumor is shown to be glycolytic (i.e., high 18F-FDG uptake), consider additional systemic targeting of substrate, such as renal glucose reabsorption or gluconeogenesis inhibitors (e.g., SGLT2 inhibitors, metformin). Direct inhibition of glycolysis should be administered only after reaching sustained therapeutic ketosis to improve safety and tolerability (“keto-adaptation”). Therapeutic targets in early clinical trials include hexokinase (2-Deoxy-D-glucose, lonidamine, 3-bromopyruvate), phosphofructokinase (3PO, ACT-PFK-158), and pyruvate kinase (gossypol/AT-101, TLN-232) [571, 572].
We propose that concurrent targeting of glutaminolysis is essential to avoid therapy resistance. At this time, one of the anti-glutaminolytic drugs considered to work best as part of KMT in preclinical models is the pan-glutaminase inhibitor 6-diazo-5-oxo-L-norleucine (DON) [143]. Any prospective compound that can safely and effectively target glutamine availability and/or utilization may elicit comparable effects, such as DON prodrugs or novel glutaminase inhibitors [573, 574]. Additionally, tumor-specific delivery of DON is being investigated as an enhancer of anti-tumor immunity [575].
After cancer cells have been rendered vulnerable by press-pulse metabolic pressure, cancer-associated pathways and the tumor microenvironment can be targeted via synergistic drug combinations.
Fig. 5.
Overview of potential drug treatments as part of KMT research. Strategies are divided into glucose targeting (red), glutamine targeting (green), and tumor microenvironment stabilization (blue). Safe administration of metabolic inhibitors will require physiological adaptation to a GKI-adjusted KD/KD-R, which can be accelerated by water-only fasting. Then, glycolysis targeting can be considered to further improve GKI and slow tumor progression (e.g., antidiabetic agents such as metformin or SGLT2 inhibitors, as well as research-phase glycolytic inhibitors). Glutaminolysis should be targeted at the same time (e.g., sodium phenylbutyrate, DON, or novel glutamine inhibitors). Finally, normalization of the tumor microenvironment can be explored in a modular fashion; for example, cell proliferation (mebendazole), inflammation (NSAIDs), hypoxia (HBOT), redox balance (DCA, intravenous vitamin C), immunotherapy, or combinatory approaches (e.g., CUSP9)
Pharmacological targeting of glycolysis
Dietary KMT shifts whole-body physiology to an evolutionarily conserved metabolic state of nutrient scarcity that is inhospitable to tumor growth, but facing advanced disease will require multimodal and combinatorial strategies [103, 105, 162, 576, 577]. Further improvements to GKI (substrate availability) and direct targeting of glycolysis can be implemented after ketogenic adaptation via drug repurposing or investigational compounds, depending on the clinical context (Additional File 5: Table S2).
Metformin at standard antidiabetic dosing improves glycemic control via mild liver gluconeogenesis inhibition and increased insulin sensitivity [578]. At realistically achievable in vivo concentrations, direct cytotoxicity via complex I inhibition is unlikely, but positive regulation of the tumor immune microenvironment has been noted [579]. Cancer therapy with metformin is being evaluated in an expanding number of clinical trials due to its good safety profile, mostly as a synergistic addition to SOC [580, 581]. Berberine, an over-the-counter alternative, exhibits similar effects on glycemic control [582]. Other biguanides may be more efficacious in lowering gluconeogenesis but also carry a higher risk of lactic acidosis, which restricts their use to research [583, 584]. At this time, we do not recommend OXPHOS inhibitors with higher potency, such as IACS-010759, due to unacceptable off-target toxicity [585, 586].
SGLT2 inhibitors (e.g., dapagliflozin, empagliflozin, canagliflozin) can be considered to further decrease GKI and insulin signaling [587–589]; dose adjustments and monitoring of ketoacidosis is required during KDs and fasting, especially in patients with a history of type 2 diabetes or prone to ketoacidosis [590–593]. SGLT2 inhibitors experienced a recent resurgence as attractive combinations with PI3K inhibitors via suppression of the insulin feedback loop; however, greater synergy was observed with the KD in certain preclinical models, even if future clinical adoption would be more demanding [68, 594]. Therefore, a dual combination of low-dose SGLT2 inhibitors and KDs warrants further research. In fact, the KD is now being rebranded as “insulin suppressing” and trials with PI3K inhibitors are underway [595]. Renewed interest in the often-overlooked intersection between diet and cancer may lead to a more universal appreciation of “how common clinical practices such as intravenous glucose administration, glucocorticoid use, or providing patients with glucose-laden nutritional supplements may impact therapeutic responses” [68].
Other antidiabetic drugs that do not act through endogenous insulin production, such as thiazolidinediones (“glitazones”), dipeptidyl peptidase-4 (DPP-4) inhibitors (“gliptins”), glucagon-like peptide 1 (GLP-1) agonists or bromocriptine, could be tools to achieve and sustain specific GKI ranges. Exogenous insulin causes surges in growth signaling that may accelerate tumor progression and chemoresistance despite transient glucose disposal, with a controversial role in cancer therapy [596]. The consequences of drug-induced insulin secretion (e.g., sulfonylureas or meglitinides) in the context of therapeutic ketosis, where insulin should be physiologically low, are not fully elucidated. MCTs and exogenous ketones can rapidly boost ketone levels and prevent hypoglycemic events during drug therapies, KDs or fasting, as well as mitigating central nervous system (CNS) oxygen toxicity in adjuvant hyperbaric medicine [277, 597].
Beyond systemic glucose availability, several research-phase chemicals that target the glycolytic pathway at the substrate, transport, or enzyme level have been explored in clinical trials, such as the classical competitive inhibitor 2-Deoxy-D-glucose [598]. However, it is important to note that systemic inhibition of glycolysis without preemptive priming to alternative energy pathways could lead to dose-limiting toxicities [66]. In our view, effective and sustained ketogenic adaptation at the biological level should be a prerequisite for the clinical testing of glycolytic inhibitors. Thus far, direct targeting of glycolysis has been relatively limited due to safety concerns [70], which could be partially offset by adjuvant dietary KMT and dose optimization, given that therapeutic ketosis also reduces glycolytic flux and increases tissue competition. Therapy resistance or prior metabolic stratification could provide a rationale for intensifying glycolysis targeting beyond substrate availability, as suggested by the principles of precision medicine [599].
Pharmacological targeting of glutaminolysis
In the context of monotherapy inhibition of glycolysis, cancer cell viability could be rescued by the other primary fermentable fuel, glutamine [33]. Even though intra-tumoral heterogeneity and clonal selection creates a potentially unlimited mutational and epigenetic landscape [600, 601], the number of metabolic substrates able to sustain proliferation is unlikely to be unlimited in light of the universal mitochondrial defects and bioenergetic/anabolic dependencies found in GBM [40, 43, 45, 115]. Currently, novel glutamine antagonists and other metabolic inhibitors such as DON prodrugs or CB-839 are being tested as monotherapy additions to SOC (Additional File 5: Table S2). Single-pathway inhibition may not be optimal due to functional redundancy: unless proven otherwise through metabolic stratification, we propose concurrent initial targeting of glucose and glutamine-driven SLP after ketogenic adaptation, given that they are the most robustly consumed for energy, biomass, and redox homeostasis [31].
DON is the prototypical drug for broad-acting glutaminolysis inhibition, targeting multiple isoforms of glutaminases and glutamine-utilizing enzymes [602]. DON is currently not FDA-approved, but has an extensive history of clinical testing, a relatively good safety profile at moderate doses, and could be revisited as a research therapy in its original or prodrug forms [603–605]. Continuous daily parenteral administration produced dose-limiting side effects in previous clinical trials (most notably, oral mucositis, nausea, vomiting, and myelosuppression; premedication with antiemetics can be implemented prophylactically) [606–608]. Instead, congruent with the short half-life (1.2 h), low-dose intermittent administration would be preferable, as suggested by initial dose-escalation studies. Thus, future research may consider parenteral or oral administration in the 0.2 to 1.1 mg/kg/day range, adjusted to individual tolerance [609, 610]. Dosing frequency (continuous or intermittent) will be contingent upon route of administration, anti-tumor response, and safety. In more recent phase IIa studies, DON has been administered at 140 mg/m2 (twice weekly) in 15-min infusions, combined with plasma glutamine depletion [611].
While DON prodrugs with improved oral bioavailability are being developed, the original compound demonstrated biological activity at oral doses up to 1.1 mg/kg/day for a duration of two or more weeks [603, 612]. DON has been administered orally as a single daily dose (without resting periods), continuous split doses every 4–6 h (with a higher incidence of side effects, such as oral mucositis), or as single or split doses given intermittently every 2–4 days (lowest incidence of side effects) [609]. Preclinical evidence from our group suggests that the KD-R may increase DON concentrations across the blood–brain barrier and reduce dosing requirements when administered on a per-need basis [143]. Based on previous clinical testing, the recommended starting point would be a lower daily dose taken with a fatty meal vehicle in a single (e.g., 0.4 mg/kg q24h) or split schedule (e.g., 0.1 mg/kg q6h), with a 1–3-day resting period upon side effects, increasing to 1.1 mg/kg (or higher) based on tolerance and pharmacokinetics. Single 1.2–2.5 mg/kg oral doses were necessary to reach serum peak concentrations comparable to 0.6–1.2 mg/kg intravenous infusion; consequently, rather than oral administration, subcutaneous delivery starting in the 0.2 to 0.4 mg/kg range (twice or thrice weekly) may be preferable for improved bioavailability and convenience in outpatient care [609, 613]. Ideally, DON would be administered after confirming stable therapeutic ketosis as a synergistic potentiation strategy. It has been suggested that supplementing DON with adenine (400 mg/day) or 4-amino-5-imidazole carboxamide (800 mg/day), gastric pH-buffering (due to DON acid-labile properties), and hypoxanthine and increased fiber intake, may reduce off-target damage to the oral and intestinal mucosa; nevertheless, the mechanisms underlying these protective effects and their relevance on therapeutic efficacy will need to be confirmed in future studies [609, 614]. The immunomodulatory effects of DON should also be considered in the context of checkpoint inhibition, neoantigen vaccines, and adoptive cell therapy [615, 616].
Sodium phenylbutyrate is a clinically approved orphan drug for urea cycle disorders and neurodegenerative diseases, with potential anti-tumor effects as a single agent or coadjuvant with glutamine antagonism [617, 618]. Phenylbutyrate rapidly metabolizes to phenylacetate, conjugated with phenylacetyl-CoA and glutamine, acting as an ammonia scavenger and inducing durable plasma glutamine depletion [619]. It is also being investigated as a histone deacetylase (HDAC) inhibitor [620]. Clinical trials in solid tumors noted a sustained dose-dependent reduction in plasma glutamine with oral doses between 180 and 360 mg/kg/day, up to a maximally tolerated dose of 27 g/day [617, 621–623]. Phenylbutyrate decreases systemic availability of glutamine, resulting in substrate competition; thus, similar to PEG-glutaminase, phenylbutyrate-induced glutamine depletion may be explored to reduce dosing requirements of DON or other enzyme-level inhibitors of glutaminolysis [624]. Interestingly, the administration of phenylacetate was feasible after prolonged fasting, accompanied by counterregulatory hormonal responses to maintain fuel homeostasis [625].
L-asparaginase, a first-line treatment for a variety of lymphoproliferative disorders, induces acute extracellular glutamine depletion through conversion to glutamate, a mechanism hypothesized to play a significant role in its antineoplastic benefits [626, 627]. L-asparaginase requires parenteral administration and is currently available in three formulations (including generic drugs) [628]. Clinical trials in solid malignancies have focused primarily on single addition to chemotherapy in pancreatic cancer, yielding only marginal improvements in survival [629]. Consequently, it has been proposed that combinations with specific glutaminolysis inhibitors such as DON may further improve therapeutic efficacy [630–632].
Other research-phase glutamine inhibitors include the aforementioned DON prodrugs (e.g., JHU-083 and DRP-104, which contain the same active compound but aim to improve bioavailability and pharmacodynamics; Azo-DON, which is selectively reduced to DON by azo-reductases in hypoxic environments; as well as azotomycin, a tripeptide diazo analog) [605, 613, 617, 633], CB-839 (telaglenastat), IPN60090, BPTES, and compound 968 (glutaminase inhibitors) [574, 634–636], V-9302 (glutamine transport inhibitor) [637], azaserine and acivicin (glutamine mimics) [638], and caudatan A [639], physapubescin K [640], and aspulvinone O [641]. Blood–brain barrier permeability as well as isoform specificity are limiting factors, given that targeting all glutaminases (rather than specific isoforms) may be preferable to avoid therapy resistance. Telaglenastat is an investigational, first-in-clinic, small molecule oral selective inhibitor of GLS1, which has reached up to phase II clinical trials in advanced solid and hematological malignancies, including IDH-mutant astrocytoma [157, 634, 642, 643]. Most active trials are now focusing on combinations with targeted therapies and immunotherapies, but we hypothesize that glycolytic compensation may also play a role in the mixed efficacy reported so far [644, 645]. Likewise, the orphan drug CPI-613 is a lipoic acid analog that targets alpha-ketoglutarate dehydrogenase (α-KGDH), inhibiting both mitochondrial SLP and TCA cycle flux, with a relatively good safety profile but disappointing performance in metastatic pancreatic cancer [646–648]. Despite failure as a single agent, we have observed a promising synergistic interaction when CPI-613 was combined with the KD-R in a pediatric glioma model [649].
Repurposed drugs with potential inhibitory effects on the glutaminolytic pathway include aminooxyacetate, apomorphine, tamoxifen/raloxifene, sulfasalazine, and ceftriaxone [36, 650–652]. Over-the-counter nutraceuticals with direct or indirect effects include EGCG [653], xanthohumol and hesperidin [654], ursolic acid [655, 656], caffeic acid [657], curcumin [658], apigenin [659], berberine [660], and other compounds with only preliminary mechanistic evidence [661]. Achieving effective inhibition of glutamine metabolism through supplementation may be difficult, unless standardized for equivalent biological activity. Patients are therefore encouraged to inquire about ongoing clinical trials or compassionate use of glutaminolysis inhibitors (such as DON, novel DON prodrugs, or CB-839). When enrolling into clinical trials, participants should be offered flexibility to implement dietary KMT with additional glycolysis targeting, given that monotherapy inhibition has only produced modest clinical benefits thus far [71].
Pharmacological targeting of the tumor microenvironment and cancer-associated pathways
The tumor microenvironment has a profound impact on therapeutic outcomes and is influenced by factors such as hypoxia [662], redox balance [663], immune function [664], inflammation [665], angiogenesis [666], autophagy [667], epigenetic signaling [668], radiation-induced senescence [669], the gut-brain signaling axis [670], tumor-synaptic networks [671], and concomitant infections (e.g., GBM exhibits a high detection rate of cytomegalovirus, which can contribute to increased oncogenic signaling, and has been clinically targeted using antivirals such as valganciclovir or adoptive cell therapy) [672–674]. The patient’s internal “macroenvironment,” that is, whole-body physiology and its exposome, also plays an undeniable but often forgotten role, especially if envisioning cancer as a competing “ecological” process between normal and malignant cells [675–677]. For example, insulin resistance and the accompanying hyperinsulinemia have been correlated with poor prognosis and can substantially reduce the efficacy of certain therapeutic approaches, such as inhibition of the insulin/PI3K axis [678–680]. GKI-adjusted KDs and fasting promote a wide-ranging normalization of the patient’s physiological macroenvironment, as well as the local tumor microenvironment, at all the levels described above [129].
Beyond metabolism, several targeted therapies based on mutational heterogeneity have been evaluated in clinical trials with arguably underwhelming results; these include growth and signaling pathways with known alterations in GBM, as well as multi-kinase inhibitors and immunotherapies [9, 681]. A lack of multi-targeted approaches has been highlighted as one of the possible reasons for this failure [682]. We hypothesize that classical antineoplastics and targeted efforts would be enhanced if applied on a baseline of dietary KMT with effective SLP targeting [102, 170, 221, 683]. For example, tumor neoantigen heterogeneity could be reduced by clonal selection through metabolic pressure, potentially improving immune recognition [684]. Early trials of checkpoint inhibitors in GBM failed to show efficacy due to the relatively immunoprivileged nature of the CNS [685, 686]. Treatment strategies aiming to overcome this site-specific limitation are underway, such as neoantigen-derived peptide and dendritic cell vaccines with coadjuvant immunostimulation [23, 687, 688]. The immunomodulatory effects of dietary and pharmacological KMT could promote and maintain a tumor-suppressive phenotype [495, 604, 689–691]. It should be noted that targeted therapies and KMT are generally compatible, with further studies needed to uncover synergistic opportunities [169, 170, 683, 692–694]. However, since most targeted therapies are only available in research settings, it is also worth exploring off-label indications with putative anti-cancer effects that are more easily accessible during routine clinical practice.
Additional File 6: Table S3 summarizes clinically approved drugs and strategies that have been proposed to modulate the GBM microenvironment. We refer to additional reviews discussing novel compounds and off-label indications with preclinical evidence that may hold promise but require further clinical testing [695–697]. It is important to note that this list is based on preexisting clinical use (“drug repurposing”) and may not involve the most potent or selective compounds in their respective category; rather, the intent is to address health disparities and lower the financial burden of cancer treatments, promoting a democratization of cancer care and off-patent drug development through publicly funded research [406, 698]. Additional File 1: Appendix 3 provides further detail on an illustrative selection of repurposed drugs that have initiated pilot safety and feasibility studies in GBM.
From an experimental perspective, combining multiple therapies will make it difficult to assign causality. It is also possible that certain interventions will increase the risk of toxicity or adverse interactions without a meaningful therapeutic benefit. Successful examples of the feasibility of multi-drug protocols can be found in the CUSP9 [394], CLOVA [699], MEMMAT [700], COMBAT [701], gMDACT [702], renin-angiotensin modulators [703], and COAST (NCT05036226) clinical trials. Cancer metabolism was not the primary target in the aforementioned proof-of-concept studies, and they did not include a “metabolic priming” dietary baseline. During informed consent, the risk/benefit analysis of combining individually safe but collectively undefined off-label drugs should be weighed against the expected efficacy of SOC and the biological rationale, including preclinical evidence. The key highlighted concept in this regard is the targeting of glycolysis and glutaminolysis while under therapeutic ketosis (metabolic press), in synergy with cancer-associated pathways (microenvironment pulse), rather than endorsing any specific drug combination as the most desirable for this purpose. Metabolic and molecular analysis during this process is important to reveal if evolutionary pressure selects for therapy-resistant cells. Future clinical research will be required to establish the optimal dosing, timing, and scheduling of the most effective press-pulse KMT combinations.
A major current limitation of drug repositioning is the lack of molecularly driven stratification and robust biomarkers to guide personalized therapy. Drug selection is often based on rational combinations that have demonstrated synergistic cytotoxicity in preclinical models, rather than specific tumor characteristics [704]. Safety concerns may understandably arise in multi-drug protocols at both the pharmacokinetic and pharmacodynamic level. To isolate the strength of each variable, most clinical trials involve single drug additions to SOC. In combinatory trials, assessing the benefit of each intervention becomes increasingly difficult, even in cross-over and multi-arm designs. Furthermore, patients with cancer are often polymedicated for prior comorbidities, with overlapping antineoplastic treatments making them a particularly vulnerable population. It is therefore important to carefully evaluate participants according to baseline health status and available molecular markers, starting with the safest interventions that demonstrate the highest scientific rationale. If combinatory approaches are proposed, drug-to-drug interactions must be screened preemptively (e.g., CYP system), followed by a slow dose buildup to foster tolerability, as elegantly illustrated in the CUSP9 trial [394]. Despite these challenges, we believe that press-pulse targeting of tumor-associated pathways in synergy with KMT will be developed as an affordable and translationally viable therapeutic strategy.
Over-the-counter dietary supplementation
It is beyond the scope of these guidelines to detail all possible dietary supplements that may be of interest during multimodal cancer therapy. As a general concept, lifestyle interventions and supplementation are intended to improve the adaptive capacity of the non-tumoral cell mass (the prevailing portion of the patient’s ecology), which will compete with the tumor for bioenergetic and biosynthetic resources [705]. This also improves the likelihood that targeting of glycolysis, glutaminolysis, and the tumor microenvironment will be better tolerated by normal cells, or that synergistic opportunities may arise [706, 707].
Additional File 7: Table S4 includes common over-the-counter nutraceuticals with emerging preclinical and clinical evidence for complementary cancer use, mostly through supporting healthy tissue function. Given that this list is not intended to be exhaustive, many excellent reviews on this topic can be found elsewhere [708–711].
It is exceedingly unlikely that large randomized clinical trials will be performed for non-patentable natural products: consequently, we encourage documenting and sharing individual clinical experiences via systematic case reporting in peer-reviewed, reputable scientific journals [366, 367]. Supplementation should be disclosed to the attending medical team and reported independently for each patient. It is indispensable to review contraindications, adverse reactions, and potential drug interactions, which can be screened using online databases [712]. It is also advisable to establish a clear timeline of intake to avoid putatively antagonistic combinations (e.g., antioxidant effects during pro-oxidant therapies) [713, 714]. It must be clearly stated that supplementation is not intended to resolve advanced cancer, and owing to its namesake, it should be viewed as “supplementary”. However, when implemented judiciously, it is also unlikely to interfere with most conventional antineoplastic drugs or KMT, thus becoming a personal choice of the informed patient [715].
Physical activity
Moderate daily physical exercise is encouraged and should be tailored to the age and fitness of the patient, including both resistance/strength training for muscle maintenance as well as aerobic/high-intensity training for cardiometabolic health [716, 717]. As a core pillar of KMT, physical activity is anti-cachexic, increases insulin sensitivity, and facilitates physiological glucose and glutamine clearance [249, 718, 719]. Furthermore, low-intensity endurance exercises such as regularly spread-out walking (smaller doses but higher frequency) modulates osteocalcin and glucagon signaling, consequently lowering glucose availability and insulin secretion [720].
Recording of metabolic parameters (such as GKI) should be contextualized, as fuel utilization and transient stress responses may influence measurements in the post-exercise window. In light of the beneficial effects of exercise on reducing cancer mortality and recurrence [721–723], as well as the inverse association between muscle mass and strength with all-cause mortality [724], patients should strive for a daily dose of physical activity that is sufficient to stimulate muscle protein synthesis, adjusted to their training experience and comorbidities; for example, as per current cancer guidelines, aim for at least 150 min per week of aerobic exercise, two or more days a week of resistance training, or ≥ 10 metabolic equivalent of task (MET)-hours per week of overall physical activity [725–727].
Stress management
Facing a serious cancer diagnosis can be traumatic and emotionally distressful, impacting patients’ mental health and psychosocial wellbeing [728]. It is pivotal that patients receive appropriate mental health support that suits their preferences and beliefs, including multidisciplinary psycho-oncological care with clinical psychotherapy, sleep hygiene, breathing exercises, limbic system retraining, meditation or prayer, not least because a high level of personal motivation is required for KMT implementation [729, 730]. On a physiological level, stress management is important to stabilize the hypothalamus–pituitary–adrenal axis and sympathetic nervous system, maintaining adequate cortisol levels, immune function, and circadian rhythms [731–733].
Evaluation of outcomes and therapy adjustments
To assess the effectiveness of KMT, we recommend monitoring tumor response using non-invasive anatomical or combined metabolic imaging within the first 1–2 months (e.g., MRI with elective 18F-FDG PET at 8 weeks), and then every 2–4 months during active treatment, in line with standard guidelines [26, 734]. If changes to SOC timing are proposed in a diet-drug KMT trial (such as radiotherapy delay), neuroimaging may need to be more frequent to detect early trends in tumor progression. If the tumor is stable or shows signs of partial response, follow-up can be scheduled every 2 to 4 months for the next 2 to 3 years, and less frequently thereafter. It is important to create a schedule that would enable timely adjustments to the therapeutic plan. In extra-neural cancers, previously positive tumor markers as well as validated liquid biopsies may assist in estimating tumor burden [735–737]. Repeated surgical debulking can prevent bulk effect, especially in slow growing tumors [738]. Active monitoring and GKI-adjusted KD/KD-R should be maintained as long as there is evidence of persistent disease or risk of recurrence.
Conclusions
Ethical considerations and future directions
One of the greatest challenges in GBM therapy is the inability of the current SOC to eliminate all microscopic tumor infiltration and cancer stem cells [739, 740]. After the inevitable recurrence, patients are often confronted with salvage therapies of limited clinical utility [741]. These grim prospects make it difficult for physicians to communicate prognosis and for patients to make realistic and informed decisions about their preferred treatment plan [742–744]. It is not uncommon to avoid emerging therapies due to safety concerns (primum non nocere), fear of straying too far from the established guidelines (defensive medicine), or lack of familiarity. This may be entirely within lex artis for early-stage cancers, despite the perceived drawbacks of certain antineoplastics, which could be regarded as justifiable if durable remission is achieved [95, 745]. For terminal, incurable cancers, it is a matter of interpretation of medical ethics as to whom should be the arbiter of therapeutic decisions, especially for interventions where the risk/benefit ratio is not fully established [746–749].
This is an ethical consideration, not a scientific one, to be decided collectively at the societal and policy level. Nevertheless, from a patient advocacy perspective, advancing education about novel therapies at all the levels of the evidence-based pyramid is essential to facilitate shared decision-making. Going forward, a larger collection of clinical trials will be needed to standardize the implementation of GKI-adjusted dietary KMT with concurrent SLP targeting. This is the context where we aim to provide a comprehensive, minimally toxic, and cost-effective GBM treatment plan, with a solid theoretical background, pilot clinical studies, and ample research potential, as it is gradually developed to become part of the standard oncology toolkit. We wish to inspire patients to take a proactive and informed role in the management of their disease, physicians to make evidence-based decisions while still exercising clinical freedom, and researchers to join the quest for discovery of the many promising therapeutic avenues that are yet to come by targeting the fundamental bioenergetic dependencies of cancer cells.
A flexible and modular protocol has been presented to guide translational GBM research, based on the evidence that most of the defining hallmarks of cancer can be explained from a mitochondrial metabolic perspective [30, 35]. As predicted by evolutionary biology, cancer cells suffer from a distinctive lack of adaptive versatility due to both mitochondrial and genomic damage, as well as persistent anabolic demands. GBM cells, like most other cancers, are comparatively more dependent on SLP flux for energy and biosynthesis due to universal defects in mitochondrial number, structure, and function, despite ample downstream mutational heterogeneity, metabolic reprogramming, and single-cell heterogeneity [35, 152, 750].
KMT is conceptualized as a press-pulse therapeutic strategy. This framework can be adjusted for any cancer subtype that is unable to proliferate under the relative restriction of both glycolysis and glutaminolysis (SLP dependency), even when supplied with compensatory oxidative fuels (OXPHOS insufficiency). Given the biochemical underpinnings, it will be important to search for cancer models that retain uncontrolled cell proliferation using primarily OXPHOS after the simultaneous targeting of glycolytic and glutaminolytic SLP flux, as this would pose an exception to the mechanistic rationale. Future research, stemming from collections of case reports and clinical trials, will offer unique insights into the optimal dosing, timing, and scheduling for maximally safe and effective SLP targeting after physiological adaptation to therapeutic ketosis.
Supplementary Information
Acknowledgements
We thank the Foundation for Metabolic Cancer Therapies, The Nelson and Claudia Peltz Family Foundation, Lewis Topper, The John and Kathy Garcia Foundation, Dr. Edward Miller, Kenneth Rainin Foundation, the Corkin Family Foundation, Children with Cancer UK, the Broken Science Initiative, Delaware County Special Deputies Benevolent Fund, and the Boston College Research Expense Fund for their support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Bob Kaplan and Eric Miller for insightful discussions. Figures created with BioRender.
Abbreviations
- 2-HG
2-Hydroxyglutarate
- ADF
Alternate-day fasting
- AUC
Area under the curve
- BMI
Body mass index
- CGM
Continuous glucose monitoring
- CKM
Continuous ketone monitoring
- CNS
Central nervous system
- DCA
Dichloroacetate
- DON
6-Diazo-5-oxo-L-norleucine
- ECAR
Extracellular acidification rate
- FMD
Fasting-mimicking diet
- GBM
Glioblastoma
- GKI
Glucose-Ketone Index
- HBOT
Hyperbaric oxygen therapy
- HDAC
Histone deacetylase
- IDH
Isocitrate dehydrogenase
- IRB
Institutional Review Board
- KD
Ketogenic diet
- KD-R
Calorically restricted ketogenic diet
- KMT
Ketogenic Metabolic Therapy
- LBM
Lean body mass
- MAD
Modified Atkins diet
- MBZ
Mebendazole
- MCT
Medium‐chain triglyceride
- MKD
Modified ketogenic diet
- mOS
Median overall survival
- NSAID
Nonsteroidal anti-inflammatory drug
- OCR
Oxygen consumption rate
- OGTT
Oral glucose tolerance test
- OXPHOS
Oxidative phosphorylation
- PFS
Progression-free survival
- SLP
Substrate-level phosphorylation
- SOC
Standard of care
- SUV
Standardized uptake value
- TTF
Tumor-Treating Fields
- VEGF
Vascular endothelial growth factor
- VE-PTP
Vascular endothelial protein tyrosine phosphatase
- βHB
Beta-hydroxybutyrate
Authors’ contributions
T.D. and T.N.S. wrote the manuscript. M.K., G.Z., J.C.M., D.P.D., A.C.S., A.P., S.F.W., J.H., R.J.K., A.H., D.C.L., I.C., B.K., K.A.S., M.C.L.P., C.E.C., B.Z.-K., J.T.-S., F.M.S., E.O., G.A.-M., M.K., R.C., A.M.E.-S., A.P., E.H.M., D.W., H.S., R.I.C., J.P.S., A.K.S., M.S.I., A.Y., G.J.P., A.C., W.A.-H., A.K.E., P.K., K.H., Z.C., G.W.Y., A.E.E., J.K.N., K.S., D.F., J.D., and P.M., contributed substantially to the discussion and revision of the content. All authors read and approved the final manuscript.
Funding
Not applicable.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
A.P. is an owner of Poff Medical Consulting and Communications, LLC, which performs consulting and public speaking services related to ketogenic metabolic therapy. A.P. is a scientific advisor to Pruvit Ventures, LLC, which sells exogenous ketone products. A.P. is an owner of Metabolic Health Initiative, LLC which is a medical education company in the field of metabolic health and metabolism-based therapies. A.P. is an inventor on and receives royalties from the following patent: “Targeting Cancer with Metabolic Therapy and Hyperbaric Oxygen” (Patent Number: 9801903). D.P.D. is an inventor of patents on the use of exogenous ketones, advisor for Levels Health, and co-owner of Ketone Technologies LLC, which does consulting and public speaking events. C.E.C. receives royalties from books, consulting, and lectures on nutrition and exercise, and serves on the scientific advisory board of Simply Good Foods/Atkins. M.K. is employed by Dietary Therapies LLC. The other authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tomás Duraj, Email: durajto@gmail.com.
Thomas N. Seyfried, Email: thomas.seyfried@bc.edu
References
- 1.Fatehi M, Hunt C, Ma R, Toyota BD. Persistent Disparities in Survival for Patients with Glioblastoma. World Neurosurg. 2018;120:e511–6. [DOI] [PubMed] [Google Scholar]
- 2.Rocha Pinheiro SL, Lemos FFB, Marques HS, Silva Luz M, de Oliveira Silva LG. Faria Souza Mendes Dos Santos C, da Costa Evangelista K, Calmon MS, Sande Loureiro M, Freire de Melo F: Immunotherapy in glioblastoma treatment: Current state and future prospects. World J Clin Oncol. 2023;14(4):138–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rominiyi O, Vanderlinden A, Clenton SJ, Bridgewater C, Al-Tamimi Y, Collis SJ. Correction: Tumour treating fields therapy for glioblastoma: current advances and future directions. Br J Cancer. 2021;125(4):623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Solinge TS, Nieland L, Chiocca EA, Broekman ML. Advances in local therapy for glioblastoma—taking the fight to the tumour. Nat Rev Neurol. 2022;18(4):221–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Efremov L, Abera SF, Bedir A, Vordermark D, Medenwald D. Patterns of glioblastoma treatment and survival over a 16-years period: pooled data from the German Cancer Registries. J Cancer Res Clin Oncol. 2021;147(11):3381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCutcheon IE, Preul MC. Historical perspective on surgery and survival with glioblastoma: how far have we come? World Neurosurgery. 2021;149:148–68. [DOI] [PubMed] [Google Scholar]
- 7.Tykocki T, Eltayeb M. Ten-year survival in glioblastoma A systematic review. J Clin Neurosci. 2018;54:7–13. [DOI] [PubMed] [Google Scholar]
- 8.Hertler C, Felsberg J, Gramatzki D, Le Rhun E, Clarke J, Soffietti R, Wick W, Chinot O, Ducray F, Roth P: Long-term survival with IDH wildtype glioblastoma: first results from the ETERNITY Brain Tumor Funders’ Collaborative Consortium (EORTC 1419). Eur J Cancer 2023. [DOI] [PubMed]
- 9.Cruz Da Silva E, Mercier MC, Etienne-Selloum N, Dontenwill M, Choulier L: A Systematic Review of Glioblastoma-Targeted Therapies in Phases II, III, IV Clinical Trials. Cancers (Basel) 2021, 13(8). [DOI] [PMC free article] [PubMed]
- 10.Mandel JJ, Yust-Katz S, Patel AJ, Cachia D, Liu D, Park M, Yuan Y, Kent TA, de Groot JF. Inability of positive phase II clinical trials of investigational treatments to subsequently predict positive phase III clinical trials in glioblastoma. Neuro Oncol. 2018;20(1):113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poon MTC, Sudlow CLM, Figueroa JD, Brennan PM. Longer-term (>/= 2 years) survival in patients with glioblastoma in population-based studies pre- and post-2005: a systematic review and meta-analysis. Sci Rep. 2020;10(1):11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weeks JC, Catalano PJ, Cronin A, Finkelman MD, Mack JW, Keating NL, Schrag D. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367(17):1616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riches JC, Voigt LP. Palliative, Ethics, and End-of-Life Care Issues in the Cancer Patient. Crit Care Clin. 2021;37(1):105–15. [DOI] [PubMed] [Google Scholar]
- 14.Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C, Barnholtz-Sloan JS: CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2016—2020. Neuro Oncol 2023, 25(Supplement_4):iv1-iv99. [DOI] [PMC free article] [PubMed]
- 15.Marenco-Hillembrand L, Wijesekera O, Suarez-Meade P, Mampre D, Jackson C, Peterson J, Trifiletti D, Hammack J, Ortiz K, Lesser E, et al. Trends in glioblastoma: outcomes over time and type of intervention: a systematic evidence based analysis. J Neurooncol. 2020;147(2):297–307. [DOI] [PubMed] [Google Scholar]
- 16.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–66. [DOI] [PubMed] [Google Scholar]
- 17.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23(8):1231–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brat DJ, Aldape K, Colman H, Figrarella-Branger D, Fuller GN, Giannini C, Holland EC, Jenkins RB, Kleinschmidt-DeMasters B, Komori T, et al. cIMPACT-NOW update 5: recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol. 2020;139(3):603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown NF, Ottaviani D, Tazare J, Gregson J, Kitchen N, Brandner S, Fersht N, Mulholland P. Survival Outcomes and Prognostic Factors in Glioblastoma. Cancers (Basel). 2022;14(13):3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zinn PO, Colen RR, Kasper EM, Burkhardt JK. Extent of resection and radiotherapy in GBM: A 1973 to 2007 surveillance, epidemiology and end results analysis of 21,783 patients. Int J Oncol. 2013;42(3):929–34. [DOI] [PubMed] [Google Scholar]
- 21.Nieder C, Grosu AL, Astner S, Molls M. Treatment of unresectable glioblastoma multiforme. Anticancer Res. 2005;25(6C):4605–10. [PubMed] [Google Scholar]
- 22.Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K, et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA. 2017;318(23):2306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liau LM, Ashkan K, Brem S, Campian JL, Trusheim JE, Iwamoto FM, Tran DD, Ansstas G, Cobbs CS, Heth JA. Association of autologous tumor lysate-loaded dendritic cell vaccination with extension of survival among patients with newly diagnosed and recurrent glioblastoma: a phase 3 prospective externally controlled cohort trial. JAMA Oncol. 2023;9(1):112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philip J, Collins A, Brand C, Sundararajan V, Lethborg C, Gold M, Lau R, Moore G, Murphy M. A proposed framework of supportive and palliative care for people with high-grade glioma. Neuro Oncol. 2018;20(3):391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivoirard R, Vallard A, Boutet C, Falk AT, Garin C, Adjabi A, Hoarau D, Forest F, Fotso MJ, Rancoule C, et al. A retrospective survey of the last 3 months of life in patients carrying glioblastoma: Clinical treatments and profiles. Mol Clin Oncol. 2018;8(1):115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Network NCC: NCCN guidelines: central nervous system cancers. The NCCN Guidelines 2024, Version 2.2024.
- 27.Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven L, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18(3):170–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birzu C, French P, Caccese M, Cerretti G, Idbaih A, Zagonel V, Lombardi G. Recurrent Glioblastoma: From Molecular Landscape to New Treatment Perspectives. Cancers (Basel). 2020;13(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seyfried TN, Shelton L, Arismendi-Morillo G, Kalamian M, Elsakka A, Maroon J, Mukherjee P. Provocative Question: Should Ketogenic Metabolic Therapy Become the Standard of Care for Glioblastoma? Neurochem Res. 2019;44(10):2392–404. [DOI] [PubMed] [Google Scholar]
- 30.Warburg OJS. On the origin of cancer cells. 1956;123(3191):309–14. [DOI] [PubMed] [Google Scholar]
- 31.Seyfried TN, Flores RE, Poff AM, D’Agostino DP. Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis. 2014;35(3):515–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfeiffer T, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001;292(5516):504–7. [DOI] [PubMed] [Google Scholar]
- 33.Chinopoulos C, Seyfried TN. Mitochondrial Substrate-Level Phosphorylation as Energy Source for Glioblastoma: Review and Hypothesis. ASN Neuro. 2018;10:1759091418818261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ravasz D, Bui D, Nazarian S, Pallag G, Karnok N, Roberts J, Marzullo BP, Tennant DA, Greenwood B, Kitayev A, et al. Residual Complex I activity and amphidirectional Complex II operation support glutamate catabolism through mtSLP in anoxia. Sci Rep. 2024;14(1):1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seyfried TN, Chinopoulos C. Can the mitochondrial metabolic theory explain better the origin and management of cancer than can the somatic mutation theory? Metabolites. 2021;11(9):572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16(10):619–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008;105(48):18782–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oizel K, Chauvin C, Oliver L, Gratas C, Geraldo F, Jarry U, Scotet E, Rabe M, Alves-Guerra MC, Teusan R, et al. Efficient Mitochondrial Glutamine Targeting Prevails Over Glioblastoma Metabolic Plasticity. Clin Cancer Res. 2017;23(20):6292–304. [DOI] [PubMed] [Google Scholar]
- 39.Maraqah HH, Abu-Asab MS, Lee HS, Aboud O. Comparative survey of mitochondrial ultrastructure in IDH1-mutant astrocytoma and IDH1-wildtype glioblastoma (GBM). Ultrastruct Pathol. 2023;47(2):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deighton RF, Le Bihan T, Martin SF, Gerth AMJ, McCulloch M, Edgar JM, Kerr LE, Whittle IR, McCulloch J. Interactions among mitochondrial proteins altered in glioblastoma. J Neurooncol. 2014;118(2):247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arismendi-Morillo G, Castellano-Ramirez A, Seyfried TN. Ultrastructural characterization of the Mitochondria-associated membranes abnormalities in human astrocytomas: Functional and therapeutics implications. Ultrastruct Pathol. 2017;41(3):234–44. [DOI] [PubMed] [Google Scholar]
- 42.Feichtinger RG, Weis S, Mayr JA, Zimmermann F, Geilberger R, Sperl W, Kofler B. Alterations of oxidative phosphorylation complexes in astrocytomas. Glia. 2014;62(4):514–25. [DOI] [PubMed] [Google Scholar]
- 43.Kossenkov AV, Milcarek A, Notta F, Jang GH, Wilson JM, Gallinger S, Zhou DC, Ding L, Ghosh JC, Perego M, et al. Mitochondrial fitness and cancer risk. PLoS ONE. 2022;17(10): e0273520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srinivasan S, Guha M, Kashina A, Avadhani NG. Mitochondrial dysfunction and mitochondrial dynamics-The cancer connection. Biochim Biophys Acta Bioenerg. 2017;1858(8):602–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghosh JC, Perego M, Agarwal E, Bertolini I, Wang Y, Goldman AR, Tang HY, Kossenkov AV, Landis CJ, Languino LR, et al. Ghost mitochondria drive metastasis through adaptive GCN2/Akt therapeutic vulnerability. Proc Natl Acad Sci U S A. 2022;119(8): e2115624119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao T, Asayama Y. Animal-cell culture media: History, characteristics, and current issues. Reprod Med Biol. 2017;16(2):99–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016;23(1):27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eagle H. Amino acid metabolism in mammalian cell cultures. Science. 1959;130(3373):432–7. [DOI] [PubMed] [Google Scholar]
- 49.Hall A, Meyle KD, Lange MK, Klima M, Sanderhoff M, Dahl C, Abildgaard C, Thorup K, Moghimi SM, Jensen PB, et al. Dysfunctional oxidative phosphorylation makes malignant melanoma cells addicted to glycolysis driven by the (V600E)BRAF oncogene. Oncotarget. 2013;4(4):584–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gouirand V, Gicquel T, Lien EC, Jaune-Pons E, Da Costa Q, Finetti P, Metay E, Duluc C, Mayers JR, Audebert S, et al. Ketogenic HMG-CoA lyase and its product beta-hydroxybutyrate promote pancreatic cancer progression. EMBO J. 2022;41(9): e110466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sperry J, Condro MC, Guo L, Braas D, Vanderveer-Harris N, Kim KKO, Pope WB, Divakaruni AS, Lai A, Christofk H et al: Glioblastoma Utilizes Fatty Acids and Ketone Bodies for Growth Allowing Progression during Ketogenic Diet Therapy. iScience 2020, 23(9):101453. [DOI] [PMC free article] [PubMed]
- 52.Santiappillai NT, Hakeem-Sanni MF, Ghasemi M, Withy A, Quek L-E, Hoy AJ: Fatty acids are not a significant contributor to the TCA cycle in cancer cell lines: evidence of incomplete fatty acid oxidation. bioRxiv 2024:2024.2003. 2025.586547.
- 53.Altea-Manzano P, Cuadros AM, Broadfield LA, Fendt SM. Nutrient metabolism and cancer in the in vivo context: a metabolic game of give and take. EMBO Rep. 2020;21(10): e50635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cluntun AA, Lukey MJ, Cerione RA, Locasale JW. Glutamine Metabolism in Cancer: Understanding the Heterogeneity. Trends Cancer. 2017;3(3):169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Faubert B, Li KY, Cai L, Hensley CT, Kim J, Zacharias LG, Yang C, Do QN, Doucette S, Burguete D et al: Lactate Metabolism in Human Lung Tumors. Cell 2017, 171(2):358–371 e359. [DOI] [PMC free article] [PubMed]
- 56.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269–70. [PubMed] [Google Scholar]
- 57.Weinhouse S. The Warburg hypothesis fifty years later. Zeitschrift fur Krebsforschung und klinische Onkologie. 1976;87(2):115–26. [DOI] [PubMed] [Google Scholar]
- 58.Bartman CR, Weilandt DR, Shen Y, Lee WD, Han Y, TeSlaa T, Jankowski CSR, Samarah L, Park NR, da Silva-Diz V, et al. Slow TCA flux and ATP production in primary solid tumours but not metastases. Nature. 2023;614(7947):349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Viale A, Pettazzoni P, Lyssiotis CA, Ying H, Sanchez N, Marchesini M, Carugo A, Green T, Seth S, Giuliani V, et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514(7524):628–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ju YS, Alexandrov LB, Gerstung M, Martincorena I, Nik-Zainal S, Ramakrishna M, Davies HR, Papaemmanuil E, Gundem G, Shlien A, et al. Origins and functional consequences of somatic mitochondrial DNA mutations in human cancer. Elife. 2014;3: e02935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duraj T, Carrion-Navarro J, Seyfried TN, Garcia-Romero N, Ayuso-Sacido A. Metabolic therapy and bioenergetic analysis: The missing piece of the puzzle. Mol Metab. 2021;54: 101389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang J, Jia PP, Liu QL, Cong MH, Gao Y, Shi HP, Yu WN, Miao MY. Low ketolytic enzyme levels in tumors predict ketogenic diet responses in cancer cell lines in vitro and in vivo. J Lipid Res. 2018;59(4):625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maurer GD, Brucker DP, Bahr O, Harter PN, Hattingen E, Walenta S, Mueller-Klieser W, Steinbach JP, Rieger J. Differential utilization of ketone bodies by neurons and glioma cell lines: a rationale for ketogenic diet as experimental glioma therapy. BMC Cancer. 2011;11(1):315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skinner R, Trujillo A, Ma X, Beierle EA: Ketone bodies inhibit the viability of human neuroblastoma cells. J Pediatr Surg 2009, 44(1):212–216; discussion 216. [DOI] [PubMed]
- 65.Artzi M, Liberman G, Vaisman N, Bokstein F, Vitinshtein F, Aizenstein O, Ben Bashat D. Changes in cerebral metabolism during ketogenic diet in patients with primary brain tumors: (1)H-MRS study. J Neurooncol. 2017;132(2):267–75. [DOI] [PubMed] [Google Scholar]
- 66.Voss M, Lorenz NI, Luger AL, Steinbach JP, Rieger J, Ronellenfitsch MW. Rescue of 2-Deoxyglucose Side Effects by Ketogenic Diet. Int J Mol Sci. 2018;19(8):2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mukherjee P, Augur ZM, Li M, Hill C, Greenwood B, Domin MA, Kondakci G, Narain NR, Kiebish MA, Bronson RT, et al. Therapeutic benefit of combining calorie-restricted ketogenic diet and glutamine targeting in late-stage experimental glioblastoma. Commun Biol. 2019;2(1):200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hopkins BD, Pauli C, Du X, Wang DG, Li X, Wu D, Amadiume SC, Goncalves MD, Hodakoski C, Lundquist MR. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature. 2018;560(7719):499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hajihassani O, Zarei M, Roichman A, Loftus A, Boutros CS, Hue J, Naji P, Boyer J, Tahan S, Gallagher P et al: A Ketogenic Diet Sensitizes Pancreatic Cancer to Inhibition of Glutamine Metabolism. bioRxiv 2024:2024.2007. 2019.604377.
- 70.Stine ZE, Schug ZT, Salvino JM, Dang CV. Targeting cancer metabolism in the era of precision oncology. Nat Rev Drug Discov. 2022;21(2):141–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang WH, Qiu Y, Stamatatos O, Janowitz T, Lukey MJ. Enhancing the Efficacy of Glutamine Metabolism Inhibitors in Cancer Therapy. Trends Cancer. 2021;7(8):790–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kawai T, Brender JR, Lee JA, Kramp T, Kishimoto S, Krishna MC, Tofilon P, Camphausen KA. Detection of metabolic change in glioblastoma cells after radiotherapy using hyperpolarized 13C-MRI. NMR Biomed. 2021;34(7): e4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wibom C, Surowiec I, Moren L, Bergstrom P, Johansson M, Antti H, Bergenheim AT. Metabolomic patterns in glioblastoma and changes during radiotherapy: a clinical microdialysis study. J Proteome Res. 2010;9(6):2909–19. [DOI] [PubMed] [Google Scholar]
- 74.Bergsneider M, Hovda DA, Shalmon E, Kelly DF, Vespa PM, Martin NA, Phelps ME, McArthur DL, Caron MJ, Kraus JF, et al. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J Neurosurg. 1997;86(2):241–51. [DOI] [PubMed] [Google Scholar]
- 75.Seyfried TN, Shelton LM, Mukherjee P. Does the existing standard of care increase glioblastoma energy metabolism? Lancet Oncol. 2010;11(9):811–3. [DOI] [PubMed] [Google Scholar]
- 76.Oliva CR, Nozell SE, Diers A, McClugage SG 3rd, Sarkaria JN, Markert JM, Darley-Usmar VM, Bailey SM, Gillespie GY, Landar A, et al. Acquisition of temozolomide chemoresistance in gliomas leads to remodeling of mitochondrial electron transport chain. J Biol Chem. 2010;285(51):39759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson BE, Mazor T, Hong C, Barnes M, Aihara K, McLean CY, Fouse SD, Yamamoto S, Ueda H, Tatsuno K, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343(6167):189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winter SF, Loebel F, Loeffler J, Batchelor TT, Martinez-Lage M, Vajkoczy P, Dietrich J. Treatment-induced brain tissue necrosis: a clinical challenge in neuro-oncology. Neuro Oncol. 2019;21(9):1118–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wen PY, Weller M, Lee EQ, Alexander BM, Barnholtz-Sloan JS, Barthel FP, Batchelor TT, Bindra RS, Chang SM, Chiocca EA, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22(8):1073–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pitter KL, Tamagno I, Alikhanyan K, Hosni-Ahmed A, Pattwell SS, Donnola S, Dai C, Ozawa T, Chang M, Chan TA, et al. Corticosteroids compromise survival in glioblastoma. Brain. 2016;139(Pt 5):1458–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klement RJ, Champ CE. Corticosteroids compromise survival in glioblastoma in part through their elevation of blood glucose levels. Brain. 2017;140(3): e16. [DOI] [PubMed] [Google Scholar]
- 82.Wong ET, Lok E, Gautam S, Swanson KD. Dexamethasone exerts profound immunologic interference on treatment efficacy for recurrent glioblastoma. Br J Cancer. 2015;113(2):232–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Decker M, Sacks P, Abbatematteo J, De Leo E, Brennan M, Rahman M. The effects of hyperglycemia on outcomes in surgical high-grade glioma patients. Clin Neurol Neurosurg. 2019;179:9–13. [DOI] [PubMed] [Google Scholar]
- 84.McGirt MJ, Chaichana KL, Gathinji M, Attenello F, Than K, Jimenez Ruiz A, Olivi A, Quinones-Hinojosa A: Persistent outpatient hyperglycemia is independently associated with decreased survival after primary resection of malignant brain astrocytomas. Neurosurgery 2008, 63(2):286–291; discussion 291. [DOI] [PubMed]
- 85.Derr RL, Ye X, Islas MU, Desideri S, Saudek CD, Grossman SA. Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J Clin Oncol. 2009;27(7):1082–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mayer A, Vaupel P, Struss HG, Giese A, Stockinger M, Schmidberger H. Strong adverse prognostic impact of hyperglycemic episodes during adjuvant chemoradiotherapy of glioblastoma multiforme. Strahlenther Onkol. 2014;190(10):933–8. [DOI] [PubMed] [Google Scholar]
- 87.Tieu MT, Lovblom LE, McNamara MG, Mason W, Laperriere N, Millar BA, Menard C, Kiehl TR, Perkins BA, Chung C. Impact of glycemia on survival of glioblastoma patients treated with radiation and temozolomide. J Neurooncol. 2015;124(1):119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Swildens KX, Sillevis Smitt PAE, van den Bent MJ, French PJ, Geurts M: The effect of dexamethasone on the microenvironment and efficacy of checkpoint inhibitors in glioblastoma: a systematic review. Neurooncol Adv 2022, 4(1):vdac087. [DOI] [PMC free article] [PubMed]
- 89.Caramanna I, de Kort JM, Brandes AA, Taal W, Platten M, Idbaih A, Frenel JS, Wick W, Preetha CJ, Bendszus M, et al. Corticosteroids use and neurocognitive functioning in patients with recurrent glioblastoma: Evidence from European Organization for Research and Treatment of Cancer (EORTC) trial 26101. Neurooncol Pract. 2022;9(4):310–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mantilla EC Jr, Abramowitz J, Dan TU, Pan E. Prolonged Steroid Dependence in Adult Patients With Glioma. Anticancer Res. 2020;40(4):2059–64. [DOI] [PubMed]
- 91.Petrelli F, De Stefani A, Ghidini A, Bruschieri L, Riboldi V, Dottorini L, Iaculli A, Zaniboni A, Trevisan F. Steroids use and survival in patients with glioblastoma multiforme: a pooled analysis. J Neurol. 2021;268(2):440–7. [DOI] [PubMed] [Google Scholar]
- 92.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Groot JF, Fuller G, Kumar AJ, Piao Y, Eterovic K, Ji Y, Conrad CA. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro Oncol. 2010;12(3):233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thompson EM, Frenkel EP, Neuwelt EA. The paradoxical effect of bevacizumab in the therapy of malignant gliomas. Neurology. 2011;76(1):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Van Kleffens T, van Baarsen B, van Leeuwen E. The medical practice of patient autonomy and cancer treatment refusals: a patients’ and physicians’ perspective. Social science medicine. 2004;58(11):2325–36. [DOI] [PubMed] [Google Scholar]
- 96.Drolet BC, White CL. Selective paternalism. Virtual Mentor. 2012;14(7):582–8. [DOI] [PubMed] [Google Scholar]
- 97.Peppercorn J. Ethics of ongoing cancer care for patients making risky decisions. J Oncol Pract. 2012;8(5):e111–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tenner L, Hlubocky FJ, Blanke CD, LeBlanc TW, Marron JM, McGinnis MM, Spence RA, Taylor LP. Let’s talk about those herbs you are taking: ethical considerations for communication with patients with cancer about complementary and alternative medicine. Journal of oncology practice. 2019;15(1):44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Winter SF, Loebel F, Dietrich J. Role of ketogenic metabolic therapy in malignant glioma: A systematic review. Crit Rev Oncol Hematol. 2017;112:41–58. [DOI] [PubMed] [Google Scholar]
- 100.Woolf EC, Syed N, Scheck AC. Tumor Metabolism, the Ketogenic Diet and beta-Hydroxybutyrate: Novel Approaches to Adjuvant Brain Tumor Therapy. Front Mol Neurosci. 2016;9:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schwartz KA, Noel M, Nikolai M, Olson LK, Hord NG, Zakem M, Clark J, Elnabtity M, Figueroa B, Chang HT. Long Term Survivals in Aggressive Primary Brain Malignancies Treated With an Adjuvant Ketogenic Diet. Front Nutr. 2022;9: 770796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hagihara K, Kajimoto K, Osaga S, Nagai N, Shimosegawa E, Nakata H, Saito H, Nakano M, Takeuchi M, Kanki H et al: Promising Effect of a New Ketogenic Diet Regimen in Patients with Advanced Cancer. Nutrients 2020, 12(5). [DOI] [PMC free article] [PubMed]
- 103.Iyikesici MS. Feasibility study of metabolically supported chemotherapy with weekly carboplatin/paclitaxel combined with ketogenic diet, hyperthermia and hyperbaric oxygen therapy in metastatic non-small cell lung cancer. Int J Hyperthermia. 2019;36(1):446–55. [DOI] [PubMed] [Google Scholar]
- 104.Iyikesici MS, Slocum AK, Slocum A, Berkarda FB, Kalamian M, Seyfried TN. Efficacy of Metabolically Supported Chemotherapy Combined with Ketogenic Diet, Hyperthermia, and Hyperbaric Oxygen Therapy for Stage IV Triple-Negative Breast Cancer. Cureus. 2017;9(7): e1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Khodabakhshi A, Akbari ME, Mirzaei HR, Seyfried TN, Kalamian M, Davoodi SH. Effects of Ketogenic metabolic therapy on patients with breast cancer: A randomized controlled clinical trial. Clin Nutr. 2021;40(3):751–8. [DOI] [PubMed] [Google Scholar]
- 106.Maroon J, Bost J, Amos A, Zuccoli G. Restricted calorie ketogenic diet for the treatment of glioblastoma multiforme. J Child Neurol. 2013;28(8):1002–8. [DOI] [PubMed] [Google Scholar]
- 107.Zuccoli G, Marcello N, Pisanello A, Servadei F, Vaccaro S, Mukherjee P, Seyfried TN. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: Case Report. Nutr Metab (Lond). 2010;7(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Panhans CM, Gresham G, Amaral LJ, Hu J. Exploring the Feasibility and Effects of a Ketogenic Diet in Patients With CNS Malignancies: A Retrospective Case Series. Front Neurosci. 2020;14:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Elsakka AMA, Bary MA, Abdelzaher E, Elnaggar M, Kalamian M, Mukherjee P, Seyfried TN. Management of Glioblastoma Multiforme in a Patient Treated With Ketogenic Metabolic Therapy and Modified Standard of Care: A 24-Month Follow-Up. Front Nutr. 2018;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martuscello RT, Vedam-Mai V, McCarthy DJ, Schmoll ME, Jundi MA, Louviere CD, Griffith BG, Skinner CL, Suslov O, Deleyrolle LP, et al. A Supplemented High-Fat Low-Carbohydrate Diet for the Treatment of Glioblastoma. Clin Cancer Res. 2016;22(10):2482–95. [DOI] [PubMed] [Google Scholar]
- 111.Rieger J, Bahr O, Maurer GD, Hattingen E, Franz K, Brucker D, Walenta S, Kammerer U, Coy JF, Weller M, et al. ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol. 2014;44(6):1843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Klein P, Tyrlikova I, Zuccoli G, Tyrlik A, Maroon JC. Treatment of glioblastoma multiforme with “classic” 4:1 ketogenic diet total meal replacement. Cancer Metab. 2020;8(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tan-Shalaby JL, Carrick J, Edinger K, Genovese D, Liman AD, Passero VA, Shah RB. Modified Atkins diet in advanced malignancies-final results of a safety and feasibility trial within the Veterans Affairs Pittsburgh Healthcare System. Nutrition metabolism. 2016;13(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Buga A, Harper DG, Sapper TN, Hyde PN, Fell B, Dickerson R, Stoner JT, Kackley ML, Crabtree CD, Decker DD, et al. Feasibility and metabolic outcomes of a well-formulated ketogenic diet as an adjuvant therapeutic intervention for women with stage IV metastatic breast cancer: The Keto-CARE trial. PLoS ONE. 2024;19(1): e0296523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Seyfried TN, Arismendi-Morillo G, Mukherjee P, Chinopoulos C: On the Origin of ATP Synthesis in Cancer. iScience 2020, 23(11):101761. [DOI] [PMC free article] [PubMed]
- 116.Maroon JC, Seyfried TN, Donohue JP, Bost J. The role of metabolic therapy in treating glioblastoma multiforme. Surg Neurol Int. 2015;6:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chang HT, Olson LK, Schwartz KA. Ketolytic and glycolytic enzymatic expression profiles in malignant gliomas: implication for ketogenic diet therapy. Nutr Metab (Lond). 2013;10(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Udumula MP, Singh H, Faraz R, Poisson L, Tiwari N, Dimitrova I, Hijaz M, Gogoi R, Swenor M, Munkarah A et al: Intermittent Fasting induced ketogenesis inhibits mouse epithelial ovarian tumors by promoting anti-tumor T cell response. bioRxiv 2023:2023.2003. 2008.531740. [DOI] [PMC free article] [PubMed]
- 119.Scheck AC, Abdelwahab MG, Fenton KE, Stafford P. The ketogenic diet for the treatment of glioma: insights from genetic profiling. Epilepsy Res. 2012;100(3):327–37. [DOI] [PubMed] [Google Scholar]
- 120.Stafford P, Abdelwahab MG, Kim DY, Preul MC, Rho JM, Scheck AC. The ketogenic diet reverses gene expression patterns and reduces reactive oxygen species levels when used as an adjuvant therapy for glioma. Nutr Metab (Lond). 2010;7(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mukherjee P, Mulrooney TJ, Marsh J, Blair D, Chiles TC, Seyfried TN. Differential effects of energy stress on AMPK phosphorylation and apoptosis in experimental brain tumor and normal brain. Mol Cancer. 2008;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mulrooney TJ, Marsh J, Urits I, Seyfried TN, Mukherjee P. Influence of caloric restriction on constitutive expression of NF-kappaB in an experimental mouse astrocytoma. PLoS ONE. 2011;6(3): e18085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shelton LM, Huysentruyt LC, Mukherjee P, Seyfried TN. Calorie restriction as an anti-invasive therapy for malignant brain cancer in the VM mouse. ASN Neuro. 2010;2(3): e00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou W, Mukherjee P, Kiebish MA, Markis WT, Mantis JG, Seyfried TN. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond). 2007;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mukherjee P, El-Abbadi MM, Kasperzyk JL, Ranes MK, Seyfried TN. Dietary restriction reduces angiogenesis and growth in an orthotopic mouse brain tumour model. Br J Cancer. 2002;86(10):1615–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Woolf EC, Curley KL, Liu Q, Turner GH, Charlton JA, Preul MC, Scheck AC. The Ketogenic Diet Alters the Hypoxic Response and Affects Expression of Proteins Associated with Angiogenesis, Invasive Potential and Vascular Permeability in a Mouse Glioma Model. PLoS ONE. 2015;10(6): e0130357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Veech RL, Todd King M, Pawlosky R, Kashiwaya Y, Bradshaw PC, Curtis W. The “great” controlling nucleotide coenzymes. IUBMB Life. 2019;71(5):565–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids. 2004;70(3):309–19. [DOI] [PubMed] [Google Scholar]
- 129.Seyfried TN, Arismendi-Morillo G, Zuccoli G, Lee DC, Duraj T, Elsakka AM, Maroon JC, Mukherjee P, Ta L, Shelton L, et al. Metabolic management of microenvironment acidity in glioblastoma. Front Oncol. 2022;12: 968351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41(3):211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35(8):427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Seyfried TN, Yu G, Maroon JC, D’Agostino DP. Press-pulse: a novel therapeutic strategy for the metabolic management of cancer. Nutr Metab (Lond). 2017;14:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Klement RJ. Beneficial effects of ketogenic diets for cancer patients: a realist review with focus on evidence and confirmation. Med Oncol. 2017;34(8):132. [DOI] [PubMed] [Google Scholar]
- 134.Seyfried TN, Shivane AG, Kalamian M, Maroon JC, Mukherjee P, Zuccoli G. Ketogenic Metabolic Therapy, Without Chemo or Radiation, for the Long-Term Management of IDH1-Mutant Glioblastoma: An 80-Month Follow-Up Case Report. Front Nutr. 2021;8: 682243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fack F, Tardito S, Hochart G, Oudin A, Zheng L, Fritah S, Golebiewska A, Nazarov PV, Bernard A, Hau AC, et al. Altered metabolic landscape in IDH-mutant gliomas affects phospholipid, energy, and oxidative stress pathways. EMBO Mol Med. 2017;9(12):1681–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van Noorden CJF, Hira VVV, van Dijck AJ, Novak M, Breznik B, Molenaar RJ. Energy Metabolism in IDH1 Wild-Type and IDH1-Mutated Glioblastoma Stem Cells: A Novel Target for Therapy? Cells. 2021;10(3):705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chesnelong C, Chaumeil MM, Blough MD, Al-Najjar M, Stechishin OD, Chan JA, Pieper RO, Ronen SM, Weiss S, Luchman HA, et al. Lactate dehydrogenase A silencing in IDH mutant gliomas. Neuro Oncol. 2014;16(5):686–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim S-H, Ito S, Yang C, Wang P, Xiao M-T. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19(1):17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Garcia CR, Highsmith KN, Knight S, Puduvalli VK, Kamiya-Matsuoka C. Single center experience of IDH inhibitors in high-grade gliomas. In.: American Society of Clinical Oncology; 2024. [Google Scholar]
- 141.Mellinghoff IK, Ellingson BM, Touat M, Maher E, De La Fuente MI, Holdhoff M, Cote GM, Burris H, Janku F, Young RJ. Ivosidenib in isocitrate dehydrogenase 1–mutated advanced glioma. J Clin Oncol. 2020;38(29):3398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kayabolen A, Yilmaz E, Bagci-Onder T. IDH Mutations in Glioma: Double-Edged Sword in Clinical Applications? Biomedicines. 2021;9(7):799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mukherjee P, Augur ZM, Li M, Hill C, Greenwood B, Domin MA, Kondakci G, Narain NR, Kiebish MA, Bronson RT, et al. Therapeutic benefit of combining calorie-restricted ketogenic diet and glutamine targeting in late-stage experimental glioblastoma. Commun Biol. 2019;2:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Javier R, Wang W, Drumm M, McCortney K, Sarkaria JN. Horbinski CJPo: The efficacy of an unrestricted cycling ketogenic diet in preclinical models of IDH wild-type and IDH mutant glioma. 2022;17(2): e0257725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Phillips MCL, Leyden J, McManus EJ, Lowyim DG, Ziad F, Moon BG. Haji Mohd Yasin NAB, Tan A, Thotathil Z, Jameson MB: Feasibility and Safety of a Combined Metabolic Strategy in Glioblastoma Multiforme: A Prospective Case Series. Journal of oncology. 2022;2022:4496734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nencioni A, Caffa I, Cortellino S, Longo VD. Fasting and cancer: molecular mechanisms and clinical application. Nat Rev Cancer. 2018;18(11):707–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Deligiorgi MV, Liapi C, Trafalis DT. How far are we from prescribing fasting as anticancer medicine? Int J Mol Sci. 2020;21(23):9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Harris AL. Development of cancer metabolism as a therapeutic target: New pathways, patient studies, stratification and combination therapy. Br J Cancer. 2020;122(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Menyhart O, Gyorffy B. Dietary approaches for exploiting metabolic vulnerabilities in cancer. Biochim Biophys Acta Rev Cancer. 2024;1879(2): 189062. [DOI] [PubMed] [Google Scholar]
- 150.Klement RJ: Anti-tumor effects of ketogenic diets and their synergism with other treatments in mice: Bayesian evidence synthesis of 1755 individual mouse survival data. Biomed J 2023:100609. [DOI] [PMC free article] [PubMed]
- 151.Xia S, Lin R, Jin L, Zhao L, Kang HB, Pan Y, Liu S, Qian G, Qian Z, Konstantakou E, et al. Prevention of Dietary-Fat-Fueled Ketogenesis Attenuates BRAF V600E Tumor Growth. Cell Metab. 2017;25(2):358–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Weber DD, Aminzadeh-Gohari S, Thapa M, Redtenbacher AS, Catalano L, Capeloa T, Vazeille T, Emberger M, Felder TK, Feichtinger RG, et al. Ketogenic diets slow melanoma growth in vivo regardless of tumor genetics and metabolic plasticity. Cancer Metab. 2022;10(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Guo A, Asztely F, Smits A, Jakola AS. Methodological Approaches to Ketogenic Dietary Treatments in Glioma Patients from a Nutritional Point of View. Nutr Cancer. 2023;75(1):112–22. [DOI] [PubMed] [Google Scholar]
- 154.Supportive PDQ, Palliative Care Editorial B: Nutrition in Cancer Care (PDQ(R)): Health Professional Version. In: PDQ Cancer Information Summaries. edn. Bethesda (MD): National Cancer Institute (US); 2002.
- 155.Klement RJ, Brehm N, Sweeney RA. Ketogenic diets in medical oncology: a systematic review with focus on clinical outcomes. Med Oncol. 2020;37(2):14. [DOI] [PubMed] [Google Scholar]
- 156.Lévesque S, Pol JG, Ferrere G, Galluzzi L, Zitvogel L, Kroemer G. Trial watch: dietary interventions for cancer therapy. Oncoimmunology. 2019;8(7): e1591878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lemberg KM, Gori SS, Tsukamoto T, Rais R, Slusher BS: Clinical development of metabolic inhibitors for oncology. J Clin Invest 2022, 132(1). [DOI] [PMC free article] [PubMed]
- 158.Zhu H, Bi D, Zhang Y, Kong C, Du J, Wu X, Wei Q, Qin H. Ketogenic diet for human diseases: the underlying mechanisms and potential for clinical implementations. Signal Transduct Target Ther. 2022;7(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Qin J, Huang X, Gou S, Zhang S, Gou Y, Zhang Q, Chen H, Sun L, Chen M, Liu D: Ketogenic diet reshapes cancer metabolism through lysine β-hydroxybutyrylation. Nature Metabolism 2024:1–24. [DOI] [PubMed]
- 160.Luengo A, Li Z, Gui DY, Sullivan LB, Zagorulya M, Do BT, Ferreira R, Naamati A, Ali A, Lewis CA, et al. Increased demand for NAD(+) relative to ATP drives aerobic glycolysis. Mol Cell. 2021;81(4):691–707 e696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest. 2013;123(9):3678–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Smith KA, Hendricks BK, DiDomenico JD, Conway BN, Smith TL, Azadi A, Fonkem E. Ketogenic Metabolic Therapy for Glioma Cureus. 2022;14(6): e26457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.McDonald TJ, Cervenka MC: Ketogenic diet therapies for seizures and status epilepticus. In: Seminars in neurology: 2020: Thieme Medical Publishers, Inc. 333 Seventh Avenue, 18th Floor, New York, NY …; 2020: 719–729. [DOI] [PMC free article] [PubMed]
- 164.Scannell JW, Blanckley A, Boldon H, Warrington B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov. 2012;11(3):191–200. [DOI] [PubMed] [Google Scholar]
- 165.Emmerich CH, Gamboa LM, Hofmann MCJ, Bonin-Andresen M, Arbach O, Schendel P, Gerlach B, Hempel K, Bespalov A, Dirnagl U, et al. Improving target assessment in biomedical research: the GOT-IT recommendations. Nat Rev Drug Discov. 2021;20(1):64–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Scheck AC, Abdelwahab MG, Fenton K, Stafford P. The ketogenic diet for the treatment of glioma: Insights from genetic profiling. Epilepsy Res. 2012;100:327–37. [DOI] [PubMed] [Google Scholar]
- 167.Lussier DM, Woolf EC, Johnson JL, Brooks KS, Blattman JN, Scheck AC. Enhanced immunity in a mouse model of malignant glioma is mediated by a therapeutic ketogenic diet. BMC Cancer. 2016;16:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Scheck AC, Syed N: Ketogenic Diet as an Adjunctuive Therapy for Malignant Brain Cancer. In: Ketogenic Diet and Metabolic Therapies: Expanded Roles in Health and Disease 2nd Edition. edn. Edited by Masino SA, Boison D, D'Agostino DP, Kossoff EH, Rho JM. New York: Oxford University Press; 2022: 125–153.
- 169.Maeyama M, Tanaka K, Nishihara M, Irino Y, Shinohara M, Nagashima H, Tanaka H, Nakamizo S, Hashiguchi M, Fujita Y, et al. Metabolic changes and anti-tumor effects of a ketogenic diet combined with anti-angiogenic therapy in a glioblastoma mouse model. Sci Rep. 2021;11(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ferrere G, Tidjani Alou M, Liu P, Goubet AG, Fidelle M, Kepp O, Durand S, Iebba V, Fluckiger A, Daillere R et al: Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight 2021, 6(2). [DOI] [PMC free article] [PubMed]
- 171.Miller VJ, Villamena FA, Volek JS. Nutritional Ketosis and Mitohormesis: Potential Implications for Mitochondrial Function and Human Health. J Nutr Metab. 2018;2018:5157645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Talib WH, Mahmod AI, Kamal A, Rashid HM, Alashqar AMD, Khater S, Jamal D, Waly M. Ketogenic Diet in Cancer Prevention and Therapy: Molecular Targets and Therapeutic Opportunities. Curr Issues Mol Biol. 2021;43(2):558–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Romer M, Dorfler J, Huebner J. The use of ketogenic diets in cancer patients: a systematic review. Clin Exp Med. 2021;21(4):501–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Meidenbauer JJ, Mukherjee P, Seyfried TN. The glucose ketone index calculator: a simple tool to monitor therapeutic efficacy for metabolic management of brain cancer. Nutr Metab (Lond). 2015;12(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Evangeliou AE, Spilioti MG, Vassilakou D, Goutsaridou F, Seyfried TN. Restricted Ketogenic Diet Therapy for Primary Lung Cancer With Metastasis to the Brain: A Case Report. Cureus. 2022;14(8): e27603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Barroso I, McCarthy MI. The Genetic Basis of Metabolic Disease. Cell. 2019;177(1):146–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M, et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell. 2015;163(5):1079–94. [DOI] [PubMed] [Google Scholar]
- 178.Westman EC, Yancy WS Jr, Humphreys M. Dietary treatment of diabetes mellitus in the pre-insulin era (1914–1922). Perspect Biol Med. 2006;49(1):77–83. [DOI] [PubMed] [Google Scholar]
- 179.Freeman JM, Kossoff EH. Ketosis and the ketogenic diet, 2010: advances in treating epilepsy and other disorders. Adv Pediatr. 2010;57(1):315–29. [DOI] [PubMed] [Google Scholar]
- 180.Roehl K, Falco-Walter J, Ouyang B, Balabanov A. Modified ketogenic diets in adults with refractory epilepsy: Efficacious improvements in seizure frequency, seizure severity, and quality of life. Epilepsy Behav. 2019;93:113–8. [DOI] [PubMed] [Google Scholar]
- 181.Scholl-Bürgi S, Höller A, Pichler K, Michel M, Haberlandt E, Karall D. Ketogenic diets in patients with inherited metabolic disorders. J Inherit Metab Dis. 2015;38:765–73. [DOI] [PubMed] [Google Scholar]
- 182.Paoli A, Rubini A, Volek JS, Grimaldi KA. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67(8):789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sarnyai Z, Palmer CM. Ketogenic Therapy in Serious Mental Illness: Emerging Evidence. Int J Neuropsychopharmacol. 2020;23(7):434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Klement RJ. Wilhelm Brünings’ forgotten contribution to the metabolic treatment of cancer utilizing hypoglycemia and a very low carbohydrate (ketogenic) diet. Journal of traditional Complementary Medicine. 2019;9(3):192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Morris AA. Cerebral ketone body metabolism. J Inherit Metab Dis. 2005;28(2):109–21. [DOI] [PubMed] [Google Scholar]
- 186.Robinson AM, Williamson DH. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev. 1980;60(1):143–87. [DOI] [PubMed] [Google Scholar]
- 187.Musa-Veloso K, Likhodii SS, Cunnane SC. Breath acetone is a reliable indicator of ketosis in adults consuming ketogenic meals. Am J Clin Nutr. 2002;76(1):65–70. [DOI] [PubMed] [Google Scholar]
- 188.Krebs H, Williamson D, Bates MW, Page MA, Hawkins R. The role of ketone bodies in caloric homeostasis. Adv Enzyme Regul. 1971;9:387–409. [Google Scholar]
- 189.Rui L. Energy metabolism in the liver. Compr Physiol. 2014;4(1):177–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.White H, Venkatesh B. Clinical review: ketones and brain injury. Crit Care. 2011;15(2):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.LaManna JC, Salem N, Puchowicz M, Erokwu B, Koppaka S, Flask C, Lee Z: Ketones suppress brain glucose consumption. In: Oxygen Transport to Tissue XXX. edn.: Springer; 2009: 301–306. [DOI] [PMC free article] [PubMed]
- 192.Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF Jr. Brain metabolism during fasting. J Clin Invest. 1967;46(10):1589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. 1999;15(6):412–26. [DOI] [PubMed] [Google Scholar]
- 194.Fine EJ, Feinman RD. Insulin, carbohydrate restriction, metabolic syndrome and cancer. Expert Rev Endocrinol Metab. 2015;10(1):15–24. [DOI] [PubMed] [Google Scholar]
- 195.Cooper ID, Brookler KH, Kyriakidou Y, Elliott BT, Crofts CAP. Metabolic Phenotypes and Step by Step Evolution of Type 2 Diabetes: A New Paradigm. Biomedicines. 2021;9(7):800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Voss M, Wagner M, von Mettenheim N, Harter PN, Wenger KJ, Franz K, Bojunga J, Vetter M, Gerlach R, Glatzel M, et al. ERGO2: A Prospective, Randomized Trial of Calorie-Restricted Ketogenic Diet and Fasting in Addition to Reirradiation for Malignant Glioma. Int J Radiat Oncol Biol Phys. 2020;108(4):987–95. [DOI] [PubMed] [Google Scholar]
- 197.Egashira R, Matsunaga M, Miyake A, Hotta S, Nagai N, Yamaguchi C, Takeuchi M, Moriguchi M, Tonari S, Nakano M, et al. Long-Term Effects of a Ketogenic Diet for Cancer. Nutrients. 2023;15(10):2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Purow B. For glioma, a sweet side to diabetes. Neuro Oncol. 2016;18(3):306–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Noch EK, Palma LN, Yim I, Bullen N, Qiu Y, Ravichandran H, Kim J, Rendeiro A, Davis MB, Elemento O, et al. Insulin feedback is a targetable resistance mechanism of PI3K inhibition in glioblastoma. Neuro Oncol. 2023;25(12):2165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yusuf S, Aretz P, Nickel AC, Westhoff P, Sharma A, Qin N, Remke M, Steiger HJ, Hanggi D, Liu H, et al. WNT/beta-Catenin-Mediated Resistance to Glucose Deprivation in Glioblastoma Stem-like Cells. Cancers (Basel). 2022;14(13):3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yamaguchi I, Yoshimura SH, Katoh H. High cell density increases glioblastoma cell viability under glucose deprivation via degradation of the cystine/glutamate transporter xCT (SLC7A11). J Biol Chem. 2020;295(20):6936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bielecka-Wajdman AM, Ludyga T, Smyk D, Smyk W, Mularska M, Swiderek P, Majewski W, Mullins CS, Linnebacher M, Obuchowicz E. Glucose Influences the Response of Glioblastoma Cells to Temozolomide and Dexamethasone. Cancer Control. 2022;29:10732748221075468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cooper ID, Kyriakidou Y, Edwards K, Petagine L, Seyfried TN, Duraj T, Soto-Mota A, Scarborough A, Jacome SL, Brookler K, et al. Ketosis Suppression and Ageing (KetoSAge): The Effects of Suppressing Ketosis in Long Term Keto-Adapted Non-Athletic Females. Int J Mol Sci. 2023;24(21):15621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Urbain P, Bertz H. Monitoring for compliance with a ketogenic diet: what is the best time of day to test for urinary ketosis? Nutrition metabolism. 2016;13(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Balasse EO, Fery F. Ketone body production and disposal: effects of fasting, diabetes, and exercise. Diabetes Metab Rev. 1989;5(3):247–70. [DOI] [PubMed] [Google Scholar]
- 206.Owen OE, Hanson RW: Ketone Bodies. In: Encyclopedia of Endocrine Diseases. edn. Edited by Martini L. New York: Elsevier; 2004: 125–136.
- 207.Kossoff EH, Zupec-Kania BA, Auvin S, Ballaban-Gil KR, Christina Bergqvist AG, Blackford R, Buchhalter JR, Caraballo RH, Cross JH, Dahlin MG, et al. Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open. 2018;3(2):175–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cervenka MC, Wood S, Bagary M, Balabanov A, Bercovici E, Brown MG, Devinsky O, Di Lorenzo C, Doherty CP, Felton E, et al. International Recommendations for the Management of Adults Treated With Ketogenic Diet Therapies. Neurol Clin Pract. 2021;11(5):385–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Watanabe M, Tuccinardi D, Ernesti I, Basciani S, Mariani S, Genco A, Manfrini S, Lubrano C, Gnessi L. Scientific evidence underlying contraindications to the ketogenic diet: An update. Obes Rev. 2020;21(10): e13053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Phinney SD, Volek JS: The art and science of low carbohydrate performance. In.: Beyond Obesity LLC, Miami, FL, USA; 2011.
- 211.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241–7. [DOI] [PubMed] [Google Scholar]
- 212.Barcellos PS, Borges N, Torres DPM. Resting energy expenditure in cancer patients: Agreement between predictive equations and indirect calorimetry. Clin Nutr ESPEN. 2021;42:286–91. [DOI] [PubMed] [Google Scholar]
- 213.McDonald TJW, Cervenka MC. Ketogenic Diets for Adults With Highly Refractory Epilepsy. Epilepsy currents / American Epilepsy Society. 2017;17(6):346–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Martin-McGill KJ, Bresnahan R, Levy RG, Cooper PN. Ketogenic diets for drug-resistant epilepsy. Cochrane Database Syst Rev. 2020;6(6):CD001903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hagstrom H, Hagfors LN, Tellstrom A, Hedelin R, Lindmark K. Low carbohydrate high fat-diet in real life assessed by diet history interviews. Nutr J. 2023;22(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Fearon K, Hutterer E, Isenring E, Kaasa S, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11–48. [DOI] [PubMed] [Google Scholar]
- 217.Capitao C, Coutinho D, Neves PM, Capelas ML, Pimenta NM, Santos T, Makitie A, Ravasco P. Protein intake and muscle mass maintenance in patients with cancer types with high prevalence of sarcopenia: a systematic review. Support Care Cancer. 2022;30(4):3007–15. [DOI] [PubMed] [Google Scholar]
- 218.Tóth C, Dabóczi A, Chanrai M, Schimmer M, Clemens Z: 38-Month long progression-free and symptom-free survival of a patient with recurrent glioblastoma multiforme: a case report of the paleolithic ketogenic diet (Pkd) used as a stand-alone treatment after failed standard oncotherapy. 2019.
- 219.O’Hearn AJCOiE, Diabetes, Obesity: Can a carnivore diet provide all essential nutrients? 2020, 27(5):312–316. [DOI] [PubMed]
- 220.Li J, Zhang H, Dai Z. Cancer Treatment With the Ketogenic Diet: A Systematic Review and Meta-analysis of Animal Studies. Front Nutr. 2021;8: 594408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Weber DD, Aminzadeh-Gohari S, Tulipan J, Catalano L, Feichtinger RG, Kofler B. Ketogenic diet in the treatment of cancer–where do we stand? Molecular metabolism. 2020;33:102–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Porper K, Shpatz Y, Plotkin L, Pechthold RG, Talianski A, Champ CE, Furman O, Shimoni-Sebag A, Symon Z, Amit U, et al. A Phase I clinical trial of dose-escalated metabolic therapy combined with concomitant radiation therapy in high-grade glioma. J Neurooncol. 2021;153(3):487–96. [DOI] [PubMed] [Google Scholar]
- 223.Lien EC, Westermark AM, Zhang Y, Yuan C, Li Z, Lau AN, Sapp KM, Wolpin BM, Vander Heiden MG. Low glycaemic diets alter lipid metabolism to influence tumour growth. Nature. 2021;599(7884):302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lv M, Zhu X, Wang H, Wang F, Guan W. Roles of caloric restriction, ketogenic diet and intermittent fasting during initiation, progression and metastasis of cancer in animal models: a systematic review and meta-analysis. PLoS ONE. 2014;9(12): e115147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhou W, Mukherjee P, Kiebish MA, Markis WT. Mantis JG. Seyfried TNJN, metabolism: The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. 2007;4(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lu Y, Tao F, Zhou MT, Tang KF. The signaling pathways that mediate the anti-cancer effects of caloric restriction. Pharmacol Res. 2019;141:512–20. [DOI] [PubMed] [Google Scholar]
- 227.Meynet O, Ricci JE. Caloric restriction and cancer: molecular mechanisms and clinical implications. Trends Mol Med. 2014;20(8):419–27. [DOI] [PubMed] [Google Scholar]
- 228.Nencioni A, Caffa I, Cortellino S. Longo VDJNRC: Fasting and cancer: molecular mechanisms and clinical application. 2018;18(11):707–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Roekenes J, Martins C. Ketogenic diets and appetite regulation. Curr Opin Clin Nutr Metab Care. 2021;24(4):359–63. [DOI] [PubMed] [Google Scholar]
- 230.Tisdale MJ, Brennan RA, Fearon KC. Reduction of weight loss and tumour size in a cachexia model by a high fat diet. Br J Cancer. 1987;56(1):39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Klement RJ, Champ CE, Kämmerer U, Koebrunner PS, Krage K, Schäfer G, Weigel M, Sweeney RA. Impact of a ketogenic diet intervention during radiotherapy on body composition: III—final results of the KETOCOMP study for breast cancer patients. Breast Cancer Res. 2020;22:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Klement RJ, Koebrunner PS, Meyer D, Kanzler S, Sweeney RA: Impact of a ketogenic diet intervention during radiotherapy on body composition: IV. Final results of the KETOCOMP study for rectal cancer patients. Clin Nutr 2021, 40(7):4674–4684. [DOI] [PubMed]
- 233.Shukla SK, Gebregiworgis T, Purohit V, Chaika NV, Gunda V, Radhakrishnan P, Mehla K, Pipinos II, Powers R, Yu F, et al. Metabolic reprogramming induced by ketone bodies diminishes pancreatic cancer cachexia. Cancer Metab. 2014;2(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Nakamura K, Tonouchi H, Sasayama A, Ashida K. A Ketogenic Formula Prevents Tumor Progression and Cancer Cachexia by Attenuating Systemic Inflammation in Colon 26 Tumor-Bearing Mice. Nutrients. 2018;10(2):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Koutnik AP, D’Agostino DP, Egan B. Anticatabolic Effects of Ketone Bodies in Skeletal Muscle. Trends Endocrinol Metab. 2019;30(4):227–9. [DOI] [PubMed] [Google Scholar]
- 236.Koutnik AP, Poff AM, Ward NP, DeBlasi JM, Soliven MA, Romero MA, Roberson PA, Fox CD, Roberts MD, D’Agostino DP. Ketone Bodies Attenuate Wasting in Models of Atrophy. J Cachexia Sarcopenia Muscle. 2020;11(4):973–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Thomsen HH, Rittig N, Johannsen M, Moller AB, Jorgensen JO, Jessen N, Moller N. Effects of 3-hydroxybutyrate and free fatty acids on muscle protein kinetics and signaling during LPS-induced inflammation in humans: anticatabolic impact of ketone bodies. Am J Clin Nutr. 2018;108(4):857–67. [DOI] [PubMed] [Google Scholar]
- 238.Youm Y-H, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D’agostino D, Planavsky N, Lupfer C, Kanneganti TD. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat Med. 2015;21(3):263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Fromentin C, Tome D, Nau F, Flet L, Luengo C, Azzout-Marniche D, Sanders P, Fromentin G, Gaudichon C. Dietary proteins contribute little to glucose production, even under optimal gluconeogenic conditions in healthy humans. Diabetes. 2013;62(5):1435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Sharma R, Tiwari S. Renal gluconeogenesis in insulin resistance: A culprit for hyperglycemia in diabetes. World J Diabetes. 2021;12(5):556–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Pillot B, Soty M, Gautier-Stein A, Zitoun C, Mithieux G. Protein feeding promotes redistribution of endogenous glucose production to the kidney and potentiates its suppression by insulin. Endocrinology. 2009;150(2):616–24. [DOI] [PubMed] [Google Scholar]
- 242.Hudson JL, Wang Y, Bergia Iii RE, Campbell WW. Protein Intake Greater than the RDA Differentially Influences Whole-Body Lean Mass Responses to Purposeful Catabolic and Anabolic Stressors: A Systematic Review and Meta-analysis. Adv Nutr. 2020;11(3):548–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Trumbo P, Schlicker S, Yates A, Poos M: Food and Nutrition Board of the Institute of Medicine, The National Academies. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc 2002, 102(11):1621–1630. [DOI] [PubMed]
- 244.Richter M, Baerlocher K, Bauer JM, Elmadfa I, Heseker H, Leschik-Bonnet E, Stangl G, Volkert D, Stehle P. on behalf of the German Nutrition S: Revised Reference Values for the Intake of Protein. Ann Nutr Metab. 2019;74(3):242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Bergstrom J, Furst P, Noree LO, Vinnars E. Intracellular free amino acid concentration in human muscle tissue. J Appl Physiol. 1974;36(6):693–7. [DOI] [PubMed] [Google Scholar]
- 246.Cruzat V, Macedo Rogero M, Noel Keane K, Curi R, Newsholme P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients. 2018;10(11):1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Gleeson M, Blannin AK, Walsh NP, Bishop NC, Clark AM. Effect of low- and high-carbohydrate diets on the plasma glutamine and circulating leukocyte responses to exercise. Int J Sport Nutr. 1998;8(1):49–59. [DOI] [PubMed] [Google Scholar]
- 248.Aoki TT, Muller WA, Cahill GF Jr. Hormonal regulation of glutamine metabolism in fasting man. Adv Enzyme Regul. 1972;10:145–51. [DOI] [PubMed] [Google Scholar]
- 249.Pedersen KS, Gatto F, Zerahn B, Nielsen J, Pedersen BK, Hojman P, Gehl J: Exercise-Mediated Lowering of Glutamine Availability Suppresses Tumor Growth and Attenuates Muscle Wasting. iScience 2020, 23(4):100978. [DOI] [PMC free article] [PubMed]
- 250.Walsh NP, Blannin AK, Robson PJ, Gleeson M. Glutamine, exercise and immune function Links and possible mechanisms. Sports Med. 1998;26(3):177–91. [DOI] [PubMed] [Google Scholar]
- 251.St-Pierre V, Vandenberghe C, Lowry CM, Fortier M, Castellano CA, Wagner R, Cunnane SC. Plasma Ketone and Medium Chain Fatty Acid Response in Humans Consuming Different Medium Chain Triglycerides During a Metabolic Study Day. Front Nutr. 2019;6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Altinoz MA, Ozpinar A, Seyfried TN. Caprylic (Octanoic) Acid as a Potential Fatty Acid Chemotherapeutic for Glioblastoma. Prostaglandins Leukot Essent Fatty Acids. 2020;159: 102142. [DOI] [PubMed] [Google Scholar]
- 253.Liu YM. Medium-chain triglyceride (MCT) ketogenic therapy. Epilepsia. 2008;49(Suppl 8):33–6. [DOI] [PubMed] [Google Scholar]
- 254.Nebeling LC, Lerner E. Implementing a ketogenic diet based on medium-chain triglyceride oil in pediatric patients with cancer. J Am Diet Assoc. 1995;95(6):693–7. [DOI] [PubMed] [Google Scholar]
- 255.Martin-McGill KJ, Marson AG, Tudur Smith C, Young B, Mills SJ, Cherry MG, Jenkinson MD. Ketogenic diets as an adjuvant therapy for glioblastoma (KEATING): a randomized, mixed methods, feasibility study. J Neurooncol. 2020;147(1):213–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.van der Louw EJ, Olieman JF, van den Bemt PM, Bromberg JE, Oomen-de Hoop E, Neuteboom RF, Catsman-Berrevoets CE, Vincent AJ. Ketogenic diet treatment as adjuvant to standard treatment of glioblastoma multiforme: a feasibility and safety study. Therapeutic advances in medical oncology. 2019;11:1758835919853958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Khodabakhshi A, Akbari ME, Mirzaei HR, Mehrad-Majd H, Kalamian M, Davoodi SH. Feasibility, Safety, and Beneficial Effects of MCT-Based Ketogenic Diet for Breast Cancer Treatment: A Randomized Controlled Trial Study. Nutr Cancer. 2020;72(4):627–34. [DOI] [PubMed] [Google Scholar]
- 258.Lindeberg S: Modern Human Physiology with Respect to Evolutionary Adaptations that Relate to Diet in the Past. Evolution of Hominin Diets 2009:43–57.
- 259.Gibson RS, Raboy V, King JC. Implications of phytate in plant-based foods for iron and zinc bioavailability, setting dietary requirements, and formulating programs and policies. Nutr Rev. 2018;76(11):793–804. [DOI] [PubMed] [Google Scholar]
- 260.Norton SK: Lost seasonality and overconsumption of plants: Risking oxalate toxicity. Journal of Evolution Health: A joint publication of the Ancestral Health Society the Society for Evolutionary Medicine Health 2017, 2(3).
- 261.Nath H, Samtiya M, Dhewa T. Beneficial attributes and adverse effects of major plant-based foods anti-nutrients on health: A review. Human Nutrition & Metabolism. 2022;28: 200147. [Google Scholar]
- 262.Poff AM, Koutnik AP, Egan B. Nutritional Ketosis with Ketogenic Diets or Exogenous Ketones: Features, Convergence, and Divergence. Curr Sports Med Rep. 2020;19(7):251–9. [DOI] [PubMed] [Google Scholar]
- 263.Nelson AB, Queathem ED, Puchalska P, Crawford PA. Metabolic Messengers: ketone bodies. Nat Metab. 2023;5(12):2062–74. [DOI] [PubMed] [Google Scholar]
- 264.Dmitrieva-Posocco O, Wong AC, Lundgren P, Golos AM, Descamps HC, Dohnalova L, Cramer Z, Tian Y, Yueh B, Eskiocak O, et al. beta-Hydroxybutyrate suppresses colorectal cancer. Nature. 2022;605(7908):160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Poff A, Koutnik AP, Egan KM, Sahebjam S, D’Agostino D, Kumar NB: Targeting the Warburg effect for cancer treatment: Ketogenic diets for management of glioma. In: Seminars in Cancer Biology: 2019: Elsevier; 2019: 135–148. [DOI] [PMC free article] [PubMed]
- 266.Poff AM, Ari C, Arnold P, Seyfried TN, D’Agostino DP. Ketone supplementation decreases tumor cell viability and prolongs survival of mice with metastatic cancer. Int J Cancer. 2014;135(7):1711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Vallejo FA, Shah SS, de Cordoba N, Walters WM, Prince J, Khatib Z, Komotar RJ, Vanni S, Graham RM. The contribution of ketone bodies to glycolytic inhibition for the treatment of adult and pediatric glioblastoma. J Neurooncol. 2020;147(2):317–26. [DOI] [PubMed] [Google Scholar]
- 268.Poff AM, Ward N, Seyfried TN, Arnold P, D’Agostino DP. Non-Toxic Metabolic Management of Metastatic Cancer in VM Mice: Novel Combination of Ketogenic Diet, Ketone Supplementation, and Hyperbaric Oxygen Therapy. PLoS ONE. 2015;10(6): e0127407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.White H, Heffernan AJ, Worrall S, Grunsfeld A, Thomas M: A Systematic Review of Intravenous β-Hydroxybutyrate Use in Humans–A Promising Future Therapy? Front Med 2021:1611. [DOI] [PMC free article] [PubMed]
- 270.Cahill GF Jr. Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. [DOI] [PubMed] [Google Scholar]
- 271.Ari C, Murdun C, Koutnik AP, Goldhagen CR, Rogers C, Park C, Bharwani S, Diamond DM, Kindy MS, D’Agostino DP, et al. Exogenous Ketones Lower Blood Glucose Level in Rested and Exercised Rodent Models. Nutrients. 2019;11(10):2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Soto-Mota A, Norwitz NG, Evans RD, Clarke K. Exogenous d-β-hydroxybutyrate lowers blood glucose in part by decreasing the availability of L-alanine for gluconeogenesis. Endocrinology, Diabetes Metabolism. 2022;5(1): e00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Kesl SL, Poff AM, Ward NP, Fiorelli TN, Ari C, Van Putten AJ, Sherwood JW, Arnold P, D’Agostino DP. Effects of exogenous ketone supplementation on blood ketone, glucose, triglyceride, and lipoprotein levels in Sprague-Dawley rats. Nutr Metab (Lond). 2016;13(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Myette-Cote E, Neudorf H, Rafiei H, Clarke K, Little J. Prior ingestion of exogenous ketone monoester attenuates the glycaemic response to an oral glucose tolerance test in healthy young individuals. J Physiol. 2018:596(8):1385–95. [DOI] [PMC free article] [PubMed]
- 275.Myette-Cote E, Caldwell HG, Ainslie PN, Clarke K, Little JP. A ketone monoester drink reduces the glycemic response to an oral glucose challenge in individuals with obesity: a randomized trial. Am J Clin Nutr. 2019;110(6):1491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Poff AM, Ari C, Seyfried TN, D’Agostino DP. The ketogenic diet and hyperbaric oxygen therapy prolong survival in mice with systemic metastatic cancer. PLoS ONE. 2013;8(6): e65522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.D’Agostino DP, Pilla R, Held HE, Landon CS, Puchowicz M, Brunengraber H, Ari C, Arnold P, Dean JB. Therapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats. Am J Physiol. 2013;304(10):R829–836. [DOI] [PubMed] [Google Scholar]
- 278.Aminzadeh-Gohari S, Feichtinger RG, Vidali S, Locker F, Rutherford T, O’Donnel M, Stoger-Kleiber A, Mayr JA, Sperl W, Kofler B. A ketogenic diet supplemented with medium-chain triglycerides enhances the anti-tumor and anti-angiogenic efficacy of chemotherapy on neuroblastoma xenografts in a CD1-nu mouse model. Oncotarget. 2017;8(39):64728–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Iyikesici MS. Long-Term Survival Outcomes of Metabolically Supported Chemotherapy with Gemcitabine-Based or FOLFIRINOX Regimen Combined with Ketogenic Diet, Hyperthermia, and Hyperbaric Oxygen Therapy in Metastatic Pancreatic Cancer. Complement Med Res. 2020;27(1):31–9. [DOI] [PubMed] [Google Scholar]
- 280.Curtis W, Kemper M, Miller A, Pawlosky R, King MT, Veech R: Mitigation of damage from reactive oxygen species and ionizing radiation by ketone body esters: Oxford University Press, New York, NY; 2017.
- 281.Roehl K, Sewak SL. Practice Paper of the Academy of Nutrition and Dietetics: Classic and Modified Ketogenic Diets for Treatment of Epilepsy. J Acad Nutr Diet. 2017;117(8):1279–92. [DOI] [PubMed] [Google Scholar]
- 282.Johnstone AM, Horgan GW, Murison SD, Bremner DM, Lobley GE. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am J Clin Nutr. 2008;87(1):44–55. [DOI] [PubMed] [Google Scholar]
- 283.Tóth C, Clemens Z. Treatment of rectal cancer with the paleolithic ketogenic diet: a 24-months follow-up. Am J Med Case Reports. 2017;5(8):205–16. [Google Scholar]
- 284.Paoli A, Cenci L, Grimaldi KA. Effect of ketogenic Mediterranean diet with phytoextracts and low carbohydrates/high-protein meals on weight, cardiovascular risk factors, body composition and diet compliance in Italian council employees. Nutr J. 2011;10(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ferraris C, Guglielmetti M, Neri LCL, Allehdan S, Mohsin Albasara JM, Fareed Alawadhi HH, Trentani C, Perna S, Tagliabue A. A Review of Ketogenic Dietary Therapies for Epilepsy and Neurological Diseases: A Proposal to Implement an Adapted Model to Include Healthy Mediterranean Products. Foods. 2023;12(9):1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kirkpatrick CF, Bolick JP, Kris-Etherton PM, Sikand G, Aspry KE, Soffer DE, Willard KE, Maki KC: Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: A scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J Clin Lipidol 2019, 13(5):689–711 e681. [DOI] [PubMed]
- 287.Jenkins DJ, Wong JM, Kendall CW, Esfahani A, Ng VW, Leong TC, Faulkner DA, Vidgen E, Paul G, Mukherjea R, et al. Effect of a 6-month vegan low-carbohydrate ('Eco-Atkins’) diet on cardiovascular risk factors and body weight in hyperlipidaemic adults: a randomised controlled trial. BMJ Open. 2014;4(2): e003505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Shilpa J, Mohan V. Ketogenic diets: Boon or bane? Indian J Med Res. 2018;148(3):251–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Brandhorst S. Fasting and fasting-mimicking diets for chemotherapy augmentation. Geroscience. 2021;43(3):1201–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Taylor SR, Falcone JN, Cantley LC, Goncalves MD. Developing dietary interventions as therapy for cancer. Nat Rev Cancer. 2022;22(8):452–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Jong CJ, Sandal P, Schaffer SW. The Role of Taurine in Mitochondria Health: More Than Just an Antioxidant. Molecules. 2021;26(16):4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Groesbeck DK, Bluml RM, Kossoff EH. Long-term use of the ketogenic diet in the treatment of epilepsy. Dev Med Child Neurol. 2006;48(12):978–81. [DOI] [PubMed] [Google Scholar]
- 293.Batch JT, Lamsal SP, Adkins M, Sultan S, Ramirez MN. Advantages and Disadvantages of the Ketogenic Diet: A Review Article. Cureus. 2020;12(8): e9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Lindefeldt M, Eng A, Darban H, Bjerkner A, Zetterstrom CK, Allander T, Andersson B, Borenstein E, Dahlin M, Prast-Nielsen S. The ketogenic diet influences taxonomic and functional composition of the gut microbiota in children with severe epilepsy. NPJ Biofilms Microbiomes. 2019;5(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.McNally MA, Pyzik PL, Rubenstein JE, Hamdy RF, Kossoff EH. Empiric use of potassium citrate reduces kidney-stone incidence with the ketogenic diet. Pediatrics. 2009;124(2):e300–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.De Vivo DC, Bohan TP, Coulter DL, Dreifuss FE, Greenwood RS, Nordli DR Jr, Shields WD, Stafstrom CE, Tein I. L-carnitine supplementation in childhood epilepsy: current perspectives. Epilepsia. 1998;39(11):1216–25. [DOI] [PubMed] [Google Scholar]
- 297.Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, Peters C, Zhyzhneuskaya S, Al-Mrabeh A, Hollingsworth KG, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391(10120):541–51. [DOI] [PubMed] [Google Scholar]
- 298.Yeboah J, Young R, McClelland RL, Delaney JC, Polonsky TS, Dawood FZ, Blaha MJ, Miedema MD, Sibley CT, Carr JJ, et al. Utility of Nontraditional Risk Markers in Atherosclerotic Cardiovascular Disease Risk Assessment. J Am Coll Cardiol. 2016;67(2):139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Hallberg SJ, McKenzie AL, Williams PT, Bhanpuri NH, Peters AL, Campbell WW, Hazbun TL, Volk BM, McCarter JP, Phinney SD. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Therapy. 2018;9(2):583–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Yetley EA, DeMets DL, Harlan WR Jr. Surrogate disease markers as substitutes for chronic disease outcomes in studies of diet and chronic disease relations. Am J Clin Nutr. 2017;106(5):1175–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Klein KR, Walker CP, McFerren AL, Huffman H, Frohlich F, Buse JB. Carbohydrate Intake Prior to Oral Glucose Tolerance Testing. J Endocr Soc. 2021;5(5):bvab049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Cao J, Lei S, Wang X, Cheng S. The Effect of a Ketogenic Low-Carbohydrate, High-Fat Diet on Aerobic Capacity and Exercise Performance in Endurance Athletes: A Systematic Review and Meta-Analysis. Nutrients. 2021;13(8):2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Rodbard D. Continuous Glucose Monitoring: A Review of Recent Studies Demonstrating Improved Glycemic Outcomes. Diabetes Technol Ther. 2017;19(S3):S25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Yost O, DeJonckheere M, Stonebraker S, Ling G, Buis L, Pop-Busui R, Kim N, Mizokami-Stout K, Richardson C. Continuous Glucose Monitoring With Low-Carbohydrate Diet Coaching in Adults With Prediabetes: Mixed Methods Pilot Study. JMIR Diabetes. 2020;5(4): e21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Nguyen KT, Xu NY, Zhang JY, Shang T, Basu A, Bergenstal RM, Castorino K, Chen KY, Kerr D, Koliwad SK, et al. Continuous Ketone Monitoring Consensus Report 2021. J Diabetes Sci Technol. 2022;16(3):689–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.SIBIONICS to Make Debut at the 59th Annual Meeting of the European Association for the Study of Diabetes (EASD). In: PR Newswire Europe. 2023: NA.
- 307.Alva S, Brazg R, Castorino K, Kipnes M, Liljenquist DR, Liu H. Accuracy of the Third Generation of a 14-Day Continuous Glucose Monitoring System. Diabetes Ther. 2023;14(4):767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Alva S, Castorino K, Cho H, Ou J. Feasibility of Continuous Ketone Monitoring in Subcutaneous Tissue Using a Ketone Sensor. J Diabetes Sci Technol. 2021;15(4):768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Toth C, Clemens Z. Halted Progression of Soft Palate Cancer in a Patient Treated with the Paleolithic Ketogenic Diet Alone: A 20-months Follow-up. American Journal of Medical Case Reports. 2016;4(8):288–92. [Google Scholar]
- 310.Yakupova EI, Bocharnikov AD, Plotnikov EY. Effects of Ketogenic Diet on Muscle Metabolism in Health and Disease. Nutrients. 2022;14(18):3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Kalamian M: KETO for CANCER: Ketogenic Metabolic Therapy as a Targeted Nutritional Strategy. White River Junction, VT: Chelsea Green; 2017.
- 312.van Vliet S, Burd NA, van Loon LJ. The skeletal muscle anabolic response to plant-versus animal-based protein consumption. J Nutr. 2015;145(9):1981–91. [DOI] [PubMed] [Google Scholar]
- 313.Shin E, Kang H, Lee H, Lee S, Jeon J, Seong K, Youn H, Youn B: Exosomal Plasminogen Activator Inhibitor-1 Induces Ionizing Radiation-Adaptive Glioblastoma Cachexia. Cells 2022, 11(19). [DOI] [PMC free article] [PubMed]
- 314.Sizoo EM, Braam L, Postma TJ, Pasman HR, Heimans JJ, Klein M, Reijneveld JC, Taphoorn MJ. Symptoms and problems in the end-of-life phase of high-grade glioma patients. Neuro Oncol. 2010;12(11):1162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Furtner J, Genbrugge E, Gorlia T, Bendszus M, Nowosielski M, Golfinopoulos V, Weller M, Van Den Bent MJ, Wick W, Preusser M. Temporal muscle thickness is an independent prognostic marker in patients with progressive glioblastoma: translational imaging analysis of the EORTC 26101 trial. Neuro Oncol. 2019;21(12):1587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Cui P, Shao W, Huang C, Wu CJ, Jiang B, Lin D. Metabolic derangements of skeletal muscle from a murine model of glioma cachexia. Skelet Muscle. 2019;9(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Klement RJ, Sweeney RA: Impact of a ketogenic diet intervention during radiotherapy on body composition: I. Initial clinical experience with six prospectively studied patients. BMC Res Notes 2016, 9:143. [DOI] [PMC free article] [PubMed]
- 318.Tomasin R, Martin ACBM, Cominetti MR. Metastasis and cachexia: alongside in clinics, but not so in animal models. J Cachexia Sarcopenia Muscle. 2019;10(6):1183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Ferrer M, Mourikis N, Davidson EE, Kleeman SO, Zaccaria M, Habel J, Rubino R, Gao Q, Flint TR, Young L: Ketogenic diet promotes tumor ferroptosis but induces relative corticosterone deficiency that accelerates cachexia. Cell Metab 2023. [DOI] [PMC free article] [PubMed]
- 320.Cortez NE, Mackenzie GG. Ketogenic Diets in Pancreatic Cancer and Associated Cachexia: Cellular Mechanisms and Clinical Perspectives. Nutrients. 2021;13(9):3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.van de Worp W, Schols A, Theys J, van Helvoort A, Langen RCJ. Nutritional Interventions in Cancer Cachexia: Evidence and Perspectives From Experimental Models. Front Nutr. 2020;7: 601329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Setiawan T, Sari IN, Wijaya YT, Julianto NM, Muhammad JA, Lee H, Chae JH, Kwon HY. Cancer cachexia: molecular mechanisms and treatment strategies. J Hematol Oncol. 2023;16(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Christiansen AR, Lipshultz LI, Hotaling JM, Pastuszak AW. Selective androgen receptor modulators: the future of androgen therapy? Transl Androl Urol. 2020;9(Suppl 2):S135–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.O’Hearn A. Can a carnivore diet provide all essential nutrients? Current Opinion in Endocrinology, Diabetes Obesity. 2020;27(5):312–6. [DOI] [PubMed] [Google Scholar]
- 325.Churuangsuk C, Griffiths D, Lean MEJ, Combet E. Impacts of carbohydrate-restricted diets on micronutrient intakes and status: A systematic review. Obes Rev. 2019;20(8):1132–47. [DOI] [PubMed] [Google Scholar]
- 326.Chu DY, Ravelli MN, Faltersack KM, Woods AL, Almane D, Li Z, Sampene E, Felton EA. Hypocarnitinemia and its effect on seizure control in adult patients with intractable epilepsy on the modified Atkins diet. Front Nutr. 2023;10:1304209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Ferrara G, Kim J, Lin S, Hua J, Seto E. A Focused Review of Smartphone Diet-Tracking Apps: Usability, Functionality, Coherence With Behavior Change Theory, and Comparative Validity of Nutrient Intake and Energy Estimates. JMIR Mhealth Uhealth. 2019;7(5): e9232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Chin SO, Keum C, Woo J, Park J, Choi HJ, Woo JT, Rhee SY. Successful weight reduction and maintenance by using a smartphone application in those with overweight and obesity. Sci Rep. 2016;6(1):34563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Shah UA, Iyengar NM. Plant-Based and Ketogenic Diets As Diverging Paths to Address Cancer: A Review. JAMA Oncol. 2022;8(8):1201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Ligibel JA, Bohlke K, May AM, Clinton SK, Demark-Wahnefried W, Gilchrist SC, Irwin ML, Late M, Mansfield S, Marshall TF. Exercise, diet, and weight management during cancer treatment: ASCO guideline. J Clin Oncol. 2022;40(22):2491–507. [DOI] [PubMed] [Google Scholar]
- 331.Mittelman SD. The Role of Diet in Cancer Prevention and Chemotherapy Efficacy. Annu Rev Nutr. 2020;40:273–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Champ CE, Palmer JD, Volek JS, Werner-Wasik M, Andrews DW, Evans JJ, Glass J, Kim L, Shi W. Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J Neurooncol. 2014;117(1):125–31. [DOI] [PubMed] [Google Scholar]
- 333.Seyfried TN, Flores R, Poff AM, D'Agostino DP, Mukherjee P: Metabolic therapy: a new paradigm for managing malignant brain cancer. Cancer Lett 2015, 356(2 Pt A):289–300. [DOI] [PubMed]
- 334.Lane J, Brown NI, Williams S, Plaisance EP, Fontaine KR. Ketogenic Diet for Cancer: Critical Assessment and Research Recommendations. Nutrients. 2021;13(10):3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Yang YF, Mattamel PB, Joseph T, Huang J, Chen Q, Akinwunmi BO, Zhang CJP, Ming WK. Efficacy of Low-Carbohydrate Ketogenic Diet as an Adjuvant Cancer Therapy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2021;13(5):1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Arora N, Pulimamidi S, Yadav H, Jain S, Glover J, Dombrowski K, Hernandez B, Sarma AK, Aneja R. Intermittent fasting with ketogenic diet: A combination approach for management of chronic diseases. Clin Nutr ESPEN. 2023;54:166–74. [DOI] [PubMed] [Google Scholar]
- 337.Phillips MCL, Thotathil Z, Dass PH, Ziad F, Moon BG. Ketogenic metabolic therapy in conjunction with standard treatment for glioblastoma: A case report. Oncol Lett. 2024;27(5):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Tian Q, Price ND, Hood L. Systems cancer medicine: towards realization of predictive, preventive, personalized and participatory (P4) medicine. J Intern Med. 2012;271(2):111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Martinez-Garay C, Djouder N. Dietary interventions and precision nutrition in cancer therapy. Trends Mol Med. 2023;29(7):489–511. [DOI] [PubMed] [Google Scholar]
- 340.Schwartz KA, Noel M, Nikolai M, Chang HT. Investigating the Ketogenic Diet As Treatment for Primary Aggressive Brain Cancer: Challenges and Lessons Learned. Front Nutr. 2018;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Blumenthal DT, Won M, Mehta MP, Curran WJ, Souhami L, Michalski JM, Rogers CL, Corn BW. Short delay in initiation of radiotherapy may not affect outcome of patients with glioblastoma: a secondary analysis from the radiation therapy oncology group database. J Clin Oncol. 2009;27(5):733–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Magrowski L, Nowicka E, Masri O, Tukiendorf A, Tarnawski R, Miszczyk M. The survival impact of significant delays between surgery and radiochemotherapy in glioblastoma patients: A retrospective analysis from a large tertiary center. J Clin Neurosci. 2021;90:39–47. [DOI] [PubMed] [Google Scholar]
- 343.Zur I, Tzuk-Shina T, Guriel M, Eran A, Kaidar-Person O. Survival impact of the time gap between surgery and chemo-radiotherapy in glioblastoma patients. Sci Rep. 2020;10(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Sun MZ, Oh T, Ivan ME, Clark AJ, Safaee M, Sayegh ET, Kaur G, Parsa AT, Bloch O. Survival impact of time to initiation of chemoradiotherapy after resection of newly diagnosed glioblastoma. J Neurosurg. 2015;122(5):1144–50. [DOI] [PubMed] [Google Scholar]
- 345.Katsigiannis S, Krischek B, Barleanu S, Grau S, Galldiks N, Timmer M, Kabbasch C, Goldbrunner R, Stavrinou P. Impact of time to initiation of radiotherapy on survival after resection of newly diagnosed glioblastoma. Radiat Oncol. 2019;14(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Zhang M, Xu F, Ni W, Qi W, Cao W, Xu C, Chen J, Gao Y. Survival impact of delaying postoperative chemoradiotherapy in newly-diagnosed glioblastoma patients. Transl Cancer Res. 2020;9(9):5450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.De Barros A, Attal J, Roques M, Nicolau J, Sol J-C, Cohen-Jonathan-Moyal E, Roux F-E. Impact on survival of early tumor growth between surgery and radiotherapy in patients with de novo glioblastoma. J Neurooncol. 2019;142:489–97. [DOI] [PubMed] [Google Scholar]
- 348.Burnet NG, Jena R, Jefferies SJ, Stenning SP, Kirkby NF. Mathematical modelling of survival of glioblastoma patients suggests a role for radiotherapy dose escalation and predicts poorer outcome after delay to start treatment. Clin Oncol (R Coll Radiol). 2006;18(2):93–103. [DOI] [PubMed] [Google Scholar]
- 349.Mellinghoff IK, van den Bent MJ, Blumenthal DT, Touat M, Peters KB, Clarke J, Mendez J, Yust-Katz S, Welsh L, Mason WP, et al. Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma. N Engl J Med. 2023;389(7):589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Chaichana KL, Zadnik P, Weingart JD, Olivi A, Gallia GL, Blakeley J, Lim M, Brem H, Quinones-Hinojosa A. Multiple resections for patients with glioblastoma: prolonging survival. J Neurosurg. 2013;118(4):812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Burnett T, Mozgunov P, Pallmann P, Villar SS, Wheeler GM, Jaki T. Adding flexibility to clinical trial designs: an example-based guide to the practical use of adaptive designs. BMC Med. 2020;18(1):352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Pitre T, Cheng S, Cusano E, Khan N, Mikhail D, Leung G, Vernooij RWM, Yarnell CJ, Goligher E, Murthy S, et al. Methodology and design of platform trials: a meta-epidemiological study. J Clin Epidemiol. 2023;157:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Most MM, Ershow AG, Clevidence BA. An overview of methodologies, proficiencies, and training resources for controlled feeding studies. J Am Diet Assoc. 2003;103(6):729–35. [DOI] [PubMed] [Google Scholar]
- 354.Cruwys T, Norwood R, Chachay VS, Ntontis E, Sheffield J. “An Important Part of Who I am”: The Predictors of Dietary Adherence among Weight-Loss, Vegetarian, Vegan, Paleo, and Gluten-Free Dietary Groups. Nutrients. 2020;12(4):970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Kiessling L, Radbruch J, Schaube S: The impact of self-selection on performance. 2018.
- 356.Carrera PM, Kantarjian HM, Blinder VS. The financial burden and distress of patients with cancer: Understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J Clin. 2018;68(2):153–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Abrams HR, Durbin S, Huang CX, Johnson SF, Nayak RK, Zahner GJ, Peppercorn J. Financial toxicity in cancer care: origins, impact, and solutions. Transl Behav Med. 2021;11(11):2043–54. [DOI] [PubMed] [Google Scholar]
- 358.Klement RJ, Weigel MM, Sweeney RA. A ketogenic diet consumed during radiotherapy improves several aspects of quality of life and metabolic health in women with breast cancer. Clin Nutr. 2021;40(6):4267–74. [DOI] [PubMed] [Google Scholar]
- 359.Augustus E, Granderson I, Rocke KD. The Impact of a Ketogenic Dietary Intervention on the Quality of Life of Stage II and III Cancer Patients: A Randomized Controlled Trial in the Caribbean. Nutr Cancer. 2021;73(9):1590–600. [DOI] [PubMed] [Google Scholar]
- 360.Tulipan J, Kofler B. Implementation of a Low-Carbohydrate Diet Improves the Quality of Life of Cancer Patients–An Online Survey. Front Nutr. 2021;8: 661253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Kammerer U, Klement RJ, Joos FT, Sutterlin M, Reuss-Borst M. Low Carb and Ketogenic Diets Increase Quality of Life, Physical Performance, Body Composition, and Metabolic Health of Women with Breast Cancer. Nutrients. 2021;13(3):1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Chung VCH, Ho LTF, Leung TH, Wong CHL. Designing delivery models of traditional and complementary medicine services: a review of international experiences. Br Med Bull. 2021;137(1):70–81. [DOI] [PubMed] [Google Scholar]
- 363.Lakomy R, Kazda T, Selingerova I, Poprach A, Pospisil P, Belanova R, Fadrus P, Vybihal V, Smrcka M, Jancalek R, et al. Real-World Evidence in Glioblastoma: Stupp’s Regimen After a Decade. Front Oncol. 2020;10:840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Sheikh S, Radivoyevitch T, Barnholtz-Sloan JS, Vogelbaum M. Long-term trends in glioblastoma survival: implications for historical control groups in clinical trials. Neurooncol Pract. 2020;7(2):158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Schork NJ. Personalized medicine: Time for one-person trials. Nature. 2015;520(7549):609–11. [DOI] [PubMed] [Google Scholar]
- 366.Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, Kiene H, Helfand M, Altman DG, Sox H, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. 2017;89:218–35. [DOI] [PubMed] [Google Scholar]
- 367.Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. The CARE guidelines: consensus-based clinical case reporting guideline development. Global advances in health medicine. 2013;2(5):38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Vernieri C, Fuca G, Ligorio F, Huber V, Vingiani A, Iannelli F, Raimondi A, Rinchai D, Frige G, Belfiore A, et al. Fasting-Mimicking Diet Is Safe and Reshapes Metabolism and Antitumor Immunity in Patients with Cancer. Cancer Discov. 2022;12(1):90–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.de Toledo FW, Grundler F, Bergouignan A, Drinda S, Michalsen A. Safety, health improvement and well-being during a 4 to 21-day fasting period in an observational study including 1422 subjects. PLoS ONE. 2019;14(1): e0209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Kreitzman SN, Coxon AY, Szaz KF. Glycogen storage: illusions of easy weight loss, excessive weight regain, and distortions in estimates of body composition. Am J Clin Nutr. 1992;56(1 Suppl):292S–293S. [DOI] [PubMed] [Google Scholar]
- 371.Dai Z, Zhang H, Wu F, Chen Y, Yang C, Wang H, Sui X, Guo Y, Xin B, Guo Z, et al. Effects of 10-Day Complete Fasting on Physiological Homeostasis, Nutrition and Health Markers in Male Adults. Nutrients. 2022;14(18):3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Ogłodek E, Pilis P. Wiesław: Is Water-Only Fasting Safe? Global Advances in Health Medicine. 2021;10:21649561211031176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Cuthbertson DJ, Steele T, Wilding JP, Halford J, Harrold JA, Hamer M, Karpe F. What have human experimental overfeeding studies taught us about adipose tissue expansion and susceptibility to obesity and metabolic complications? Int J Obes. 2017;41(6):853–65. [DOI] [PubMed] [Google Scholar]
- 374.Fearon KC, Borland W, Preston T, Tisdale MJ, Shenkin A, Calman KC. Cancer cachexia: influence of systemic ketosis on substrate levels and nitrogen metabolism. Am J Clin Nutr. 1988;47(1):42–8. [DOI] [PubMed] [Google Scholar]
- 375.Finnell JS, Saul BC, Goldhamer AC, Myers TR. Is fasting safe? A chart review of adverse events during medically supervised, water-only fasting. BMC complementary alternative medicine. 2018;18(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Valdemarin F, Caffa I, Persia A, Cremonini AL, Ferrando L, Tagliafico L, Tagliafico A, Guijarro A, Carbone F, Ministrini S, et al. Safety and Feasibility of Fasting-Mimicking Diet and Effects on Nutritional Status and Circulating Metabolic and Inflammatory Factors in Cancer Patients Undergoing Active Treatment. Cancers (Basel). 2021;13(16):4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Geurts M, van den Bent MJ. Timing of radiotherapy in newly diagnosed glioblastoma: no need to rush? Neuro Oncol. 2018;20(7):868–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Frenkel M. Refusing treatment. Oncologist. 2013;18(5):634–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Senderovitch H. The Ethical and Legal Dilemma in Terminating the Physician-Patient Relationship. Health Law Can. 2016;36(4):168–73. [PubMed] [Google Scholar]
- 380.Prabhakar A, Quach A, Zhang H, Terrera M, Jackemeyer D, Xian X, Tsow F, Tao N, Forzani ES. Acetone as biomarker for ketosis buildup capability–a study in healthy individuals under combined high fat and starvation diets. Nutr J. 2015;14(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Gibson AA, Eroglu EI, Rooney K, Harper C, McClintock S, Franklin J, Markovic TP, Seimon RV, Sainsbury A. Urine dipsticks are not accurate for detecting mild ketosis during a severely energy restricted diet. Obes Sci Pract. 2020;6(5):544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Dalakleidi KV, Papadelli M, Kapolos I, Papadimitriou K. Applying Image-Based Food-Recognition Systems on Dietary Assessment: A Systematic Review. Adv Nutr. 2022;13(6):2590–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Tahir GA, Loo CK: A comprehensive survey of image-based food recognition and volume estimation methods for dietary assessment. In: Healthcare: 2021: Multidisciplinary Digital Publishing Institute; 2021: 1676. [DOI] [PMC free article] [PubMed]
- 384.Athinarayanan SJ, Adams RN, Hallberg SJ, McKenzie AL, Bhanpuri NH, Campbell WW, Volek JS, Phinney SD, McCarter JP. Long-Term Effects of a Novel Continuous Remote Care Intervention Including Nutritional Ketosis for the Management of Type 2 Diabetes: A 2-Year Non-randomized Clinical Trial. Front Endocrinol (Lausanne). 2019;10:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL: 1. Improving Care and Promoting Health in Populations: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46(Supplement_1):S10-S18. [DOI] [PMC free article] [PubMed]
- 386.Masino SA: Ketogenic diet and metabolic therapies: expanded roles in health and disease: Oxford University Press; 2022.
- 387.Kossoff EH, Zahava Turner R, Cervenka MC, Barron BJ: Ketogenic Diet Therapies for Epilepsy and Other Conditions: Springer Publishing Company; 2020.
- 388.Attina A, Leggeri C, Paroni R, Pivari F, Dei Cas M, Mingione A, Dri M, Marchetti M, Di Renzo L. Fasting: How to Guide. Nutrients. 2021;13(5):1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Stafstrom CE, Rho JM: Epilepsy and the ketogenic diet: Springer Science & Business Media; 2004.
- 390.Volek J, Phinney SD, Kossoff E, Eberstein JA, Moore J: The Art and Science of Low Carbohydrate Living: An Expert Guide to Making the Life-saving Benefits of Carbohydrate Restriction Sustainable and Enjoyable: Beyond Obesity; 2011.
- 391.Bender JL, Babinski S, Wong G, Tricco AC, Englesakis M, Cyr AB, Potts H, Perski O, Esplen MJ, Young C, et al. Establishing best practices in cancer online support groups: protocol for a realist review. BMJ Open. 2021;11(11): e053916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Young JS, Al-Adli N, Sibih YE, Scotford KL, Casey M, James S, Berger MS: Recognizing the psychological impact of a glioma diagnosis on mental and behavioral health: a systematic review of what neurosurgeons need to know. Journal of Neurosurgery 2022, 1(aop):1–9. [DOI] [PMC free article] [PubMed]
- 393.Tlusty G, Hanna KM. Health Ownership: A Concept Analysis. Nurs Sci Q. 2021;34(4):413–9. [DOI] [PubMed] [Google Scholar]
- 394.Halatsch ME, Kast RE, Karpel-Massler G, Mayer B, Zolk O, Schmitz B, Scheuerle A, Maier L, Bullinger L, Mayer-Steinacker R et al: A phase Ib/IIa trial of 9 repurposed drugs combined with temozolomide for the treatment of recurrent glioblastoma: CUSP9v3. Neurooncol Adv 2021, 3(1):vdab075. [DOI] [PMC free article] [PubMed]
- 395.Agrawal S, Vamadevan P, Mazibuko N, Bannister R, Swery R, Wilson S, Edwards S. A New Method for Ethical and Efficient Evidence Generation for Off-Label Medication Use in Oncology (A Case Study in Glioblastoma). Front Pharmacol. 2019;10:681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.İyikesici MS, Slocum AK, Slocum A, Berkarda FB, Kalamian M, Seyfried TN: Efficacy of metabolically supported chemotherapy combined with ketogenic diet, hyperthermia, and hyperbaric oxygen therapy for stage IV triple-negative breast cancer. Cureus 2017, 9(7). [DOI] [PMC free article] [PubMed]
- 397.Jansen N, Walach H. The development of tumours under a ketogenic diet in association with the novel tumour marker TKTL1: a case series in general practice. Oncol Lett. 2016;11(1):584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Medlinskiene K, Tomlinson J, Marques I, Richardson S, Stirling K, Petty D. Barriers and facilitators to the uptake of new medicines into clinical practice: a systematic review. BMC Health Serv Res. 2021;21(1):1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Veatch RM. Doctor does not know best: why in the new century physicians must stop trying to benefit patients. The Journal of medicine philosophy. 2000;25(6):701–21. [DOI] [PubMed] [Google Scholar]
- 400.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, Rubin HR. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–65. [DOI] [PubMed] [Google Scholar]
- 401.Noakes TD, Kalamian M, Seyfried TN, Mukherjee P, D’Agostino DP, Arismendi-Morillo G, Chinopoulos C, Tettenborn M, Winters N: Cancer. In: Ketogenic. edn. Edited by Noakes TD, Murphy T, Wellington N, Kajee H, Rice SM: Academic Press; 2023: 307–362.
- 402.Wittich CM, Burkle CM, Lanier WL: Ten common questions (and their answers) about off-label drug use. In: Mayo Clin Proc: 2012: Elsevier; 2012: 982–990. [DOI] [PMC free article] [PubMed]
- 403.Borysowski J, Gorski A. Compassionate use of unauthorized drugs: Legal regulations and ethical challenges. Eur J Intern Med. 2019;65:12–6. [DOI] [PubMed] [Google Scholar]
- 404.Fontanals S, Esteve A, Gonzalez A, Ibanez C, Martinez J, Mesia R, Clopes A. Real-world treatment outcomes of medicines used in special situations (off-label and compassionate use) in oncology and hematology: A retrospective study from a comprehensive cancer institution. Cancer Med. 2023;12(16):17112–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Krzyzanowska MK. Off-label use of cancer drugs: a benchmark is established. J Clin Oncol. 2013;31(9):1125–7. [DOI] [PubMed] [Google Scholar]
- 406.Organization WH: Repurposing of medicines–the underrated champion of sustainable innovation: policy brief. In.: World Health Organization. Regional Office for Europe; 2021.
- 407.Lynch HF, Zettler PJ, Sarpatwari A. Promoting Patient Interests in Implementing the Federal Right to Try Act. JAMA. 2018;320(9):869–70. [DOI] [PubMed] [Google Scholar]
- 408.Trojanowski P, Jarosz B, Szczepanek D. The diagnostic quality of needle brain biopsy specimens obtained with different sampling methods - Experimental study. Sci Rep. 2019;9(1):8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Alieva M, Margarido AS, Wieles T, Abels ER, Colak B, Boquetale C, Jan Noordmans H, Snijders TJ, Broekman ML, van Rheenen J. Preventing inflammation inhibits biopsy-mediated changes in tumor cell behavior. Sci Rep. 2017;7(1):7529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Kameyama H, Dondapati P, Simmons R, Leslie M, Langenheim JF, Sun Y, Yi M, Rottschaefer A, Pathak R, Nuguri S, et al. Needle biopsy accelerates pro-metastatic changes and systemic dissemination in breast cancer: Implications for mortality by surgery delay. Cell Rep Med. 2023;4(12): 101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Noch EK, Sait SF, Farooq S, Trippett TM, Miller AM. A case series of extraneural metastatic glioblastoma at Memorial Sloan Kettering Cancer Center. Neurooncol Pract. 2021;8(3):325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Ronvaux L, Riva M, Coosemans A, Herzog M, Rommelaere G, Donis N, D’Hondt L, Douxfils J. Liquid Biopsy in Glioblastoma Cancers (Basel). 2022;14(14):3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Gritsch S, Batchelor TT, Gonzalez Castro LN. Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer. 2022;128(1):47–58. [DOI] [PubMed] [Google Scholar]
- 414.Agarwal A, Edgar MA, Desai A, Gupta V, Soni N, Bathla G. Molecular GBM versus Histopathological GBM: Radiology-Pathology-Genetic Correlation and the New WHO 2021 Definition of Glioblastoma. AJNR Am J Neuroradiol. 2024;45(8):1006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Costelloe CM, Amini B, Madewell JE: Risks and benefits of gadolinium-based contrast-enhanced MRI. In: Seminars in Ultrasound, CT and MRI: 2020: Elsevier; 2020: 170–182. [DOI] [PubMed]
- 416.Brown SD. Professional norms regarding how radiologists handle incidental findings. J Am Coll Radiol. 2013;10(4):253–7. [DOI] [PubMed] [Google Scholar]
- 417.Doubeni CA, Gabler NB, Wheeler CM, McCarthy AM, Castle PE, Halm EA, Schnall MD, Skinner CS, Tosteson ANA, Weaver DL, et al. Timely follow-up of positive cancer screening results: A systematic review and recommendations from the PROSPR Consortium. CA Cancer J Clin. 2018;68(3):199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Lee TH, Brennan TA. Direct-to-consumer marketing of high-technology screening tests. N Engl J Med. 2002;346(7):529–31. [DOI] [PubMed] [Google Scholar]
- 419.Hitzeman N, Cotton E. Incidentalomas: initial management. Am Fam Physician. 2014;90(11):784–9. [PubMed] [Google Scholar]
- 420.Aghi M, Barker Ii FG. Benign adult brain tumors: an evidence-based medicine review. Prog Neurol Surg. 2006;19:80–96. [DOI] [PubMed] [Google Scholar]
- 421.Michelson N, Rincon-Torroella J, Quinones-Hinojosa A, Greenfield JP. Exploring the role of inflammation in the malignant transformation of low-grade gliomas. J Neuroimmunol. 2016;297:132–40. [DOI] [PubMed] [Google Scholar]
- 422.Zhao H, Jin H, Xian J, Zhang Z, Shi J, Bai X. Effect of Ketogenic Diets on Body Composition and Metabolic Parameters of Cancer Patients: A Systematic Review and Meta-Analysis. Nutrients. 2022;14(19):4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Chaudhary R: Ketogenic Diet as a Treatment and Prevention Strategy for Cancer: A Therapeutic Alternative. Nutrition 2024:112427.
- 424.Mishra A, Giuliani G, Longo VD. Nutrition and dietary restrictions in cancer prevention. Biochim Biophys Acta Rev Cancer. 2024;1879(1): 189063. [DOI] [PubMed] [Google Scholar]
- 425.Kwok KHF, Ip EC, Lee SF. The conundrums of the reasonable patient standard in English medical law. BMC Med Ethics. 2023;24(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Akeeb AA, King SM, Olaku O, White JD. Communication between cancer patients and physicians about complementary and alternative medicine: A systematic review. Journal of Integrative Complementary Medicine. 2023;29(2):80–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Rosenbaum L. The paternalism preference—choosing unshared decision making. Obstetrical Gynecological Survey. 2015;70(12):739–40. [DOI] [PubMed] [Google Scholar]
- 428.Cherny NI, Sullivan R, Dafni U, Kerst JM, Sobrero A, Zielinski C, de Vries EG, Piccart MJ. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann Oncol. 2015;26(8):1547–73. [DOI] [PubMed] [Google Scholar]
- 429.Stahl D, Tomlinson T. Is there a right not to know? Nat Rev Clin Oncol. 2017;14(5):259–60. [DOI] [PubMed] [Google Scholar]
- 430.Helgesson G. What is a reasonable framework for new non-validated treatments? Theoretical Medicine Bioethics. 2020;41:239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Neal EG, Zupec-Kania B, Pfeifer HH. Carnitine, nutritional supplementation and discontinuation of ketogenic diet therapies. Epilepsy Res. 2012;100(3):267–71. [DOI] [PubMed] [Google Scholar]
- 432.Elisia I, Krystal G. The Pros and Cons of Low Carbohydrate and Ketogenic Diets in the Prevention and Treatment of Cancer. Front Nutr. 2021;8: 634845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020;20(1):26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Pierscianek D, Ahmadipour Y, Oppong MD, Rauschenbach L, Kebir S, Glas M, Sure U, Jabbarli R. Blood-Based Biomarkers in High Grade Gliomas: a Systematic Review. Mol Neurobiol. 2019;56(9):6071–9. [DOI] [PubMed] [Google Scholar]
- 435.Zachariah MA, Oliveira-Costa JP, Carter BS, Stott SL, Nahed BV. Blood-based biomarkers for the diagnosis and monitoring of gliomas. Neuro Oncol. 2018;20(9):1155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Miller AM, Shah RH, Pentsova EI, Pourmaleki M, Briggs S, Distefano N, Zheng Y, Skakodub A, Mehta SA, Campos C, et al. Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature. 2019;565(7741):654–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Müller Bark J, Kulasinghe A, Chua B, Day BW, Punyadeera C. Circulating biomarkers in patients with glioblastoma. Br J Cancer. 2020;122(3):295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Jelski W, Mroczko B. Molecular and Circulating Biomarkers of Brain Tumors. Int J Mol Sci. 2021;22(13):7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Cescon DW, Bratman SV, Chan SM, Siu LL. Circulating tumor DNA and liquid biopsy in oncology. Nat Cancer. 2020;1(3):276–90. [DOI] [PubMed] [Google Scholar]
- 440.Hoffer OA, Ben-David MA, Katz E, Zoltnik Kirshenabum D, Alezra D, Zimmer Y, Kelson I, Gannot I. Thermal imaging as a tool for evaluating tumor treatment efficacy. J Biomed Opt. 2018;23(5):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Sadeghi-Naini A, Sannachi L, Tadayyon H, Tran WT, Slodkowska E, Trudeau M, Gandhi S, Pritchard K, Kolios MC, Czarnota GJ. Chemotherapy-Response Monitoring of Breast Cancer Patients Using Quantitative Ultrasound-Based Intra-Tumour Heterogeneities. Sci Rep. 2017;7(1):10352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Zhang Y, Zhao J, Wang Y, Cai W, Zhang X, Li K, Liu W, Zhao Y, Kang H. Changes of Tumor Markers in Patients with Breast Cancer during Postoperative Adjuvant Chemotherapy. Dis Markers. 2022;2022:7739777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Venniyoor A, Al Bahrani B, Rajan B. The Dilemma of Serum Tumor Marker (STM) Flares. Gulf J Oncolog. 2014;1(15):63–7. [PubMed] [Google Scholar]
- 444.Kurebayashi J, Nishimura R, Tanaka K, Kohno N, Kurosumi M, Moriya T, Ogawa Y, Taguchi T. Significance of serum tumor markers in monitoring advanced breast cancer patients treated with systemic therapy: a prospective study. Breast Cancer. 2004;11(4):389–95. [DOI] [PubMed] [Google Scholar]
- 445.Kim HS, Park YH, Park MJ, Chang MH, Jun HJ, Kim KH, Ahn JS, Kang WK, Park K, Im YH. Clinical significance of a serum CA15-3 surge and the usefulness of CA15-3 kinetics in monitoring chemotherapy response in patients with metastatic breast cancer. Breast Cancer Res Treat. 2009;118(1):89–97. [DOI] [PubMed] [Google Scholar]
- 446.Yasasever V, Dincer M, Camlica H, Karaloglu D, Dalay N. Utility of CA 15–3 and CEA in monitoring breast cancer patients with bone metastases: special emphasis on “spiking” phenomena. Clin Biochem. 1997;30(1):53–6. [DOI] [PubMed] [Google Scholar]
- 447.Kim HJ, Lee KW, Kim YJ, Oh DY, Kim JH, Im SA, Lee JS. Chemotherapy-induced transient CEA and CA19-9 surges in patients with metastatic or recurrent gastric cancer. Acta Oncol. 2009;48(3):385–90. [DOI] [PubMed] [Google Scholar]
- 448.Guadagni S, Masedu F, Fiorentini G, Sarti D, Fiorentini C, Guadagni V, Apostolou P, Papasotiriou I, Parsonidis P, Valenti M, et al. Circulating tumour cell gene expression and chemosensitivity analyses: predictive accuracy for response to multidisciplinary treatment of patients with unresectable refractory recurrent rectal cancer or unresectable refractory colorectal cancer liver metastases. BMC Cancer. 2022;22(1):660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Anichini G, Fulmali P, O'Neill K, Datta V, Crook T, Syed N: PATH-24. ACCURATE IDENTIFICATION OF GLIAL MALIGNANCIES FROM PERIPHERAL BLOOD. Neuro Oncol 2022, 24(Supplement_7):vii155-vii155.
- 450.Valentini MC, Mellai M, Annovazzi L, Melcarne A, Denysenko T, Cassoni P, Casalone C, Maurella C, Grifoni S, Fania P, et al. Comparison among conventional and advanced MRI, (18)F-FDG PET/CT, phenotype and genotype in glioblastoma. Oncotarget. 2017;8(53):91636–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Venneti S, Dunphy MP, Zhang H, Pitter KL, Zanzonico P, Campos C, Carlin SD, La Rocca G, Lyashchenko S, Ploessl K et al: Glutamine-based PET imaging facilitates enhanced metabolic evaluation of gliomas in vivo. Sci Transl Med 2015, 7(274):274ra217. [DOI] [PMC free article] [PubMed]
- 452.Albert NL, Weller M, Suchorska B, Galldiks N, Soffietti R, Kim MM, la Fougere C, Pope W, Law I, Arbizu J, et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016;18(9):1199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Galldiks N, Niyazi M, Grosu AL, Kocher M, Langen K-J, Law I, Minniti G, Kim MM, Tsien C, Dhermain F. Contribution of PET imaging to radiotherapy planning and monitoring in glioma patients-a report of the PET/RANO group. Neuro Oncol. 2021;23(6):881–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Okumura W, Iwasaki T, Toyama T, Iso T, Arai M, Oriuchi N, Endo K, Yokoyama T, Suzuki T, Kurabayashi M. Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med. 2004;45(12):1989–98. [PubMed] [Google Scholar]
- 455.Lee KH, Ko BH, Paik JY, Jung KH, Choe YS, Choi Y, Kim BT. Effects of anesthetic agents and fasting duration on 18F-FDG biodistribution and insulin levels in tumor-bearing mice. J Nucl Med. 2005;46(9):1531–6. [PubMed] [Google Scholar]
- 456.Bennett OA, Ramsay SC, Malacova E, Bourgeat P, Goodman SJ, Dunn CJ, Robinson BM, Lee K, Pattison DA. Regional differences in the reduction in cerebral FDG uptake induced by the ketogenic diet. Eur J Hybrid Imaging. 2022;6(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Doche E, Phlip M, Cammilleri S, Suissa L, GUEDJ E: Regional brain glucose metabolism is differentially affected by ketogenic diet: A human semiquantitative positron emission tomography. 2022. [DOI] [PubMed]
- 458.Kim M, Eleftheriou A, Ravotto L, Weber B, Rivlin M, Navon G, Capozza M, Anemone A, Longo DL, Aime S. What do we know about dynamic glucose-enhanced (DGE) MRI and how close is it to the clinics? Horizon 2020 GLINT consortium report. Magnetic Resonance Materials in Physics, Biology Medicine. 2022;35(1):87–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Schmidt CA, Fisher-Wellman KH, Neufer PD. From OCR and ECAR to energy: Perspectives on the design and interpretation of bioenergetics studies. J Biol Chem. 2021;297(4): 101140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Wang Y, Patti GJ. The Warburg effect: a signature of mitochondrial overload. Trends Cell Biol. 2023;33(12):1014–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Qian L, Li Y, Cao Y, Meng G, Peng J, Li H, Wang Y, Xu T, Zhang L, Sun B, et al. Pan-Cancer Analysis of Glycolytic and Ketone Bodies Metabolic Genes: Implications for Response to Ketogenic Dietary Therapy. Front Oncol. 2021;11: 689068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Vallejo FA, Shah SS, de Cordoba N, Walters WM, Prince J, Khatib Z, Komotar RJ, Vanni S, Graham RM. The contribution of ketone bodies to glycolytic inhibition for the treatment of adult and pediatric glioblastoma. J Neurooncol. 2020;147:317–26. [DOI] [PubMed] [Google Scholar]
- 463.Ludikhuize MC, Meerlo M, Burgering BMT, Rodriguez Colman MJ. Protocol to profile the bioenergetics of organoids using Seahorse. STAR Protoc. 2021;2(1): 100386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Boykov I, Montgomery M, Hagen J, Aruleba R, McLaughlin K, Coalson H, Nelson M, Pereyra A, Ellis J, Zeczycki T et al: Pan-tissue mitochondrial phenotyping reveals lower OXPHOS expression and function across tumor types. 2023:2023.2006.2004.542600. [DOI] [PMC free article] [PubMed]
- 465.Sainero-Alcolado L, Liano-Pons J, Ruiz-Perez MV, Arsenian-Henriksson M. Targeting mitochondrial metabolism for precision medicine in cancer. Cell Death Differ. 2022;29(7):1304–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Butler M, van der Meer LT, van Leeuwen FN. Amino Acid Depletion Therapies: Starving Cancer Cells to Death. Trends Endocrinol Metab. 2021;32(6):367–81. [DOI] [PubMed] [Google Scholar]
- 467.Lee H, Woo SM, Jang H, Kang M, Kim S-Y: Cancer depends on fatty acids for ATP production: A possible link between cancer and obesity. In: Seminars in Cancer Biology: 2022: Elsevier; 2022. [DOI] [PubMed]
- 468.Ying M, You D, Zhu X, Cai L, Zeng S, Hu X. Lactate and glutamine support NADPH generation in cancer cells under glucose deprived conditions. Redox Biol. 2021;46: 102065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Zhang X, Dang CV. Time to hit pause on mitochondria-targeting cancer therapies. Nat Med. 2023;29(1):29–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Wang ZH, Chen L, Li W, Chen L, Wang YP. Mitochondria transfer and transplantation in human health and diseases. Mitochondrion. 2022;65:80–7. [DOI] [PubMed] [Google Scholar]
- 471.Zhou W, Zhao Z, Yu Z, Hou Y, Keerthiga R, Fu A. Mitochondrial transplantation therapy inhibits the proliferation of malignant hepatocellular carcinoma and its mechanism. Mitochondrion. 2022;65:11–22. [DOI] [PubMed] [Google Scholar]
- 472.Chang JC, Chang HS, Wu YC, Cheng WL, Lin TT, Chang HJ, Kuo SJ, Chen ST, Liu CS. Mitochondrial transplantation regulates antitumour activity, chemoresistance and mitochondrial dynamics in breast cancer. J Exp Clin Cancer Res. 2019;38(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Koh S, Dupuis N, Auvin S. Ketogenic diet and Neuroinflammation. Epilepsy Res. 2020;167: 106454. [DOI] [PubMed] [Google Scholar]
- 474.Han C. Potential Value of Positron Emission Tomography (PET) in Evaluating the Ketogenic Diet as Anticancer Therapy. Journal of Nutritional Oncology. 2018;3(2):49–54. [Google Scholar]
- 475.Cussó L, Musteanu M, Mulero F, Barbacid M, Desco M. Effects of a ketogenic diet on [18 F] FDG-PET imaging in a mouse model of lung cancer. Mol Imag Biol. 2019;21:279–85. [DOI] [PubMed] [Google Scholar]
- 476.Martin-McGill KJ, Marson AG, Tudur Smith C, Jenkinson MD. Ketogenic diets as an adjuvant therapy in glioblastoma (the KEATING trial): study protocol for a randomised pilot study. Pilot feasibility studies. 2017;3:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Stummer W. Extent of resection and survival in glioblastoma multiforme. Neurosurgery. 2009;64(6):E1206. [DOI] [PubMed] [Google Scholar]
- 478.Baig Mirza A, Christodoulides I, Lavrador JP, Giamouriadis A, Vastani A, Boardman T, Ahmed R, Norman I, Murphy C, Devi S. 5-Aminolevulinic acid-guided resection improves the overall survival of patients with glioblastoma—A comparative cohort study of 343 patients. Neuro-oncology advances. 2021;3(1):vdab047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Voskuil FJ, Steinkamp PJ, Zhao T, van der Vegt B, Koller M, Doff JJ, Jayalakshmi Y, Hartung JP, Gao J, Sumer BD, et al. Exploiting metabolic acidosis in solid cancers using a tumor-agnostic pH-activatable nanoprobe for fluorescence-guided surgery. Nat Commun. 2020;11(1):3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Netufo O, Connor K, Shiels LP, Sweeney KJ, Wu D, O’Shea DF, Byrne AT, Miller IS. Refining Glioblastoma Surgery through the Use of Intra-Operative Fluorescence Imaging Agents. Pharmaceuticals (Basel). 2022;15(5):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Barbagallo GM, Jenkinson MD, Brodbelt AR. ‘Recurrent’glioblastoma multiforme, when should we reoperate? Br J Neurosurg. 2008;22(3):452–5. [DOI] [PubMed] [Google Scholar]
- 482.De Bonis P, Anile C, Pompucci A, Fiorentino A, Balducci M, Chiesa S, Lauriola L, Maira G, Mangiola A. The influence of surgery on recurrence pattern of glioblastoma. Clin Neurol Neurosurg. 2013;115(1):37–43. [DOI] [PubMed] [Google Scholar]
- 483.Dejaegher J, De Vleeschouwer S: Recurring glioblastoma: A case for reoperation? Exon Publications 2017:281–296. [PubMed]
- 484.Germano IM, Johnson DR, Patrick HH, Goodman AL, Ziu M, Ormond DR, Olson JJ. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines on the Management of Progressive Glioblastoma in Adults: Update of the 2014 Guidelines. Neurosurgery. 2022;90(5):e112–5. [DOI] [PubMed] [Google Scholar]
- 485.Valencia I, Pfeifer H, Thiele EA. General anesthesia and the ketogenic diet: clinical experience in nine patients. Epilepsia. 2002;43(5):525–9. [DOI] [PubMed] [Google Scholar]
- 486.Albanese A, Prevedello L, Markovich M, Busetto L, Vettor R, Foletto M: Pre-operative Very Low Calorie Ketogenic Diet (VLCKD) vs. Very Low Calorie Diet (VLCD): Surgical Impact. Obes Surg 2019, 29(1):292–296. [DOI] [PubMed]
- 487.Forsythe CE, Phinney SD, Fernandez ML, Quann EE, Wood RJ, Bibus DM, Kraemer WJ, Feinman RD, Volek JS. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids. 2008;43(1):65–77. [DOI] [PubMed] [Google Scholar]
- 488.Jordan S, Tung N, Casanova-Acebes M, Chang C, Cantoni C, Zhang D, Wirtz TH, Naik S, Rose SA, Brocker CN et al: Dietary Intake Regulates the Circulating Inflammatory Monocyte Pool. Cell 2019, 178(5):1102–1114 e1117. [DOI] [PMC free article] [PubMed]
- 489.Abd Ellatif RA, Ibrahim MA: Ketogenic diet enhances delayed wound healing in immunocompromised rats: A histological and immunohistochemical study. Egyptian Journal of Histology 2021.
- 490.Kesl S, Jung M, Prather J, Sherwood J, Gould L, D'Agostino D: Sustaining dietary ketosis to improve blood flow and wound healing in young and aged Fisher rats (734.7). The FASEB Journal 2014, 28:734.737.
- 491.Lu Y, Yang Y-Y, Zhou M-W, Liu N, Xing H-Y, Liu X-X, Li F. Ketogenic diet attenuates oxidative stress and inflammation after spinal cord injury by activating Nrf2 and suppressing the NF-κB signaling pathways. Neurosci Lett. 2018;683:13–8. [DOI] [PubMed] [Google Scholar]
- 492.Sayadi JJ, Sayadi L, Satteson E, Chopan M. Nerve injury and repair in a ketogenic milieu: A systematic review of traumatic injuries to the spinal cord and peripheral nervous tissue. PLoS ONE. 2021;16(1): e0244244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Masino SA, Ruskin DN. Ketogenic diets and pain. J Child Neurol. 2013;28(8):993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Włodarczyk A, Cubała WJ, Stawicki M. Ketogenic diet for depression: A potential dietary regimen to maintain euthymia? Progress in Neuro-Psychopharmacology Biological Psychiatry. 2021;109: 110257. [DOI] [PubMed] [Google Scholar]
- 495.Lussier DM, Woolf EC, Johnson JL, Brooks KS, Blattman JN, Scheck AC. Enhanced immunity in a mouse model of malignant glioma is mediated by a therapeutic ketogenic diet. BMC Cancer. 2016;16(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Gomez-Arbelaez D, Bellido D, Castro AI, Ordonez-Mayan L, Carreira J, Galban C, Martinez-Olmos MA, Crujeiras AB, Sajoux I, Casanueva FF. Body Composition Changes After Very-Low-Calorie Ketogenic Diet in Obesity Evaluated by 3 Standardized Methods. J Clin Endocrinol Metab. 2017;102(2):488–98. [DOI] [PubMed] [Google Scholar]
- 497.Masood W, Annamaraju P, Uppaluri KR: Ketogenic diet. In: StatPearls. edn.: StatPearls Publishing; 2021. [PubMed]
- 498.James LJ, Shirreffs SM. Effect of electrolyte addition to rehydration drinks consumed after severe fluid and energy restriction. J Strength Cond Res. 2015;29(2):521–7. [DOI] [PubMed] [Google Scholar]
- 499.Watanabe M, Tozzi R, Risi R, Tuccinardi D, Mariani S, Basciani S, Spera G, Lubrano C, Gnessi L. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: A comprehensive review of the literature. Obes Rev. 2020;21(8): e13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Pietzner M, Uluvar B, Kolnes KJ, Jeppesen PB, Frivold SV, Skattebo O, Johansen EI, Skalhegg BS, Wojtaszewski JFP, Kolnes AJ, et al. Systemic proteome adaptions to 7-day complete caloric restriction in humans. Nat Metab. 2024;6(4):764–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Alirezaei M, Kemball CC, Flynn CT, Wood MR, Whitton JL, Kiosses WB. Short-term fasting induces profound neuronal autophagy. Autophagy. 2010;6(6):702–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19(2):181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Ibrahim EM, Al-Foheidi MH, Al-Mansour MM. Energy and caloric restriction, and fasting and cancer: a narrative review. Support Care Cancer. 2021;29(5):2299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Kikomeko J, Schutte T, van Velzen MJM, Seefat R, van Laarhoven HWM. Short-term fasting and fasting mimicking diets combined with chemotherapy: a narrative review. Ther Adv Med Oncol. 2023;15:17588359231161418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.de Groot S, Pijl H, van der Hoeven JJM, Kroep JR. Effects of short-term fasting on cancer treatment. J Exp Clin Cancer Res. 2019;38(1):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Wilhelmi de Toledo F, Buchinger A, Burggrabe H, Holz G, Kuhn C, Lischka E, Lischka N, Lutzner H, May W, Ritzmann-Widderich M et al: Fasting therapy - an expert panel update of the 2002 consensus guidelines. Forsch Komplementmed 2013, 20(6):434–443. [DOI] [PubMed]
- 507.Nishimuta M, Kodama N, Yoshitake Y, Shimada M, Serizawa N. Dietary Salt (Sodium Chloride) Requirement and Adverse Effects of Salt Restriction in Humans. J Nutr Sci Vitaminol. 2018;64(2):83–9. [DOI] [PubMed] [Google Scholar]
- 508.Garg R, Williams GH, Hurwitz S, Brown NJ, Hopkins PN, Adler GK. Low-salt diet increases insulin resistance in healthy subjects. Metabolism. 2011;60(7):965–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Lloyd-Mostyn RH, Lord PS, Glover R, West C, Gilliland IC. Uric acid metabolism in starvation. Ann Rheum Dis. 1970;29(5):553–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Teruya T, Chaleckis R, Takada J, Yanagida M, Kondoh H. Diverse metabolic reactions activated during 58-hr fasting are revealed by non-targeted metabolomic analysis of human blood. Sci Rep. 2019;9(1):854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Gohari S, Ghobadi S, Jafari A, Ahangar H, Gohari S, Mahjani M. The effect of dietary approaches to stop hypertension and ketogenic diets intervention on serum uric acid concentration: a systematic review and meta-analysis of randomized controlled trials. Sci Rep. 2023;13(1):10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Anton SD, Moehl K, Donahoo WT, Marosi K, Lee SA, Mainous AG 3rd, Leeuwenburgh C, Mattson MP. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity (Silver Spring). 2018;26(2):254–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Hall KD, Chen KY, Guo J, Lam YY, Leibel RL, Mayer LE, Reitman ML, Rosenbaum M, Smith SR, Walsh BT, et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am J Clin Nutr. 2016;104(2):324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Zupec-Kania BA, Aldaz V, Montgomery ME, Kostas KC: Enteral and parenteral applications of ketogenic diet therapy: experiences from four centers. ICAN: Infant, Child, Adolescent Nutrition 2011, 3(5):274–281.
- 515.Kaul N, Nation J, Laing J, Nicolo JP, Deane AM, Udy AA, Kwan P, O’Brien TJ. Modified low ratio ketogenic therapy in the treatment of adults with super-refractory status epilepticus. JPEN J Parenter Enteral Nutr. 2022;46(8):1819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.van der Louw E, Aldaz V, Harvey J, Roan M, van den Hurk D, Cross JH, Auvin S, Review G. Optimal clinical management of children receiving ketogenic parenteral nutrition: a clinical practice guide. Dev Med Child Neurol. 2020;62(1):48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5(10):1725–31. [DOI] [PubMed] [Google Scholar]
- 518.Gzell C, Back M, Wheeler H, Bailey D, Foote M. Radiotherapy in Glioblastoma: the Past, the Present and the Future. Clin Oncol (R Coll Radiol). 2017;29(1):15–25. [DOI] [PubMed] [Google Scholar]
- 519.Haggiagi A, Lassman AB: Newly diagnosed glioblastoma in the elderly: when is temozolomide alone enough? In., vol. 22: Oxford University Press US; 2020: 1058–1059. [DOI] [PMC free article] [PubMed]
- 520.Wick A, Kessler T, Platten M, Meisner C, Bamberg M, Herrlinger U, Felsberg J, Weyerbrock A, Papsdorf K, Steinbach JP, et al. Superiority of temozolomide over radiotherapy for elderly patients with RTK II methylation class, MGMT promoter methylated malignant astrocytoma. Neuro Oncol. 2020;22(8):1162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Gupta K, Vuckovic I, Zhang S, Xiong Y, Carlson BL, Jacobs J, Olson I, Petterson XM, Macura SI, Sarkaria J, et al. Radiation Induced Metabolic Alterations Associate With Tumor Aggressiveness and Poor Outcome in Glioblastoma. Front Oncol. 2020;10:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Tabatabaei P, Visse E, Bergstrom P, Brannstrom T, Siesjo P, Bergenheim AT. Radiotherapy induces an immediate inflammatory reaction in malignant glioma: a clinical microdialysis study. J Neurooncol. 2017;131(1):83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Arnold KM, Flynn NJ, Raben A, Romak L, Yu Y, Dicker AP, Mourtada F, Sims-Mourtada J. The Impact of Radiation on the Tumor Microenvironment: Effect of Dose and Fractionation Schedules. Cancer Growth Metastasis. 2018;11:1179064418761639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Sundahl N, Duprez F, Ost P, De Neve W, Mareel M. Effects of radiation on the metastatic process. Mol Med. 2018;24(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Duan C, Yang R, Yuan L, Engelbach JA, Tsien CI, Rich KM, Dahiya SM, Johanns TM, Ackerman JJH, Garbow JR. Late Effects of Radiation Prime the Brain Microenvironment for Accelerated Tumor Growth. Int J Radiat Oncol Biol Phys. 2019;103(1):190–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Shankar A, Kumar S, Iskander AS, Varma NR, Janic B, deCarvalho A, Mikkelsen T, Frank JA, Ali MM, Knight RA, et al. Subcurative radiation significantly increases cell proliferation, invasion, and migration of primary glioblastoma multiforme in vivo. Chin J Cancer. 2014;33(3):148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Gupta K, Burns TC. Radiation-Induced Alterations in the Recurrent Glioblastoma Microenvironment: Therapeutic Implications. Front Oncol. 2018;8:503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Betlazar C, Middleton RJ, Banati RB, Liu GJ. The impact of high and low dose ionising radiation on the central nervous system. Redox Biol. 2016;9:144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Cuccurullo V, Di Stasio GD, Cascini GL, Gatta G, Bianco C. The Molecular Effects of Ionizing Radiations on Brain Cells: Radiation Necrosis vs. Tumor Recurrence Diagnostics (Basel). 2019;9(4):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Carr CM, Benson JC, DeLone DR, Diehn FE, Kim DK, Merrell KW, Nagelschneider AA, Madhavan AA, Johnson DR. Intracranial long-term complications of radiation therapy: an image-based review. Neuroradiology. 2021;63(4):471–82. [DOI] [PubMed] [Google Scholar]
- 531.Makale MT, McDonald CR, Hattangadi-Gluth JA, Kesari S. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat Rev. 2017;13(1):52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Kazda T, Dziacky A, Burkon P, Pospisil P, Slavik M, Rehak Z, Jancalek R, Slampa P, Slaby O, Lakomy R. Radiotherapy of glioblastoma 15 years after the landmark Stupp’s trial: more controversies than standards? Radiology oncology. 2018;52(2):121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Ziu M, Kim BYS, Jiang W, Ryken T, Olson JJ. The role of radiation therapy in treatment of adults with newly diagnosed glioblastoma multiforme: a systematic review and evidence-based clinical practice guideline update. J Neurooncol. 2020;150(2):215–67. [DOI] [PubMed] [Google Scholar]
- 534.Colangelo NW, Azzam EI. Extracellular vesicles originating from glioblastoma cells increase metalloproteinase release by astrocytes: the role of CD147 (EMMPRIN) and ionizing radiation. Cell Commun Signal. 2020;18(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Lawrie TA, Gillespie D, Dowswell T, Evans J, Erridge S, Vale L, Kernohan A, Grant R. Long-term neurocognitive and other side effects of radiotherapy, with or without chemotherapy, for glioma. Cochrane Database Syst Rev. 2019;8(8):CD013047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Guram K, Smith M, Ginader T, Bodeker K, Pelland D, Pennington E, Buatti JM. Using smaller-than-standard radiation treatment margins does not change survival outcomes in patients with high-grade gliomas. Pract Radiat Oncol. 2019;9(1):16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Zeng Q, Stylianou T, Preston J, Glover S, O’Neill K, Woolf EC, Scheck AC, Syed N: The ketogenic diet alters the epigenetic landscape of GBM to potentiate the effects of chemotherapy and radiotherapy. Neuro Oncol 2019, 21(Supplement_4):iv8-iv8.
- 538.Abdelwahab MG, Fenton KE, Preul MC, Rho JM, Lynch A, Stafford P, Scheck AC. The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS ONE. 2012;7(5): e36197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Balducci M, Chiesa S, Diletto B, D’Agostino GR, Mangiola A, Manfrida S, Mantini G, Albanese A, Fiorentino A, Frascino V, et al. Low-dose fractionated radiotherapy and concomitant chemotherapy in glioblastoma multiforme with poor prognosis: a feasibility study. Neuro Oncol. 2012;14(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Bergman D, Modh A, Schultz L, Snyder J, Mikkelsen T, Shah M, Ryu S, Siddiqui MS, Walbert T. Randomized prospective trial of fractionated stereotactic radiosurgery with chemotherapy versus chemotherapy alone for bevacizumab-resistant high-grade glioma. J Neurooncol. 2020;148(2):353–61. [DOI] [PubMed] [Google Scholar]
- 541.Klement RJ. The influence of ketogenic therapy on the 5 R’s of radiobiology. Int J Radiat Biol. 2019;95(4):394–407. [DOI] [PubMed] [Google Scholar]
- 542.Valayer S, Kim D, Fogtman A, Straube U, Winnard A, Caplan N, Green DA, van Leeuwen FHP, Weber T. The Potential of Fasting and Caloric Restriction to Mitigate Radiation Damage-A Systematic Review. Front Nutr. 2020;7: 584543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Icard P, Ollivier L, Forgez P, Otz J, Alifano M, Fournel L, Loi M, Thariat J. Perspective: Do fasting, caloric restriction, and diets increase sensitivity to radiotherapy? A literature review. Adv Nutr. 2020;11(5):1089–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Beauchamp T, Childress J. Principles of Biomedical Ethics: Marking Its Fortieth Anniversary. Am J Bioeth. 2019;19(11):9–12. [DOI] [PubMed] [Google Scholar]
- 545.Association WM. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. [DOI] [PubMed] [Google Scholar]
- 546.Matsuyama R, Reddy S, Smith TJ. Why do patients choose chemotherapy near the end of life? A review of the perspective of those facing death from cancer. J Clin Oncol. 2006;24(21):3490–6. [DOI] [PubMed] [Google Scholar]
- 547.Winter SF, Vaios EJ, Shih HA, Grassberger C, Parsons MW, Gardner MM, Ehret F, Kaul D, Boehmerle W, Endres M, et al. Mitigating Radiotoxicity in the Central Nervous System: Role of Proton Therapy. Curr Treat Options Oncol. 2023;24(11):1524–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Giles AJ, Hutchinson MND, Sonnemann HM, Jung J, Fecci PE, Ratnam NM, Zhang W, Song H, Bailey R, Davis D, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Stokum JA, Gerzanich V, Sheth KN, Kimberly WT, Simard JM. Emerging Pharmacological Treatments for Cerebral Edema: Evidence from Clinical Studies. Annu Rev Pharmacol Toxicol. 2020;60:291–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Carpentier AF, Ferrari D, Bailon O, Ursu R, Banissi C, Dubessy AL, Belin C, Levy C. Steroid-sparing effects of angiotensin-II inhibitors in glioblastoma patients. Eur J Neurol. 2012;19(10):1337–42. [DOI] [PubMed] [Google Scholar]
- 551.Goldman M, Lucke-Wold B, Martinez-Sosa M, Katz J, Mehkri Y, Valisno J, Quintin S. Steroid utility, immunotherapy, and brain tumor management: an update on conflicting therapies. Explor Target Antitumor Ther. 2022;3(5):659–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Rangwala BS, Shakil A, Mustafa MS, Rangwala HS, Fatima H, Siddiq MA. Losartan and Immune Checkpoint Inhibitors in Glioblastoma: An Appropriate Substitute for Steroids. Ann Neurosci. 2024;31(3):152–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Kirste S, Treier M, Wehrle SJ, Becker G, Abdel-Tawab M, Gerbeth K, Hug MJ, Lubrich B, Grosu AL, Momm F. Boswellia serrata acts on cerebral edema in patients irradiated for brain tumors: a prospective, randomized, placebo-controlled, double-blind pilot trial. Cancer. 2011;117(16):3788–95. [DOI] [PubMed] [Google Scholar]
- 554.Dubinski D, Hattingen E, Senft C, Seifert V, Peters KG, Reiss Y, Devraj K, Plate KH. Controversial roles for dexamethasone in glioblastoma - Opportunities for novel vascular targeting therapies. J Cereb Blood Flow Metab. 2019;39(8):1460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Lim-Fat MJ, Bi WL, Lo J, Lee EQ, Ahluwalia MS, Batchelor TT, Chang SM, Chiocca EA, Chukwueke U, Cloughesy TF, et al. Letter: When Less is More: Dexamethasone Dosing for Brain Tumors. Neurosurgery. 2019;85(3):E607–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Amaral L, Gresham G, Kim S, Tighiouart M, Nelson T, Welborn A, Lockshon L, Noorvash B, Rudnick JD, Irwin SA. The ketogenic diet plus standard of care for adults with recently diagnosed glioblastoma: Results from a phase 1 trial. In.: American Society of Clinical Oncology; 2023. [Google Scholar]
- 557.Klement RJ. The emerging role of ketogenic diets in cancer treatment. Curr Opin Clin Nutr Metab Care. 2019;22(2):129–34. [DOI] [PubMed] [Google Scholar]
- 558.Soeters MR, Soeters PB, Schooneman MG, Houten SM, Romijn JA. Adaptive reciprocity of lipid and glucose metabolism in human short-term starvation. Am J Physiol. 2012;303(12):E1397–1407. [DOI] [PubMed] [Google Scholar]
- 559.Reinfeld BI, Madden MZ, Wolf MM, Chytil A, Bader JE, Patterson AR, Sugiura A, Cohen AS, Ali A, Do BT, et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature. 2021;593(7858):282–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Cohen AS, Grudzinski J, Smith GT, Peterson TE, Whisenant JG, Hickman TL, Ciombor KK, Cardin D, Eng C, Goff LW, et al. First-in-Human PET Imaging and Estimated Radiation Dosimetry of l-[5-(11)C]-Glutamine in Patients with Metastatic Colorectal Cancer. J Nucl Med. 2022;63(1):36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Alomari S, Zhang I, Hernandez A, Kraft CY, Raj D, Kedda J, Tyler B. Drug Repurposing for Glioblastoma and Current Advances in Drug Delivery-A Comprehensive Review of the Literature. Biomolecules. 2021;11(12):1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Basso J, Miranda A, Sousa J, Pais A, Vitorino C. Repurposing drugs for glioblastoma: From bench to bedside. Cancer Lett. 2018;428:173–83. [DOI] [PubMed] [Google Scholar]
- 563.Serafin MB, Bottega A, da Rosa TF, Machado CS, Foletto VS, Coelho SS, da Mota AD, Horner R. Drug Repositioning in Oncology. Am J Ther. 2021;28(1):e111–7. [DOI] [PubMed] [Google Scholar]
- 564.Harrison RK. Phase II and phase III failures: 2013–2015. Nat Rev Drug Discov. 2016;15(12):817–8. [DOI] [PubMed] [Google Scholar]
- 565.Ranganathan S, Haslam A, Tuia J, Prasad V. Characteristics and outcomes of new molecular oncology drug approvals, in combination or monotherapy. J Cancer Policy. 2024;39: 100462. [DOI] [PubMed] [Google Scholar]
- 566.Hall PE, Lewis R, Syed N, Shaffer R, Evanson J, Ellis S, Williams M, Feng X, Johnston A, Thomson JA, et al. A Phase I Study of Pegylated Arginine Deiminase (Pegargiminase), Cisplatin, and Pemetrexed in Argininosuccinate Synthetase 1-Deficient Recurrent High-grade Glioma. Clin Cancer Res. 2019;25(9):2708–16. [DOI] [PubMed] [Google Scholar]
- 567.Breckenridge A, Jacob R. Overcoming the legal and regulatory barriers to drug repurposing. Nat Rev Drug Discov. 2019;18(1):1–2. [DOI] [PubMed] [Google Scholar]
- 568.Oprea TI, Mestres J. Drug repurposing: far beyond new targets for old drugs. AAPS J. 2012;14(4):759–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Cha GD, Jung S, Choi SH, Kim DH. Local Drug Delivery Strategies for Glioblastoma Treatment. Brain Tumor Res Treat. 2022;10(3):151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Verbaanderd C, Rooman I, Meheus L, Huys I. On-Label or Off-Label? Overcoming Regulatory and Financial Barriers to Bring Repurposed Medicines to Cancer Patients. Front Pharmacol. 2019;10:1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Zhang Y, Li Q, Huang Z, Li B, Nice EC, Huang C, Wei L, Zou B. Targeting glucose metabolism enzymes in cancer treatment: current and emerging strategies. Cancers (Basel). 2022;14(19):4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Fiveash JB, Ye X, Peerboom DM, Mikkelsen T, Chowdhary S, Rosenfeld M, Lesser GJ, Fisher J, Desideri S, Grossman S, et al. Clinical trials of R-(-)-gossypol (AT-101) in newly diagnosed and recurrent glioblastoma: NABTT 0602 and NABTT 0702. PLoS ONE. 2024;19(1): e0291128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Wang Z, Liu F, Fan N, Zhou C, Li D, Macvicar T, Dong Q, Bruns CJ, Zhao Y. Targeting Glutaminolysis: New Perspectives to Understand Cancer Development and Novel Strategies for Potential Target Therapies. Front Oncol. 2020;10: 589508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Yap TA, Dumbrava EE, Rodon Ahnert J, Hong DS, Pant S, Karp DD, Piha-Paul SAA, Subbiah V, Tsimberidou AM, Fu S: First-in-human biomarker-driven phase I trial of the potent and selective glutaminase-1 (GLS1) inhibitor IACS-6274 (IPN60090) in patients (pts) with molecularly selected advanced solid tumors. In.: Wolters Kluwer Health; 2021.
- 575.DeBerardinis RJ. Tumor Microenvironment, Metabolism, and Immunotherapy. N Engl J Med. 2020;382(9):869–71. [DOI] [PubMed] [Google Scholar]
- 576.Bhatia K. Bhumika, Das A: Combinatorial drug therapy in cancer - New insights. Life Sci. 2020;258: 118134. [DOI] [PubMed] [Google Scholar]
- 577.Ngoi NYL, Eu JQ, Hirpara J, Wang L, Lim JSJ, Lee SC, Lim YC, Pervaiz S, Goh BC, Wong ALA. Targeting Cell Metabolism as Cancer Therapy. Antioxid Redox Signal. 2020;32(5):285–308. [DOI] [PubMed] [Google Scholar]
- 578.Takhwifa F, Aninditha T, Setiawan H, Sauriasari R. The potential of metformin as an antineoplastic in brain tumors: A systematic review. Heliyon. 2021;7(4): e06558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Wang S, Lin Y, Xiong X, Wang L, Guo Y, Chen Y, Chen S, Wang G, Lin P, Chen H. Low-Dose Metformin Reprograms the Tumor Immune Microenvironment in Human Esophageal Cancer: Results of a Phase II Clinical TrialLow-Dose Metformin Turns TIME against Cancer. Clin Cancer Res. 2020;26(18):4921–32. [DOI] [PubMed] [Google Scholar]
- 580.Kim HS, Kim JH, Jang HJ, Lee J. The addition of metformin to systemic anticancer therapy in advanced or metastatic cancers: a meta-analysis of randomized controlled trials. Int J Med Sci. 2020;17(16):2551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Fuentes-Fayos AC, ME GG, Perez-Gomez JM, Montero-Hidalgo AJ, Martin-Colom J, Doval-Rosa C, Blanco-Acevedo C, Torres E, Toledano-Delgado A, Sanchez-Sanchez R et al: Metformin and simvastatin exert additive antitumour effects in glioblastoma via senescence-state: clinical and translational evidence. EBioMedicine 2023, 90:104484. [DOI] [PMC free article] [PubMed]
- 582.Xie W, Su F, Wang G, Peng Z, Xu Y, Zhang Y, Xu N, Hou K, Hu Z, Chen Y, et al. Glucose-lowering effect of berberine on type 2 diabetes: A systematic review and meta-analysis. Front Pharmacol. 2022;13:1015045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Jiang W, Finniss S, Cazacu S, Xiang C, Brodie Z, Mikkelsen T, Poisson L, Shackelford DB, Brodie C. Repurposing phenformin for the targeting of glioma stem cells and the treatment of glioblastoma. Oncotarget. 2016;7(35):56456–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Yendapally R, Sikazwe D, Kim SS, Ramsinghani S, Fraser-Spears R, Witte AP, La-Viola B. A review of phenformin, metformin, and imeglimin. Drug Dev Res. 2020;81(4):390–401. [DOI] [PubMed] [Google Scholar]
- 585.Yap TA, Daver N, Mahendra M, Zhang J, Kamiya-Matsuoka C, Meric-Bernstam F, Kantarjian HM, Ravandi F, Collins ME, Francesco MED, et al. Complex I inhibitor of oxidative phosphorylation in advanced solid tumors and acute myeloid leukemia: phase I trials. Nat Med. 2023;29(1):115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Machado ND, Heather LC, Harris AL, Higgins GS. Targeting mitochondrial oxidative phosphorylation: lessons, advantages, and opportunities. Br J Cancer. 2023;129(6):897–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Lau KTK, Ng L, Wong JWH, Loong HHF, Chan WWL, Lee CH, Wong CKH. Repurposing sodium-glucose co-transporter 2 inhibitors (SGLT2i) for cancer treatment - A Review. Rev Endocr Metab Disord. 2021;22(4):1121–36. [DOI] [PubMed] [Google Scholar]
- 588.Park LK, Lim KH, Volkman J, Abdiannia M, Johnston H, Nigogosyan Z, Siegel MJ, McGill JB, McKee AM, Salam M, et al. Safety, tolerability, and effectiveness of the sodium-glucose cotransporter 2 inhibitor (SGLT2i) dapagliflozin in combination with standard chemotherapy for patients with advanced, inoperable pancreatic adenocarcinoma: a phase 1b observational study. Cancer Metab. 2023;11(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Luo J, Hendryx M, Dong Y. Sodium-glucose cotransporter 2 (SGLT2) inhibitors and non-small cell lung cancer survival. Br J Cancer. 2023;128(8):1541–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Kapila V, Topf J. Sodium-Glucose Co-transporter 2 Inhibitor-Associated Euglycemic Diabetic Ketoacidosis After Bariatric Surgery: A Case and Literature Review. Cureus. 2021;13(8): e17093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Hendrickson AL, Ye XQ, Kalra SS, Franck AJ, Urbine D. Euglycemic Diabetic Ketoacidosis in a Patient Prescribed Empagliflozin and a Ketogenic Diet: A Case of Misdiagnosed Type 1 Diabetes. Clin Diabetes. 2021;39(1):121–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Dorcely B, Nitis J, Schwartzbard A, Newman JD, Goldberg IJ, Sum M. A Case Report: Euglycemic Diabetic Ketoacidosis Presenting as Chest Pain in a Patient on a Low Carbohydrate Diet. Curr Diabetes Rev. 2021;17(2):243–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Guirguis H, Beroukhim Afrahimi S, Pham C. The Use of SGLT-2 Inhibitors Coupled With a Strict Low-Carbohydrate Diet: A Set-Up for Inducing Severe Diabetic Ketoacidosis. Clin Med Insights Case Rep. 2022;15:11795476221090044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Liu D, Weintraub MA, Garcia C, Goncalves MD, Sisk AE, Casas A, Harding JJ, Harnicar S, Drilon A, Jhaveri K, et al. Characterization, management, and risk factors of hyperglycemia during PI3K or AKT inhibitor treatment. Cancer Med. 2022;11(8):1796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Senior M. Precision nutrition to boost cancer treatments. Nat Biotechnol. 2022;40(10):1422–4. [DOI] [PubMed] [Google Scholar]
- 596.Sissung TM, Schmidt KT, Figg WD. Insulin potentiation therapy for cancer? Lancet Oncol. 2019;20(2):191–2. [DOI] [PubMed] [Google Scholar]
- 597.Poff AM, Moss S, Soliven M, D’Agostino DP. Ketone Supplementation: Meeting the Needs of the Brain in an Energy Crisis. Front Nutr. 2021;8: 783659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Pajak B, Siwiak E, Soltyka M, Priebe A, Zielinski R, Fokt I, Ziemniak M, Jaskiewicz A, Borowski R, Domoradzki T, et al. 2-Deoxy-d-Glucose and Its Analogs: From Diagnostic to Therapeutic Agents. Int J Mol Sci. 2019;21(1):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Patil N, Howe O, Cahill P, Byrne HJ. Monitoring and modelling the dynamics of the cellular glycolysis pathway: A review and future perspectives. Mol Metab. 2022;66: 101635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Becker AP, Sells BE, Haque SJ, Chakravarti A. Tumor Heterogeneity in Glioblastomas: From Light Microscopy to Molecular Pathology. Cancers (Basel). 2021;13(4):761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Yuan S, Almagro J, Fuchs E. Beyond genetics: driving cancer with the tumour microenvironment behind the wheel. Nat Rev Cancer. 2024;24(4):274–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Pittillo R, Hunt D: Azaserine and 6-diazo-5-oxo-L-norleucine (DON). In: Antibiotics: Volume I Mechanism of Action. edn.: Springer; 1967: 481–493.
- 603.Lemberg KM, Vornov JJ, Rais R, Slusher BS. We’re not “DON” yet: optimal dosing and prodrug delivery of 6-Diazo-5-oxo-L-norleucine. Mol Cancer Ther. 2018;17(9):1824–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Rais R, Lemberg KM, Tenora L, Arwood ML, Pal A, Alt J, Wu Y, Lam J, Aguilar JMH, Zhao L et al: Discovery of DRP-104, a tumor-targeted metabolic inhibitor prodrug. Sci Adv 2022, 8(46):eabq5925. [DOI] [PMC free article] [PubMed]
- 605.Xu H, Zheng M, Yang C, Wang K, Lv Z, Liu Z, Tang Z, Chen X. Azo-based hypoxic-activated 6-diazo-5-oxo-L-norleucine (DON) prodrug combined with vascular disrupting agent nanoparticles for tumor-selective glutamine metabolism blockade. Chem Eng J. 2024;481: 148281. [Google Scholar]
- 606.Sklaroff RB, Casper ES, Magill GB, Young CW. Phase I study of 6-diazo-5-oxo-L-norleucine (DON). Cancer Treat Rep. 1980;64(12):1247–51. [PubMed] [Google Scholar]
- 607.Unger C, Müller C, Jäger E, Bausch M, Roberts J, Al-Batran S, Sethuraman N: Results from a phase I dose escalation study of PEGylated glutaminase in combination with 6-diazo-5-oxo-L-norleucine (DON) in advanced malignant solid tumors. Journal of Clinical Oncology 2006, 24(18_suppl):13017–13017.
- 608.Nampota-Nkomba N, Nyirenda OM, Mallewa J, Chimalizeni Y, Dzabala N, Fay MP, Gopalakrishnan M, Laurens MB, O’Brien NF, Miller LH, et al. DON in pediatric cerebral malaria, a phase I/IIA dose-escalation safety study: study protocol for a clinical trial. Trials. 2024;25(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Magill G, Myers W, Reilly H, Putnam R, Magill J, Sykes M, Escher G, Karnofsky D, Burchenal J. Pharmacological and initial therapeutic observations on 6-Diazo-5-Oxo-L-Norleucine (Don) in human neoplastic disease. Cancer. 1957;10(6):1138–50. [DOI] [PubMed] [Google Scholar]
- 610.Alt J, Potter MC, Rojas C, Slusher BS. Bioanalysis of 6-diazo-5-oxo-l-norleucine in plasma and brain by ultra-performance liquid chromatography mass spectrometry. Anal Biochem. 2015;474:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Mueller C, Al-Batran S, Jaeger E, Schmidt B, Bausch M, Unger C, Sethuraman N: A phase IIa study of PEGylated glutaminase (PEG-PGA) plus 6-diazo-5-oxo-L-norleucine (DON) in patients with advanced refractory solid tumors. Journal of Clinical Oncology 2008, 26(15_suppl):2533–2533.
- 612.Hanaford AR, Alt J, Rais R, Wang SZ, Kaur H, Thorek DLJ, Eberhart CG, Slusher BS, Martin AM, Raabe EH. Orally bioavailable glutamine antagonist prodrug JHU-083 penetrates mouse brain and suppresses the growth of MYC-driven medulloblastoma. Translational oncology. 2019;12(10):1314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Ahluwalia GS, Grem JL, Hao Z, Cooney DA. Metabolism and action of amino acid analog anti-cancer agents. Pharmacol Ther. 1990;46(2):243–71. [DOI] [PubMed] [Google Scholar]
- 614.Sullivan MP, Nelson JA, Feldman S, Van Nguyen B. Pharmacokinetic and phase I study of intravenous DON (6-diazo-5-oxo-L-norleucine) in children. Cancer Chemother Pharmacol. 1988;21:78–84. [DOI] [PubMed] [Google Scholar]
- 615.Pillai R, LeBoeuf SE, Hao Y, New C, Blum JLE, Rashidfarrokhi A, Huang SM, Bahamon C, Wu WL, Karadal-Ferrena B et al: Glutamine antagonist DRP-104 suppresses tumor growth and enhances response to checkpoint blockade in KEAP1 mutant lung cancer. Sci Adv 2024, 10(13):eadm9859. [DOI] [PMC free article] [PubMed]
- 616.Yokoyama Y, Estok TM, Wild R. Sirpiglenastat (DRP-104) induces antitumor efficacy through direct, broad antagonism of glutamine metabolism and stimulation of the innate and adaptive immune systems. Mol Cancer Ther. 2022;21(10):1561–72. [DOI] [PubMed] [Google Scholar]
- 617.Iannitti T, Palmieri B. Clinical and experimental applications of sodium phenylbutyrate. Drugs R D. 2011;11(3):227–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Heo YA. Sodium Phenylbutyrate and Ursodoxicoltaurine: First Approval. CNS Drugs. 2022;36(9):1007–13. [DOI] [PubMed] [Google Scholar]
- 619.Palir N, Ruiter JPN, Wanders RJA, Houtkooper RH. Identification of enzymes involved in oxidation of phenylbutyrate. J Lipid Res. 2017;58(5):955–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Kusaczuk M, Kretowski R, Bartoszewicz M, Cechowska-Pasko M. Phenylbutyrate-a pan-HDAC inhibitor-suppresses proliferation of glioblastoma LN-229 cell line. Tumour Biol. 2016;37(1):931–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Phuphanich S, Baker SD, Grossman SA, Carson KA, Gilbert MR, Fisher JD, Carducci MA. Oral sodium phenylbutyrate in patients with recurrent malignant gliomas: a dose escalation and pharmacologic study. Neuro Oncol. 2005;7(2):177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Darmaun D, Welch S, Rini A, Sager BK, Altomare A, Haymond MW. Phenylbutyrate-induced glutamine depletion in humans: effect on leucine metabolism. Am J Physiol. 1998;274(5):E801–807. [DOI] [PubMed] [Google Scholar]
- 623.Gilbert J, Baker SD, Bowling MK, Grochow L, Figg WD, Zabelina Y, Donehower RC, Carducci MA. A phase I dose escalation and bioavailability study of oral sodium phenylbutyrate in patients with refractory solid tumor malignancies. Clin Cancer Res. 2001;7(8):2292–300. [PubMed] [Google Scholar]
- 624.Mueller C, Al-Batran S, Jaeger E, Schmidt B, Bausch M, Unger C, Sethuraman N: A phase IIa study of PEGylated glutaminase (PEG-PGA) plus 6-diazo-5-oxo-L-norleucine (DON) in patients with advanced refractory solid tumors. In: ASCO. vol. 26: J Clin Oncol 2008.
- 625.Owen OE, Smalley KJ, D’Alessio DA, Mozzoli MA, Dawson EK. Protein, fat, and carbohydrate requirements during starvation: anaplerosis and cataplerosis. Am J Clin Nutr. 1998;68(1):12–34. [DOI] [PubMed] [Google Scholar]
- 626.Emadi A, Zokaee H, Sausville EA. Asparaginase in the treatment of non-ALL hematologic malignancies. Cancer Chemother Pharmacol. 2014;73(5):875–83. [DOI] [PubMed] [Google Scholar]
- 627.Chan WK, Horvath TD, Tan L, Link T, Harutyunyan KG, Pontikos MA, Anishkin A, Du D, Martin LA, Yin E, et al. Glutaminase Activity of L-Asparaginase Contributes to Durable Preclinical Activity against Acute Lymphoblastic Leukemia. Mol Cancer Ther. 2019;18(9):1587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Sankaran H, Sengupta S, Purohit V, Kotagere A, Moulik NR, Prasad M, Dhamne C, Narula G, Banavali S, Gota V: A comparison of asparaginase activity in generic formulations of E.coli derived L- asparaginase: In-vitro study and retrospective analysis of asparaginase monitoring in pediatric patients with leukemia. Br J Clin Pharmacol 2020, 86(6):1081–1088. [DOI] [PMC free article] [PubMed]
- 629.P Hammel I El-Hariry T Macarulla R Garcia-Carbonero J-P Metges O Bouché F Portales RA Pazo Cid L Mineur AM Cubillo Gracian 2022 Trybeca-1: A randomized, phase 3 study of eryaspase in combination with chemotherapy versus chemotherapy alone as second-line treatment in patients with advanced pancreatic adenocarcinoma (NCT03665441) American Society of Clinical Oncology In.
- 630.Ohba S, Hirose Y. L-asparaginase and 6-diazo-5-oxo-L-norleucine synergistically inhibit the growth of glioblastoma cells. J Neurooncol. 2020;146(3):469–75. [DOI] [PubMed] [Google Scholar]
- 631.Van Trimpont M, Peeters E, De Visser Y, Schalk AM, Mondelaers V, De Moerloose B, Lavie A, Lammens T, Goossens S, Van Vlierberghe P. Novel insights on the use of L-asparaginase as an efficient and safe anti-cancer therapy. Cancers (Basel). 2022;14(4):902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Recouvreux MV, Grenier SF, Zhang Y, Esparza E, Lambies G, Galapate CM, Maganti S, Duong-Polk K, Bhullar D, Naeem R, et al. Glutamine mimicry suppresses tumor progression through asparagine metabolism in pancreatic ductal adenocarcinoma. Nat Cancer. 2024;5(1):100–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Yamashita AS, da Costa Rosa M, Stumpo V, Rais R, Slusher BS, Riggins GJ. The glutamine antagonist prodrug JHU-083 slows malignant glioma growth and disrupts mTOR signaling. Neurooncol Adv. 2021;3(1):vdaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Harding JJ, Telli M, Munster P, Voss MH, Infante JR, DeMichele A, Dunphy M, Le MH, Molineaux C, Orford K, et al. A Phase I Dose-Escalation and Expansion Study of Telaglenastat in Patients with Advanced or Metastatic Solid Tumors. Clin Cancer Res. 2021;27(18):4994–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Gross MI, Demo SD, Dennison JB, Chen L, Chernov-Rogan T, Goyal B, Janes JR, Laidig GJ, Lewis ER, Li J. Antitumor Activity of the Glutaminase Inhibitor CB-839 in Triple-Negative Breast CancerAntitumor Activity of the Glutaminase Inhibitor CB-839 in TNBC. Mol Cancer Ther. 2014;13(4):890–901. [DOI] [PubMed] [Google Scholar]
- 636.Katt WP, Cerione RA. Glutaminase regulation in cancer cells: a druggable chain of events. Drug Discovery Today. 2014;19(4):450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Schulte ML, Fu A, Zhao P, Li J, Geng L, Smith ST, Kondo J, Coffey RJ, Johnson MO, Rathmell JC, et al. Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nat Med. 2018;24(2):194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Hidalgo M, Rodriguez G, Kuhn JG, Brown T, Weiss G, MacGovren JP, Von Hoff DD, Rowinsky EK. A Phase I and pharmacological study of the glutamine antagonist acivicin with the amino acid solution aminosyn in patients with advanced solid malignancies. Clin Cancer Res. 1998;4(11):2763–70. [PubMed] [Google Scholar]
- 639.Sun Y, Feng X, Liu X, Qian C, Che X, Cao F, Jin S, Meng D. Caudatan A, an undescribed human kidney-type glutaminase inhibitor with tetracyclic flavan from Ohwia caudata. Phytochemistry. 2018;152:22–8. [DOI] [PubMed] [Google Scholar]
- 640.Wu C, Zheng M, Gao S, Luan S, Cheng L, Wang L, Li J, Chen L, Li H. A natural inhibitor of kidney-type glutaminase: a withanolide from Physalis pubescens with potent anti-tumor activity. Oncotarget. 2017;8(69):113516–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Sun W, Luan S, Qi C, Tong Q, Yan S, Li H, Zhang Y. Aspulvinone O, a natural inhibitor of GOT1 suppresses pancreatic ductal adenocarcinoma cells growth by interfering glutamine metabolism. Cell Commun Signal. 2019;17(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.SH Kizilbash S McBrayer J Port JM Reid I Lanza JB Allred A Chakravarti C Kunos AA Adjei 2019 A phase Ib trial of CB-839 (telaglenastat) in combination with radiation therapy and temozolomide in patients with IDH-mutated diffuse astrocytoma and anaplastic astrocytoma (NCT03528642) American Society of Clinical Oncology In.
- 643.Konopleva M, DiNardo C, Bhagat T, Baran N, Lodi A, Saxena K, Cai T, Su X, Skwarska A, Guerra V et al: Glutaminase inhibition in combination with azacytidine in myelodysplastic syndromes: Clinical efficacy and correlative analyses. Res Sq 2023. [DOI] [PubMed]
- 644.Tannir NM, Agarwal N, Porta C, Lawrence NJ, Motzer R, McGregor B, Lee RJ, Jain RK, Davis N, Appleman LJ, et al. Efficacy and Safety of Telaglenastat Plus Cabozantinib vs Placebo Plus Cabozantinib in Patients With Advanced Renal Cell Carcinoma: The CANTATA Randomized Clinical Trial. JAMA Oncol. 2022;8(10):1411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Spigel DR, Akerley W, Evangelist M, Johnson M, Levy B, Owonikoko T, Paik P, Papagiannakopoulos T, Reckamp K, Akella L: P47. 07 KEAPSAKE Study of Telaglenastat vs Placebo Plus Standard-of-Care in 1L KEAP1/NRF2-Mutated Non-Squamous Metastatic NSCLC. Journal of Thoracic Oncology 2021, 16(10):S1099.
- 646.Philip PA, Bahary N, Mahipal A, Kasi A, Rocha Lima CMSP, Alistar AT, Oberstein PE, Golan T, Sahai V, Metges JP. Phase 3, multicenter, randomized study of CPI-613 with modified FOLFIRINOX (mFFX) versus FOLFIRINOX (FFX) as first-line therapy for patients with metastatic adenocarcinoma of the pancreas (AVENGER500). In.: American Society of Clinical Oncology; 2022. [Google Scholar]
- 647.Pardee TS, Lee K, Luddy J, Maturo C, Rodriguez R, Isom S, Miller LD, Stadelman KM, Levitan D, Hurd D. A Phase I Study of the First-in-Class Antimitochondrial Metabolism Agent, CPI-613, in Patients with Advanced Hematologic MalignanciesA Phase I Study of CPI-613. Clin Cancer Res. 2014;20(20):5255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Alistar A, Morris BB, Desnoyer R, Klepin HD, Hosseinzadeh K, Clark C, Cameron A, Leyendecker J, D’Agostino R Jr, Topaloglu U, et al. Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: a single-centre, open-label, dose-escalation, phase 1 trial. Lancet Oncol. 2017;18(6):770–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Mukherjee P, Greenwood B, Henao J, Kiebish MA, Seyfried TN: Ketogenic diet as a metabolic vehicle for enhancing the therapeutic efficacy of mebendazole and devimistat in preclinical pediatric glioma. bioRxiv 2023:2023.2006. 2009.544252.
- 650.Jin L, Alesi GN, Kang S. Glutaminolysis as a target for cancer therapy. Oncogene. 2016;35(28):3619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Lewerenz J, Albrecht P, Tien ML, Henke N, Karumbayaram S, Kornblum HI, Wiedau-Pazos M, Schubert D, Maher P, Methner A. Induction of Nrf2 and xCT are involved in the action of the neuroprotective antibiotic ceftriaxone in vitro. J Neurochem. 2009;111(2):332–43. [DOI] [PubMed] [Google Scholar]
- 652.Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433(7021):73–7. [DOI] [PubMed] [Google Scholar]
- 653.Li C, Allen A, Kwagh J, Doliba NM, Qin W, Najafi H, Collins HW, Matschinsky FM, Stanley CA, Smith TJ. Green tea polyphenols modulate insulin secretion by inhibiting glutamate dehydrogenase. J Biol Chem. 2006;281(15):10214–21. [DOI] [PubMed] [Google Scholar]
- 654.Carmo F, Silva C, Martel F. Inhibition of Glutamine Cellular Uptake Contributes to the Cytotoxic Effect of Xanthohumol in Triple-Negative Breast Cancer Cells. Nutr Cancer. 2022;74(9):3413–30. [DOI] [PubMed] [Google Scholar]
- 655.Lodi A, Saha A, Lu X, Wang B, Sentandreu E, Collins M, Kolonin MG, DiGiovanni J, Tiziani S. Combinatorial treatment with natural compounds in prostate cancer inhibits prostate tumor growth and leads to key modulations of cancer cell metabolism. NPJ precision oncology. 2017;1(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Tabrez S, Zughaibi TA, Hoque M, Suhail M, Khan MI, Khan AU. Targeting Glutaminase by Natural Compounds: Structure-Based Virtual Screening and Molecular Dynamics Simulation Approach to Suppress Cancer Progression. Molecules. 2022;27(15):5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Tyszka-Czochara M, Bukowska-Strakova K, Kocemba-Pilarczyk KA, Majka M. Caffeic acid targets AMPK signaling and regulates tricarboxylic acid cycle anaplerosis while metformin downregulates HIF-1α-induced glycolytic enzymes in human cervical squamous cell carcinoma lines. Nutrients. 2018;10(7):841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Fan W-h, Wang F-c. Jin Z, Zhu L, Zhang J-x: Curcumin Synergizes with Cisplatin to Inhibit Colon Cancer through Targeting the MicroRNA-137-Glutaminase Axis. Current Medical Science. 2022;42(1):108–17. [DOI] [PubMed] [Google Scholar]
- 659.Lee YM, Lee G, Oh TI, Kim BM, Shim DW, Lee KH, Kim YJ, Lim BO, Lim JH. Inhibition of glutamine utilization sensitizes lung cancer cells to apigenin-induced apoptosis resulting from metabolic and oxidative stress. Int J Oncol. 2016;48(1):399–408. [DOI] [PubMed] [Google Scholar]
- 660.Zhang P, Wang Q, Lin Z, Yang P, Dou K, Zhang R. Berberine Inhibits Growth of Liver Cancer Cells by Suppressing Glutamine Uptake. Onco Targets Ther. 2019;12:11751–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Cerella C, Radogna F, Dicato M, Diederich M: Natural compounds as regulators of the cancer cell metabolism. Int J Cell Biol 2013, 2013. [DOI] [PMC free article] [PubMed]
- 662.Colwell N, Larion M, Giles AJ, Seldomridge AN, Sizdahkhani S, Gilbert MR, Park DM. Hypoxia in the glioblastoma microenvironment: shaping the phenotype of cancer stem-like cells. Neuro Oncol. 2017;19(7):887–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Olivier C, Oliver L, Lalier L, Vallette FM. Drug Resistance in Glioblastoma: The Two Faces of Oxidative Stress. Front Mol Biosci. 2020;7: 620677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Pombo Antunes AR, Scheyltjens I, Duerinck J, Neyns B, Movahedi K, Van Ginderachter JA: Understanding the glioblastoma immune microenvironment as basis for the development of new immunotherapeutic strategies. Elife 2020, 9. [DOI] [PMC free article] [PubMed]
- 665.Al-Kharboosh R, ReFaey K, Lara-Velazquez M, Grewal SS, Imitola J, Quinones-Hinojosa A. Inflammatory Mediators in Glioma Microenvironment Play a Dual Role in Gliomagenesis and Mesenchymal Stem Cell Homing: Implication for Cellular Therapy. Mayo Clin Proc Innov Qual Outcomes. 2020;4(4):443–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Mosteiro A, Pedrosa L, Ferres A, Diao D, Sierra A, Gonzalez JJ. The Vascular Microenvironment in Glioblastoma: A Comprehensive Review. Biomedicines. 2022;10(6):1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667.Jandrey EHF, Bezerra M, Inoue LT, Furnari FB, Camargo AA, Costa ET. A Key Pathway to Cancer Resilience: The Role of Autophagy in Glioblastomas. Front Oncol. 2021;11: 652133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Uribe D, Niechi I, Rackov G, Erices JI, San Martin R, Quezada C. Adapt to Persist: Glioblastoma Microenvironment and Epigenetic Regulation on Cell Plasticity. Biology (Basel). 2022;11(2):313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Fletcher-Sananikone E, Kanji S, Tomimatsu N, Di Cristofaro LFM, Kollipara RK, Saha D, Floyd JR, Sung P, Hromas R, Burns TC. Elimination of Radiation-Induced Senescence in the Brain Tumor Microenvironment Attenuates Glioblastoma RecurrenceRadiation-Induced Senescence as a Driver of GBM Recurrence. Can Res. 2021;81(23):5935–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Dono A, Nickles J, Rodriguez-Armendariz AG, McFarland BC, Ajami NJ, Ballester LY, Wargo JA, Esquenazi Y. Glioma and the gut–brain axis: opportunities and future perspectives. Neuro-Oncology Advances. 2022;4(1):vdac054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Hausmann D, Hoffmann DC, Venkataramani V, Jung E, Horschitz S, Tetzlaff SK, Jabali A, Hai L, Kessler T, Azorin DD, et al. Autonomous rhythmic activity in glioma networks drives brain tumour growth. Nature. 2023;613(7942):179–86. [DOI] [PubMed] [Google Scholar]
- 672.Yang T, Liu D, Fang S, Ma W, Wang Y. Cytomegalovirus and Glioblastoma: A Review of the Biological Associations and Therapeutic Strategies. J Clin Med. 2022;11(17):5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Peredo-Harvey I, Rahbar A, Söderberg-Nauclér C. Presence of the human cytomegalovirus in glioblastomas—A systematic review. Cancers (Basel). 2021;13(20):5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674.Hochhalter CB, Carr C, O’Neill BE, Ware ML, Strong MJ. The association between human cytomegalovirus and glioblastomas: a review. Neuroimmunology Neuroinflammation. 2017;4:96–108. [Google Scholar]
- 675.Merlo LM, Pepper JW, Reid BJ, Maley CC. Cancer as an evolutionary and ecological process. Nat Rev Cancer. 2006;6(12):924–35. [DOI] [PubMed] [Google Scholar]
- 676.Kroemer G, McQuade JL, Merad M, Andre F, Zitvogel L. Bodywide ecological interventions on cancer. Nat Med. 2023;29(1):59–74. [DOI] [PubMed] [Google Scholar]
- 677.Tamiz AP, Koroshetz WJ, Dhruv NT, Jett DA. A focus on the neural exposome. Neuron. 2022;110(8):1286–9. [DOI] [PubMed] [Google Scholar]
- 678.Hopkins BD, Goncalves MD, Cantley LC. Obesity and Cancer Mechanisms: Cancer Metabolism. J Clin Oncol. 2016;34(35):4277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Srinivasan M, Arzoun H, Gk LB, Thangaraj SR: A Systematic Review: Does Insulin Resistance Affect the Risk and Survival Outcome of Breast Cancer in Women? Cureus 2022, 14(1). [DOI] [PMC free article] [PubMed]
- 680.Goncalves MD, Farooki A. Management of Phosphatidylinositol-3-Kinase Inhibitor-Associated Hyperglycemia. Integr Cancer Ther. 2022;21:15347354211073164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Bernstock JD, Blitz SE, Hoffman SE, Gerstl JVE, Chiocca EA, Friedman GK. Recent oncolytic virotherapy clinical trials outline a roadmap for the treatment of high-grade glioma. Neurooncol Adv. 2023;5(1):vdad081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682.Sestito S, Runfola M, Tonelli M, Chiellini G, Rapposelli S. New Multitarget Approaches in the War Against Glioblastoma: A Mini-Perspective. Front Pharmacol. 2018;9:874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683.Yang L, TeSlaa T, Ng S, Nofal M, Wang L, Lan T, Zeng X, Cowan A, McBride M, Lu W, et al. Ketogenic diet and chemotherapy combine to disrupt pancreatic cancer metabolism and growth. Med. 2022;3(2):119–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Jia Q, Wang A, Yuan Y, Zhu B, Long H. Heterogeneity of the tumor immune microenvironment and its clinical relevance. Exp Hematol Oncol. 2022;11(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685.Lee AH, Sun L, Mochizuki AY, Reynoso JG, Orpilla J, Chow F, Kienzler JC, Everson RG, Nathanson DA, Bensinger SJ, et al. Neoadjuvant PD-1 blockade induces T cell and cDC1 activation but fails to overcome the immunosuppressive tumor associated macrophages in recurrent glioblastoma. Nat Commun. 2021;12(1):6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686.Arrieta VA, Dmello C, McGrail DJ, Brat DJ, Lee-Chang C, Heimberger AB, Chand D, Stupp R, Sonabend AM: Immune checkpoint blockade in glioblastoma: from tumor heterogeneity to personalized treatment. J Clin Invest 2023, 133(2). [DOI] [PMC free article] [PubMed]
- 687.Latzer P, Zelba H, Battke F, Reinhardt A, Shao B, Bartsch O, Rabsteyn A, Harter J, Schulze M, Okech T, et al. A real-world observation of patients with glioblastoma treated with a personalized peptide vaccine. Nat Commun. 2024;15(1):6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688.Hu JL, Omofoye OA, Rudnick JD, Kim S, Tighiouart M, Phuphanich S, Wang H, Mazer M, Ganaway T, Chu RM, et al. A Phase I Study of Autologous Dendritic Cell Vaccine Pulsed with Allogeneic Stem-like Cell Line Lysate in Patients with Newly Diagnosed or Recurrent Glioblastoma. Clin Cancer Res. 2022;28(4):689–96. [DOI] [PubMed] [Google Scholar]
- 689.Sims D, Liman AK, Leung V, Hwang A, Means J, Liman AD. What We Have Learned About Combining a Ketogenic Diet and Chemoimmunotherapy: a Case Report and Review of Literature. Fed Pract. 2023;40(Suppl 3):S98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 690.Leone RD, Zhao L, Englert JM, Sun IM, Oh MH, Sun IH, Arwood ML, Bettencourt IA, Patel CH, Wen J, et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. 2019;366(6468):1013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691.Murphy S, Rahmy S, Gan D, Zhu Y, Manyak M, Li J, Lu X, Lu X: Overcome Prostate Cancer Resistance to Immune Checkpoint Therapy with Ketogenic Diet-Induced Epigenetic Reprogramming. bioRxiv 2023:2023.2008. 2007.552383.
- 692.Dai X, Bu X, Gao Y, Guo J, Hu J, Jiang C, Zhang Z, Xu K, Duan J, He S, et al. Energy status dictates PD-L1 protein abundance and anti-tumor immunity to enable checkpoint blockade. Mol Cell. 2021;81(11):2317–2331 e2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.de Groot S, Lugtenberg RT, Cohen D, Welters MJ, Ehsan I, Vreeswijk MP, Smit VT, de Graaf H, Heijns JB, Portielje JE. Fasting mimicking diet as an adjunct to neoadjuvant chemotherapy for breast cancer in the multicentre randomized phase 2 DIRECT trial. Nat Commun. 2020;11(1):3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Yang H, Zingaro VA, Lincoff J, Tom H, Oikawa S, Oses-Prieto JA, Edmondson Q, Seiple I, Shah H, Kajimura S, et al. Remodelling of the translatome controls diet and its impact on tumorigenesis. Nature. 2024;633(8028):189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695.Fu L, Jin W, Zhang J, Zhu L, Lu J, Zhen Y, Zhang L, Ouyang L, Liu B, Yu H. Repurposing non-oncology small-molecule drugs to improve cancer therapy: Current situation and future directions. Acta Pharm Sin B. 2022;12(2):532–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.Lyne SB, Yamini B. An Alternative Pipeline for Glioblastoma Therapeutics: A Systematic Review of Drug Repurposing in Glioblastoma. Cancers (Basel). 2021;13(8):1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697.Armando RG, Mengual Gomez DL, Gomez DE. New drugs are not enough-drug repositioning in oncology: An update. Int J Oncol. 2020;56(3):651–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698.Leighl NB, Nirmalakumar S, Ezeife DA, Gyawali B. An Arm and a Leg: The Rising Cost of Cancer Drugs and Impact on Access. Am Soc Clin Oncol Educ Book. 2021;41:1–12. [DOI] [PubMed] [Google Scholar]
- 699.Furuta T, Sabit H, Dong Y, Miyashita K, Kinoshita M, Uchiyama N, Hayashi Y, Hayashi Y, Minamoto T, Nakada M. Biological basis and clinical study of glycogen synthase kinase- 3beta-targeted therapy by drug repositioning for glioblastoma. Oncotarget. 2017;8(14):22811–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700.Slavc I, Mayr L, Stepien N, Gojo J, Aliotti Lippolis M, Azizi AA, Chocholous M, Baumgartner A, Hedrich CS, Holm S, et al. Improved Long-Term Survival of Patients with Recurrent Medulloblastoma Treated with a “MEMMAT-like” Metronomic Antiangiogenic Approach. Cancers (Basel). 2022;14(20):5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Zapletalova D, Andre N, Deak L, Kyr M, Bajciova V, Mudry P, Dubska L, Demlova R, Pavelka Z, Zitterbart K, et al. Metronomic chemotherapy with the COMBAT regimen in advanced pediatric malignancies: a multicenter experience. Oncology. 2012;82(5):249–60. [DOI] [PubMed] [Google Scholar]
- 702.Kast RE, Alfieri A, Assi HI, Burns TC, Elyamany AM, Gonzalez-Cao M, Karpel-Massler G, Marosi C, Salacz ME, Sardi I, et al. MDACT: A New Principle of Adjunctive Cancer Treatment Using Combinations of Multiple Repurposed Drugs, with an Example Regimen. Cancers (Basel). 2022;14(10):2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703.O’Rawe M, Wickremesekera AC, Pandey R, Young D, Sim D, FitzJohn T, Burgess C, Kaye AH, Tan ST. Treatment of glioblastoma with re-purposed renin-angiotensin system modulators: Results of a phase I clinical trial. J Clin Neurosci. 2022;95:48–54. [DOI] [PubMed] [Google Scholar]
- 704.Kwt K. Cho WCS: Drug Repurposing for Cancer Therapy in the Era of Precision Medicine. Curr Mol Pharmacol. 2022;15(7):895–903. [DOI] [PubMed] [Google Scholar]
- 705.Maley CC, Aktipis A, Graham TA, Sottoriva A, Boddy AM, Janiszewska M, Silva AS, Gerlinger M, Yuan Y, Pienta KJ, et al. Classifying the evolutionary and ecological features of neoplasms. Nat Rev Cancer. 2017;17(10):605–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 706.Bonavida B, Bharti AC, Aggarwal BB: Role of nutraceuticals in cancer chemosensitization: Academic Press; 2017.
- 707.Calvaruso M, Pucci G, Musso R, Bravata V, Cammarata FP, Russo G, Forte GI, Minafra L. Nutraceutical Compounds as Sensitizers for Cancer Treatment in Radiation Therapy. Int J Mol Sci. 2019;20(21):5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Chu M, Zheng C, Chen C, Song G, Hu X, Wang Z-w: Targeting cancer stem cells by nutraceuticals for cancer therapy. In: Seminars in Cancer Biology: 2021: Elsevier; 2021. [DOI] [PubMed]
- 709.De Pergola G, Marucci S, Corbo F, Almerighi G, Cerutti N, Triggiani V, De Vito D, Castellana F, Zupo R: Nutraceuticals and Oral Supplements in Cancer Prevention: A Narrative Review. Endocrine, Metabolic Immune Disorders Drug Targets 2022. [DOI] [PubMed]
- 710.Maiuolo J, Gliozzi M, Carresi C, Musolino V, Oppedisano F, Scarano F, Nucera S, Scicchitano M, Bosco F, Macri R, et al. Nutraceuticals and Cancer: Potential for Natural Polyphenols. Nutrients. 2021;13(11):3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.Shukla Y, George J. Combinatorial strategies employing nutraceuticals for cancer development. Ann N Y Acad Sci. 2011;1229(1):162–75. [DOI] [PubMed] [Google Scholar]
- 712.Ng JY, Munford V, Thakar H. Web-based online resources about adverse interactions or side effects associated with complementary and alternative medicine: a systematic review, summarization and quality assessment. BMC Med Inform Decis Mak. 2020;20(1):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Ambrosone CB, Zirpoli GR, Hutson AD, McCann WE, McCann SE, Barlow WE, Kelly KM, Cannioto R, Sucheston-Campbell LE, Hershman DL, et al. Dietary Supplement Use During Chemotherapy and Survival Outcomes of Patients With Breast Cancer Enrolled in a Cooperative Group Clinical Trial (SWOG S0221). J Clin Oncol. 2020;38(8):804–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 714.Calvani M, Pasha A, Favre C. Nutraceutical Boom in Cancer: Inside the Labyrinth of Reactive Oxygen Species. Int J Mol Sci. 2020;21(6):1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 715.Jermini M, Dubois J, Rodondi PY, Zaman K, Buclin T, Csajka C, Orcurto A. L ER: Complementary medicine use during cancer treatment and potential herb-drug interactions from a cross-sectional study in an academic centre. Sci Rep. 2019;9(1):5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 716.Segal R, Zwaal C, Green E, Tomasone JR, Loblaw A, Petrella T. Exercise for People with Cancer Guideline Development G: Exercise for people with cancer: a systematic review. Curr Oncol. 2017;24(4):e290–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 717.Champ CE, Carpenter DJ, Diaz AK, Rosenberg J, Ackerson BG, Hyde PN. Resistance Training for Patients with Cancer: A Conceptual Framework for Maximizing Strength, Power, Functional Mobility, and Body Composition to Optimize Health and Outcomes. Sports Med. 2023;53(1):75–89. [DOI] [PubMed] [Google Scholar]
- 718.Hofmann P. Cancer and Exercise: Warburg Hypothesis, Tumour Metabolism and High-Intensity Anaerobic Exercise. Sports (Basel). 2018;6(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719.Paulusma CC, Lamers WH, Broer S, van de Graaf SFJ. Amino acid metabolism, transport and signalling in the liver revisited. Biochem Pharmacol. 2022;201: 115074. [DOI] [PubMed] [Google Scholar]
- 720.Cooper ID, Brookler KH, Crofts CAP. Rethinking Fragility Fractures in Type 2 Diabetes: The Link between Hyperinsulinaemia and Osteofragilitas. Biomedicines. 2021;9(9):1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Morishita S, Hamaue Y, Fukushima T, Tanaka T, Fu JB, Nakano J. Effect of Exercise on Mortality and Recurrence in Patients With Cancer: A Systematic Review and Meta-Analysis. Integr Cancer Ther. 2020;19:1534735420917462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722.Cannioto RA, Hutson A, Dighe S, McCann W, McCann SE, Zirpoli GR, Barlow W, Kelly KM, DeNysschen CA, Hershman DL, et al. Physical Activity Before, During, and After Chemotherapy for High-Risk Breast Cancer: Relationships With Survival. J Natl Cancer Inst. 2021;113(1):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723.Ekblom-Bak E, Bojsen-Moller E, Wallin P, Paulsson S, Lindwall M, Rundqvist H, Bolam KA. Association Between Cardiorespiratory Fitness and Cancer Incidence and Cancer-Specific Mortality of Colon, Lung, and Prostate Cancer Among Swedish Men. JAMA Netw Open. 2023;6(6): e2321102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 724.Li R, Xia J, Zhang XI, Gathirua-Mwangi WG, Guo J, Li Y, McKenzie S, Song Y. Associations of Muscle Mass and Strength with All-Cause Mortality among US Older Adults. Med Sci Sports Exerc. 2018;50(3):458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 725.Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, Zucker DS, Matthews CE, Ligibel JA, Gerber LH, et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc. 2019;51(11):2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 726.Sandler CX, Matsuyama M, Jones TL, Bashford J, Langbecker D, Hayes SC. Physical activity and exercise in adults diagnosed with primary brain cancer: a systematic review. J Neurooncol. 2021;153(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727.McTiernan A, Friedenreich CM, Katzmarzyk PT, Powell KE, Macko R, Buchner D, Pescatello LS, Bloodgood B, Tennant B, Vaux-Bjerke A, et al. Physical Activity in Cancer Prevention and Survival: A Systematic Review. Med Sci Sports Exerc. 2019;51(6):1252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 728.Cook SA, Salmon P, Hayes G, Byrne A, Fisher PL. Predictors of emotional distress a year or more after diagnosis of cancer: A systematic review of the literature. Psychooncology. 2018;27(3):791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 729.Hulbert-Williams NJ, Beatty L, Dhillon HM. Psychological support for patients with cancer: evidence review and suggestions for future directions. Curr Opin Support Palliat Care. 2018;12(3):276–92. [DOI] [PubMed] [Google Scholar]
- 730.Tan TT, Tan MP, Lam CL, Loh EC, Capelle DP, Zainuddin SI, Ang BT, Lim MA, Lai NZ, Tung YZ, et al. Mindful gratitude journaling: psychological distress, quality of life and suffering in advanced cancer: a randomised controlled trial. BMJ Support Palliat Care. 2023;13(e2):e389–96. [DOI] [PubMed] [Google Scholar]
- 731.Nevin JT, Moussa M, Corwin WL, Mandoiu II, Srivastava PK. Sympathetic nervous tone limits the development of myeloid-derived suppressor cells. Sci Immunol. 2020;5(51):eaay9368. [DOI] [PubMed] [Google Scholar]
- 732.Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, Li Y. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6(1):263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733.Shimba A, Ikuta K: Control of immunity by glucocorticoids in health and disease. In: Seminars in Immunopathology: 2020: Springer; 2020: 669–680. [DOI] [PubMed]
- 734.Thompson G, Lawrie TA, Kernohan A, Jenkinson MD. Interval brain imaging for adults with cerebral glioma. Cochrane Database Syst Rev. 2019;12(12):CD013137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 735.Reiter MJ, Costello JE, Schwope RB, Lisanti CJ, Osswald MB. Review of Commonly Used Serum Tumor Markers and Their Relevance for Image Interpretation. J Comput Assist Tomogr. 2015;39(6):825–34. [DOI] [PubMed] [Google Scholar]
- 736.Soda N, Clack K, Shiddiky MJA. Recent advances in liquid biopsy technologies for cancer biomarker detection. Sensors & Diagnostics. 2022;1(3):343–75. [Google Scholar]
- 737.Cohen JD, Li L, Wang Y, Thoburn C, Afsari B, Danilova L, Douville C, Javed AA, Wong F, Mattox A, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science. 2018;359(6378):926–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738.Lu VM, Goyal A, Graffeo CS, Perry A, Burns TC, Parney IF, Quinones-Hinojosa A, Chaichana KL. Survival Benefit of Maximal Resection for Glioblastoma Reoperation in the Temozolomide Era: A Meta-Analysis. World Neurosurg. 2019;127:31–7. [DOI] [PubMed] [Google Scholar]
- 739.Goenka A, Tiek D, Song X, Huang T, Hu B, Cheng SY. The Many Facets of Therapy Resistance and Tumor Recurrence in Glioblastoma. Cells. 2021;10(3):484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740.Osuka S, Van Meir EG. Overcoming therapeutic resistance in glioblastoma: the way forward. J Clin Invest. 2017;127(2):415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741.McBain C, Lawrie TA, Rogozinska E, Kernohan A, Robinson T, Jefferies S. Treatment options for progression or recurrence of glioblastoma: a network meta-analysis. Cochrane Database Syst Rev. 2021;5(1):CD013579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 742.Keating NL, Landrum MB, Rogers SO Jr, Baum SK, Virnig BA, Huskamp HA, Earle CC, Kahn KL. Physician factors associated with discussions about end-of-life care. Cancer. 2010;116(4):998–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 743.Daugherty CK, Hlubocky FJ. What are terminally ill cancer patients told about their expected deaths? A study of cancer physicians’ self-reports of prognosis disclosure. J Clin Oncol. 2008;26(36):5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744.Richardson TE, Kumar A, Xing C, Hatanpaa KJ, Walker JM. Overcoming the Odds: Toward a Molecular Profile of Long-Term Survival in Glioblastoma. J Neuropathol Exp Neurol. 2020;79(10):1031–7. [DOI] [PubMed] [Google Scholar]
- 745.Brenner H. Long-term survival rates of cancer patients achieved by the end of the 20th century: a period analysis. Lancet. 2002;360(9340):1131–5. [DOI] [PubMed] [Google Scholar]
- 746.Caplan AL, Ray A. The Ethical Challenges of Compassionate Use. JAMA. 2016;315(10):979–80. [DOI] [PubMed] [Google Scholar]
- 747.Ahn E, Shin DW, Choi JY, Kang J, Kim DK, Kim H, Lee E, Hwang KO, Oh B, Cho B. The impact of awareness of terminal illness on quality of death and care decision making: a prospective nationwide survey of bereaved family members of advanced cancer patients. Psychooncology. 2013;22(12):2771–8. [DOI] [PubMed] [Google Scholar]
- 748.Johnson SB, Butow PN, Kerridge I, Tattersall MHN. Patient autonomy and advance care planning: a qualitative study of oncologist and palliative care physicians’ perspectives. Support Care Cancer. 2018;26(2):565–74. [DOI] [PubMed] [Google Scholar]
- 749.Carmona-Bayonas A, Rodriguez-Gonzalez A, García-García T, Velasco-Durantez V, Hernández-San Gil R, Cruz-Castellanos P, Fernandez-Montes A, Castillo-Trujillo A, Ballester I, Rogado J: Can Oncologists Prompt Patient Prognostic Awareness to Enhance Decision-Making? Data From the Neoetic Study. Oncologist 2023:oyad100. [DOI] [PMC free article] [PubMed]
- 750.DeBerardinis RJ, Chandel NS. We need to talk about the Warburg effect. Nat Metab. 2020;2(2):127–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.