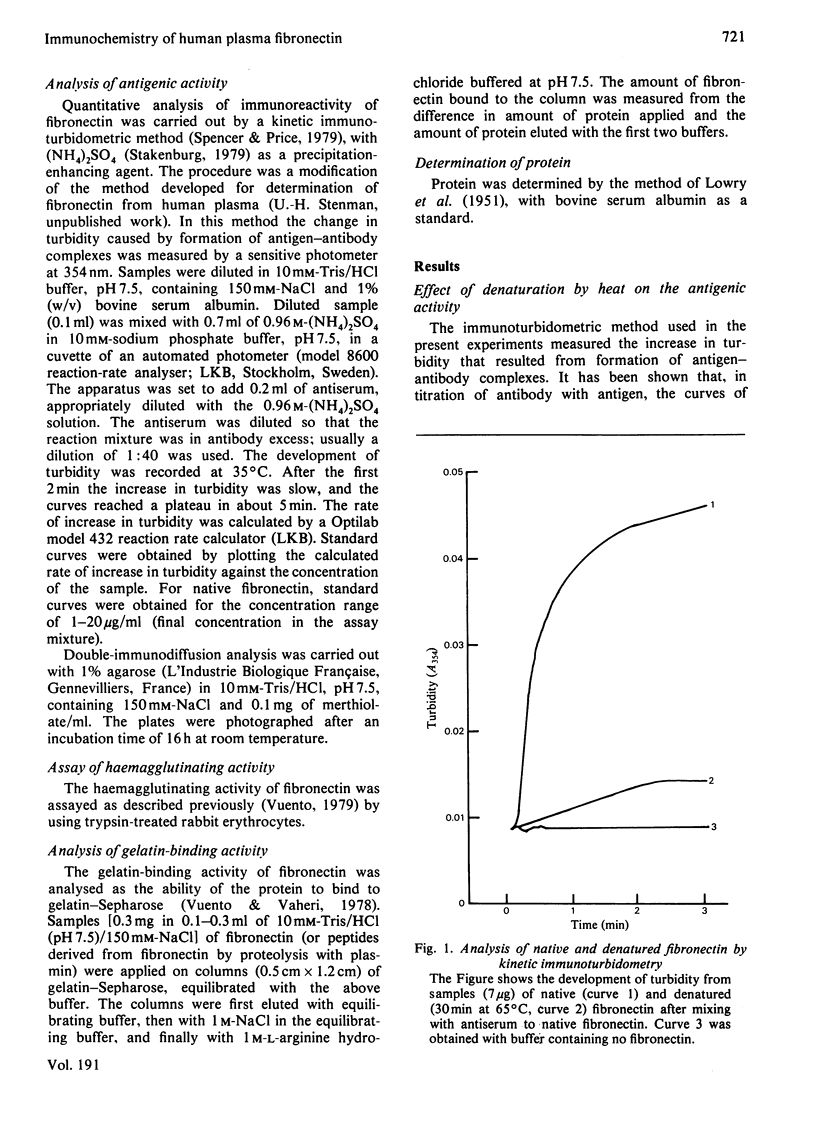
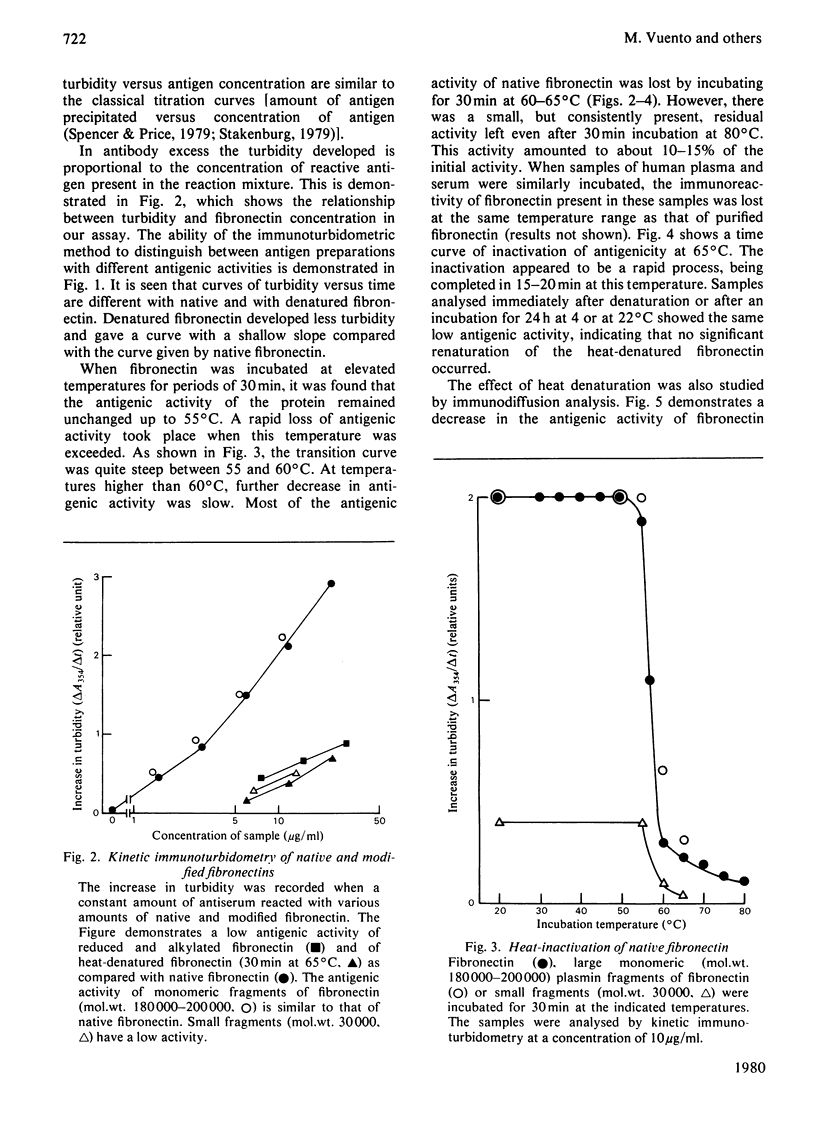
Abstract
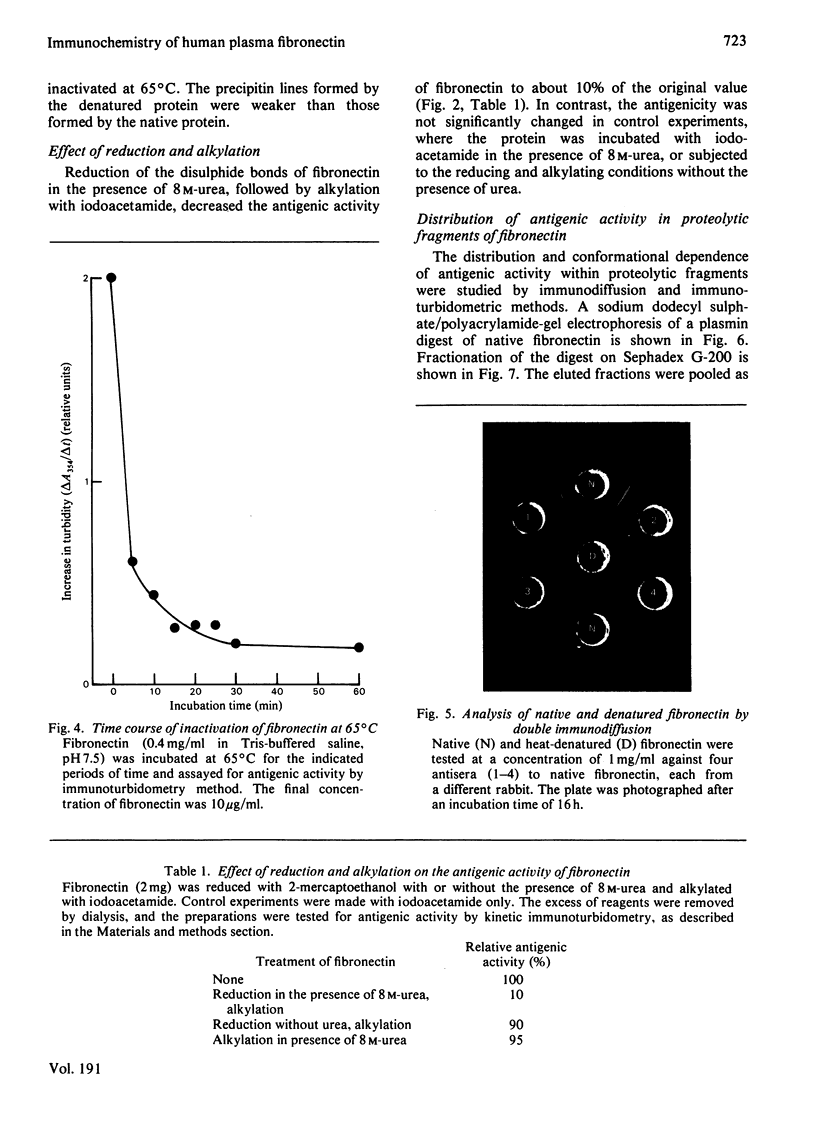
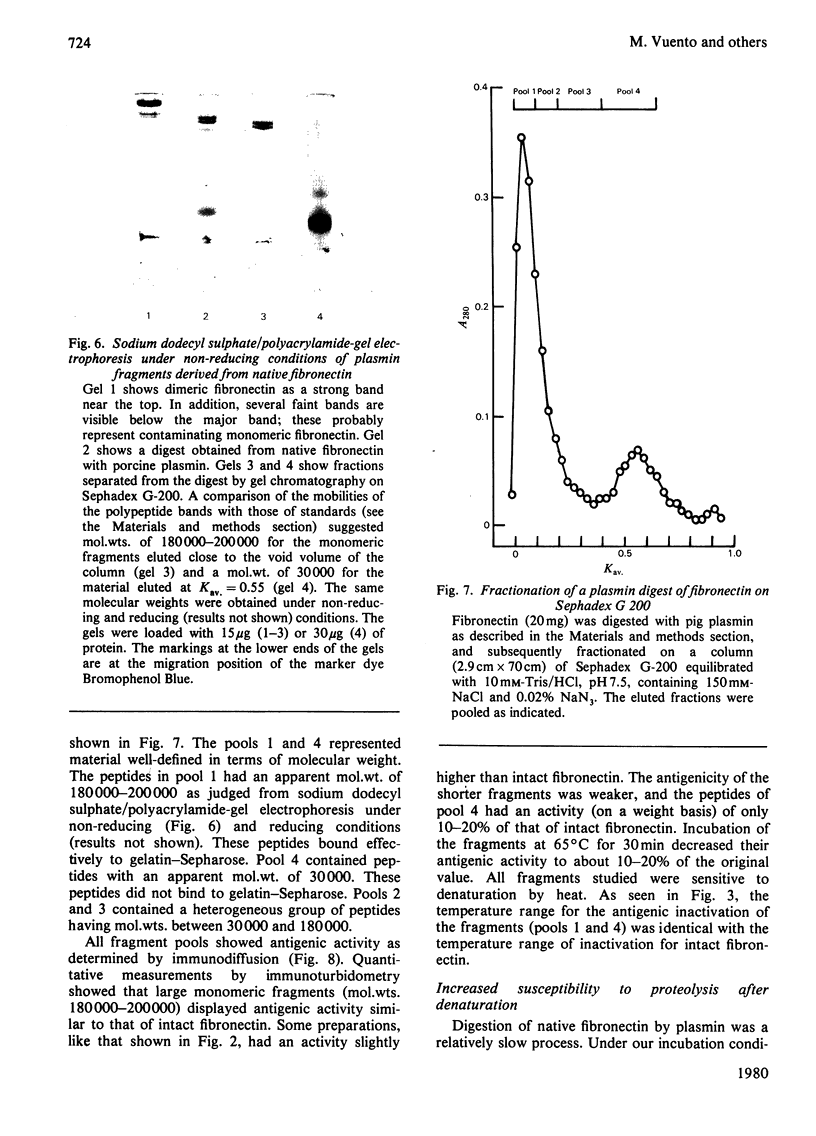
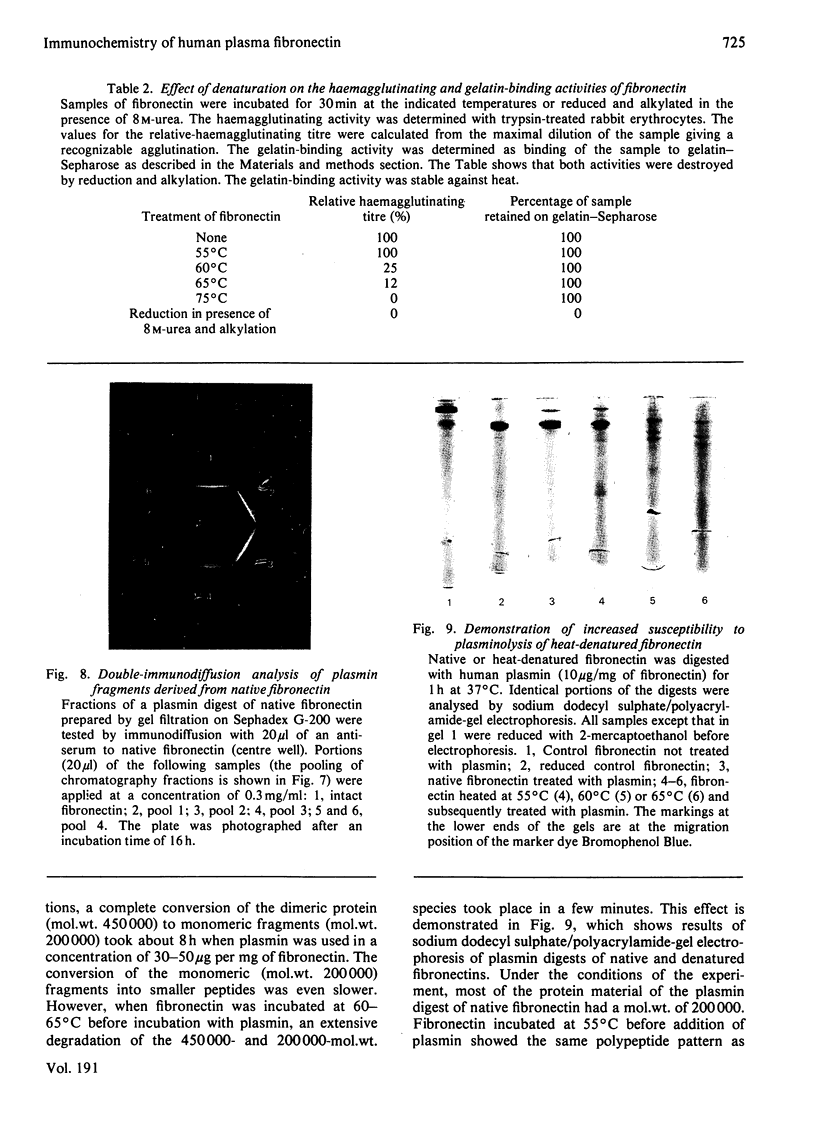
Human plasma fibronectin has been purified by a non-denaturing affinity chromatography procedure [Vuento & Vaheri, (1979) Biochem.J. 183, 331--337], and antisera have been raised by immunizing rabbits with the native protein. The antisera reacted strongly with native fibronectin, but only weakly with reduced and alkylated fibronectin or with heat-denaturated fibronectin. Denaturation also affected the haemagglutinating and gelatin-binding activities of fibronectin and increased its susceptibility to proteolytic degradation. The antisera reacted with fragments of fibronectin obtained by proteolysis with plasmin. Large fragments (mol.wt. 180000--200000), lacking the region harbouring the interchain disulphide bridges but containing the sites responsible for gelatin-binding and haemagglutinating activity, showed as intense a reaction with the antisera as intact fibronectin. Smaller peptides showed a weaker reaction. All fragments tested showed sensitivity to denaturation in their reaction with the antisera. The results were interpreted as showing that: (1) native fibronectin has an ordered conformation that is easily perturbed by denaturation; (2) most of the antigenic determinants of the protein are dependent on conformation; (3) the region of the fibronectin molecule containing the interchain disulphide bridges has only few antigenic determinants; and (4) covalent interaction of the two subunits does not contribute to the antigenic structure recognized by rabbit antisera. The observed correlation between the antigenic activity and a structural and functional intactness of fibronectin suggests that the antibodies to native fibronectin could be used as a conformational probe in studies on this protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander S. S., Jr, Colonna G., Edelhoch H. The structure and stability of human plasma cold-insoluble globulin. J Biol Chem. 1979 Mar 10;254(5):1501–1505. [PubMed] [Google Scholar]
- Balian G., Click E. M., Crouch E., Davidson J. M., Bornstein P. Isolation of a collagen-binding fragment from fibronectin and cold-insoluble globulin. J Biol Chem. 1979 Mar 10;254(5):1429–1432. [PubMed] [Google Scholar]
- Blumenstock F. A., Saba T. M., Weber P., Laffin R. Biochemical and immunological characterization of human opsonic alpha2SB glycoprotein: its identity with cold-insoluble globulin. J Biol Chem. 1978 Jun 25;253(12):4287–4291. [PubMed] [Google Scholar]
- Chen A. B., Amrani D. L., Mosesson M. W. Heterogeneity of the cold-insoluble globulin of human plasma (CIg), a circulating cell surface protein. Biochim Biophys Acta. 1977 Aug 23;493(2):310–322. doi: 10.1016/0005-2795(77)90187-8. [DOI] [PubMed] [Google Scholar]
- Colonna G., Alexander S. S., Jr, Yamada K. M., Pastan I., Edelhoch H. The stability of cell surface protein to surfactants and denaturants. J Biol Chem. 1978 Nov 10;253(21):7787–7790. [PubMed] [Google Scholar]
- Dessau W., Jilek F., Adelmann B. C., Hörmann H. Similarity of antigelatin factor and cold insoluble globulin. Biochim Biophys Acta. 1978 Mar 28;533(1):227–237. doi: 10.1016/0005-2795(78)90566-4. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Hakomori S. Proteolytic and chemical fragmentation of galactoprotein a, a major transformation-sensitive glycoprotein released from hamster embryo fibroblasts. J Biol Chem. 1979 Jun 25;254(12):5442–5450. [PubMed] [Google Scholar]
- Hahn L. H., Yamada K. M. Identification and isolation of a collagen-binding fragment of the adhesive glycoprotein fibronectin. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1160–1163. doi: 10.1073/pnas.76.3.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga S., Suzuki K., Hashimoto S. Bovine plasma cold-insoluble globulin: gross structure and function. Ann N Y Acad Sci. 1978 Jun 20;312:56–73. doi: 10.1111/j.1749-6632.1978.tb16793.x. [DOI] [PubMed] [Google Scholar]
- Jilek F., Hörmann H. Cold-insoluble globulin, II. Plasminolysis of cold-insoluble globulin. Hoppe Seylers Z Physiol Chem. 1977 Jan;358(1):133–136. [PubMed] [Google Scholar]
- Kuusela P. Fibronectin binds to Staphylococcus aureus. Nature. 1978 Dec 14;276(5689):718–720. doi: 10.1038/276718a0. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Chen A. B., Huseby R. M. The cold-insoluble globulin of human plasma: studies of its essential structural features. Biochim Biophys Acta. 1975 Apr 29;386(2):509–524. doi: 10.1016/0005-2795(75)90294-9. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G., Kuusela P., Shively J. E., Engvall E. Isolation of a tryptic fragment containing the collagen-binding site of plasma fibronectin. J Biol Chem. 1979 Jul 10;254(13):6054–6059. [PubMed] [Google Scholar]
- Ruoslahti E., Vaheri A. Interaction of soluble fibroblast surface antigen with fribrinogen and fibrin. J Exp Med. 1975 Feb 1;141(2):497–501. doi: 10.1084/jem.141.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer K., Price C. P. Kinetic immunoturbidimetry: the estimation of albumin. Clin Chim Acta. 1979 Jul 16;95(2):263–276. doi: 10.1016/0009-8981(79)90368-1. [DOI] [PubMed] [Google Scholar]
- Stakenburg J. Ammonium sulphate enhancement of the antigen-antibody reaction. Manual and automated nephelometric studies. Clin Chim Acta. 1979 Feb 1;91(3):251–261. doi: 10.1016/0009-8981(79)90481-9. [DOI] [PubMed] [Google Scholar]
- Stathakis N. E., Mosesson M. W. Interactions among heparin, cold-insoluble globulin, and fibrinogen in formation of the heparin-precipitable fraction of plasma. J Clin Invest. 1977 Oct;60(4):855–865. doi: 10.1172/JCI108840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemberger A., Hörmann H. Affinity chromatography on immobilized fibrinogen and fibrin monomer, II. The behavior of cold-insoluble globulin [1]. Hoppe Seylers Z Physiol Chem. 1976 Jul;357(7):1003–1005. [PubMed] [Google Scholar]
- Vaheri A., Mosher D. F. High molecular weight, cell surface-associated glycoprotein (fibronectin) lost in malignant transformation. Biochim Biophys Acta. 1978 Sep 18;516(1):1–25. doi: 10.1016/0304-419x(78)90002-1. [DOI] [PubMed] [Google Scholar]
- Vuento M. Hemagglutinin activity of human plasma fibronectin. Hoppe Seylers Z Physiol Chem. 1979 Sep;360(9):1327–1333. doi: 10.1515/bchm2.1979.360.2.1327. [DOI] [PubMed] [Google Scholar]
- Vuento M., Vaheri A. Dissociation of fibronectin from gelatin-agarose by amino compounds. Biochem J. 1978 Oct 1;175(1):333–336. doi: 10.1042/bj1750333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuento M., Vaheri A. Purification of fibronectin from human plasma by affinity chromatography under non-denaturing conditions. Biochem J. 1979 Nov 1;183(2):331–337. doi: 10.1042/bj1830331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Hynes R. O. Domain structure of fibronectin and its relation to function. Disulfides and sulfhydryl groups. J Biol Chem. 1979 Jul 25;254(14):6746–6754. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yamada K. M., Olden K. Fibronectins--adhesive glycoproteins of cell surface and blood. Nature. 1978 Sep 21;275(5677):179–184. doi: 10.1038/275179a0. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. The major cell surface glycoprotein of chick embryo fibroblasts is an agglutinin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3158–3162. doi: 10.1073/pnas.72.8.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]