Abstract
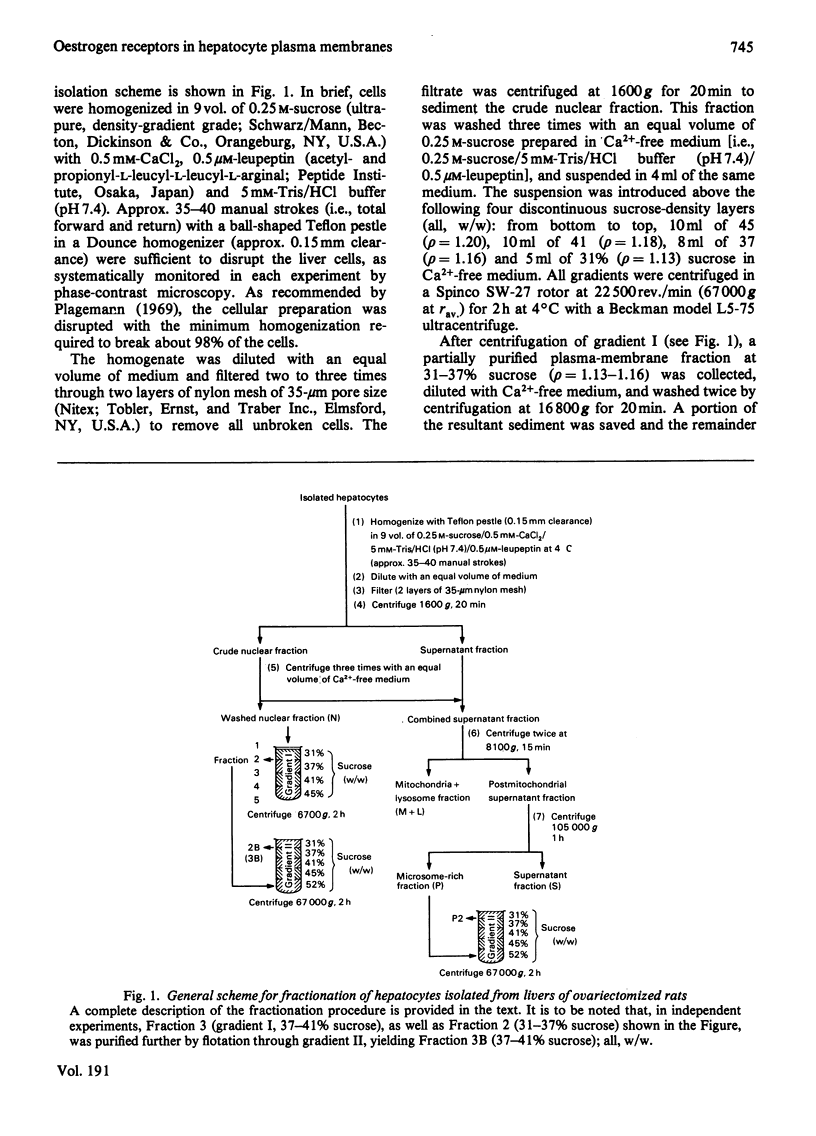
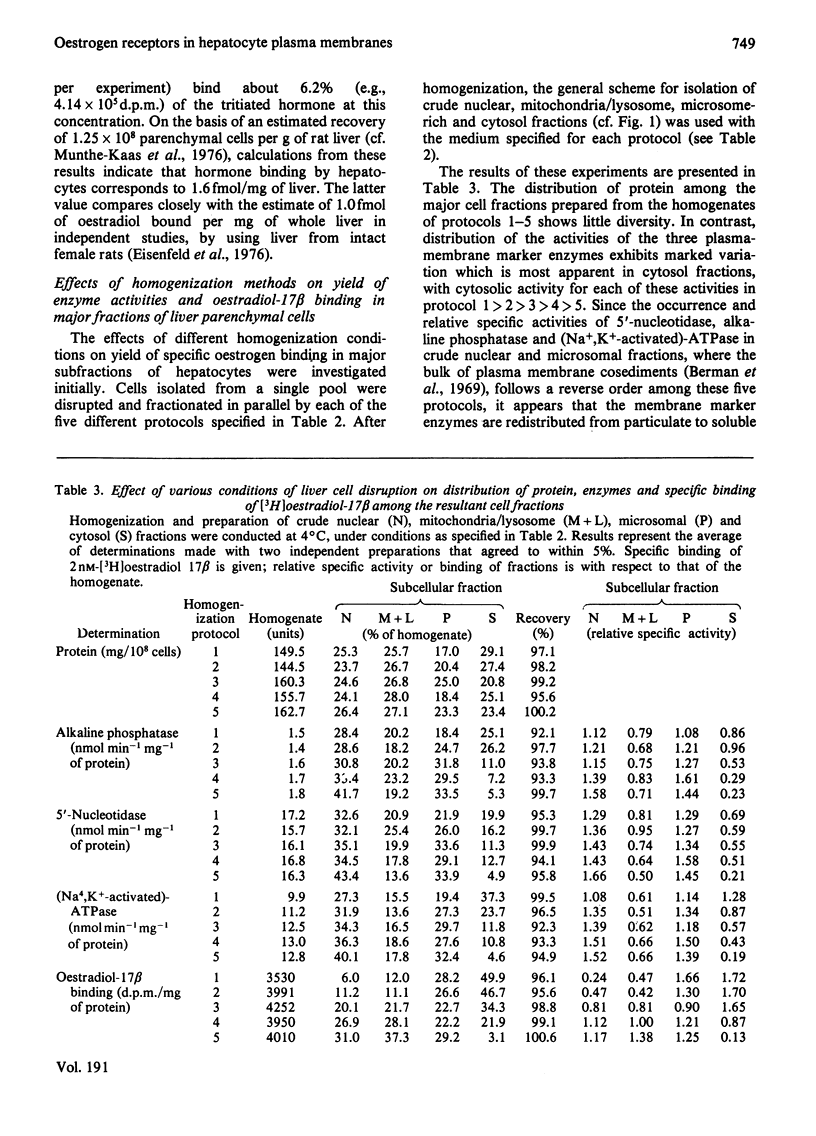
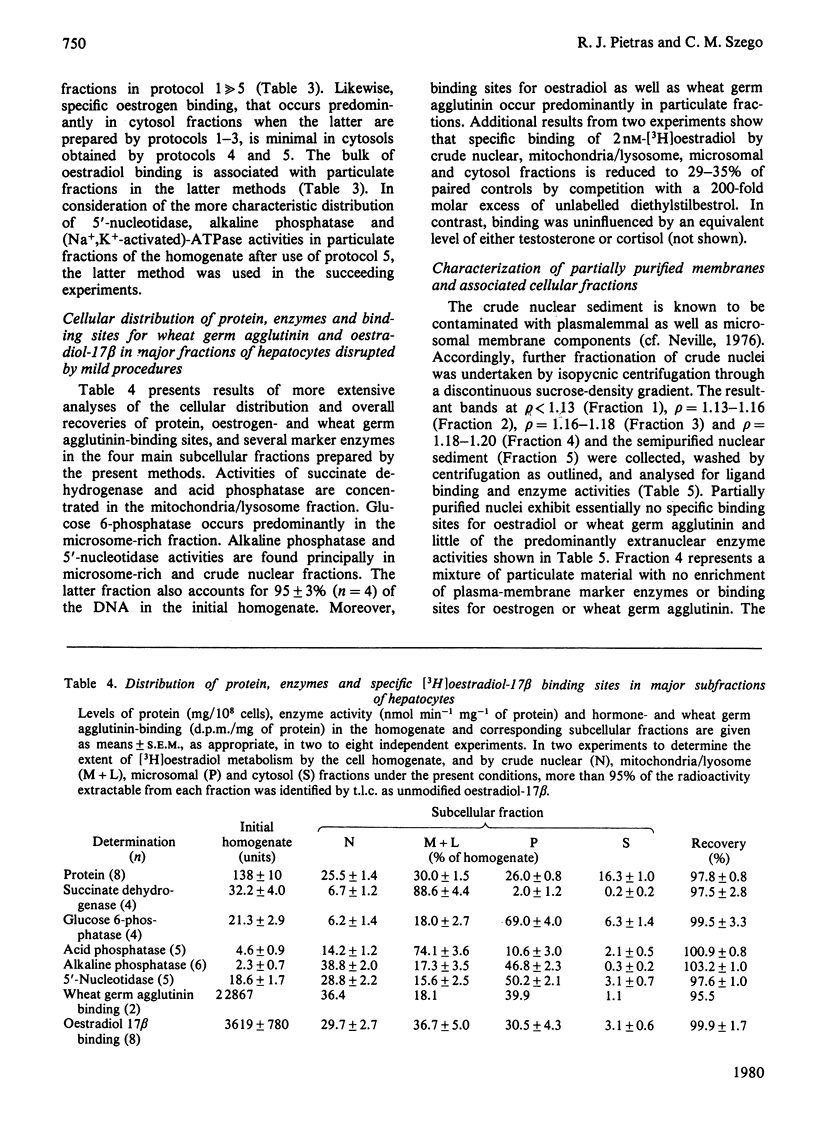
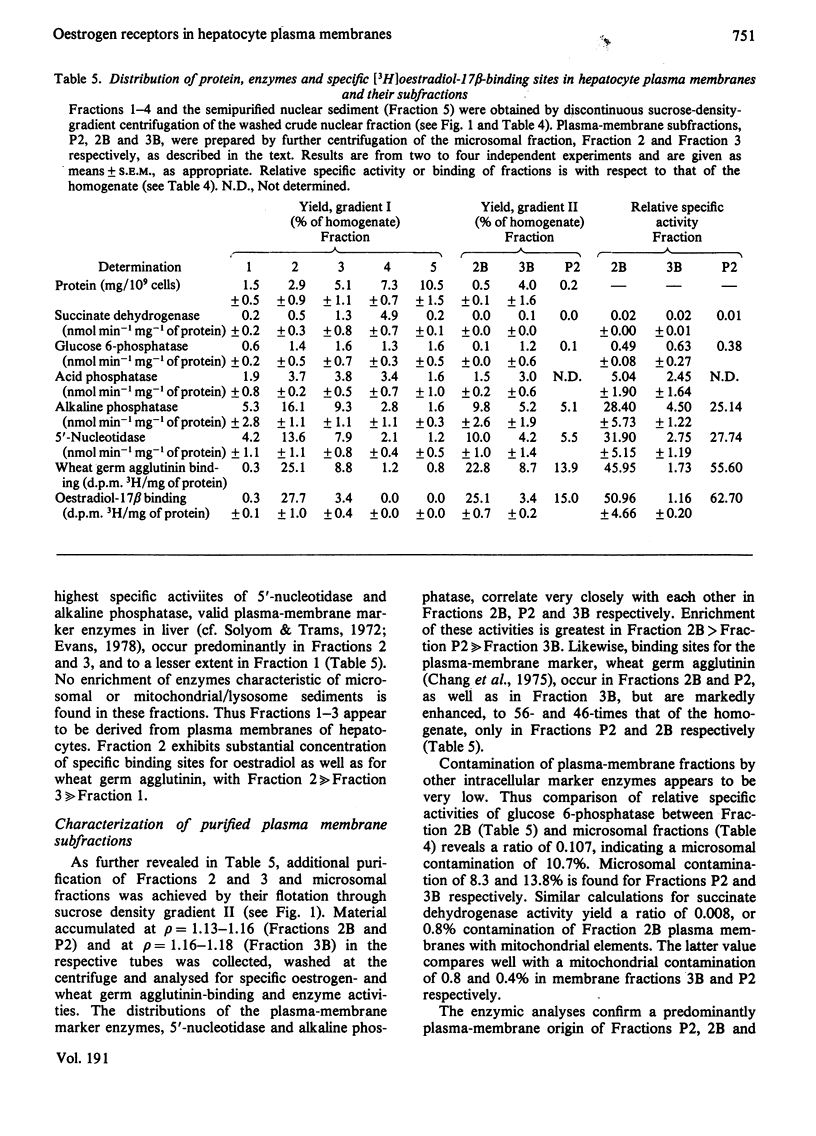
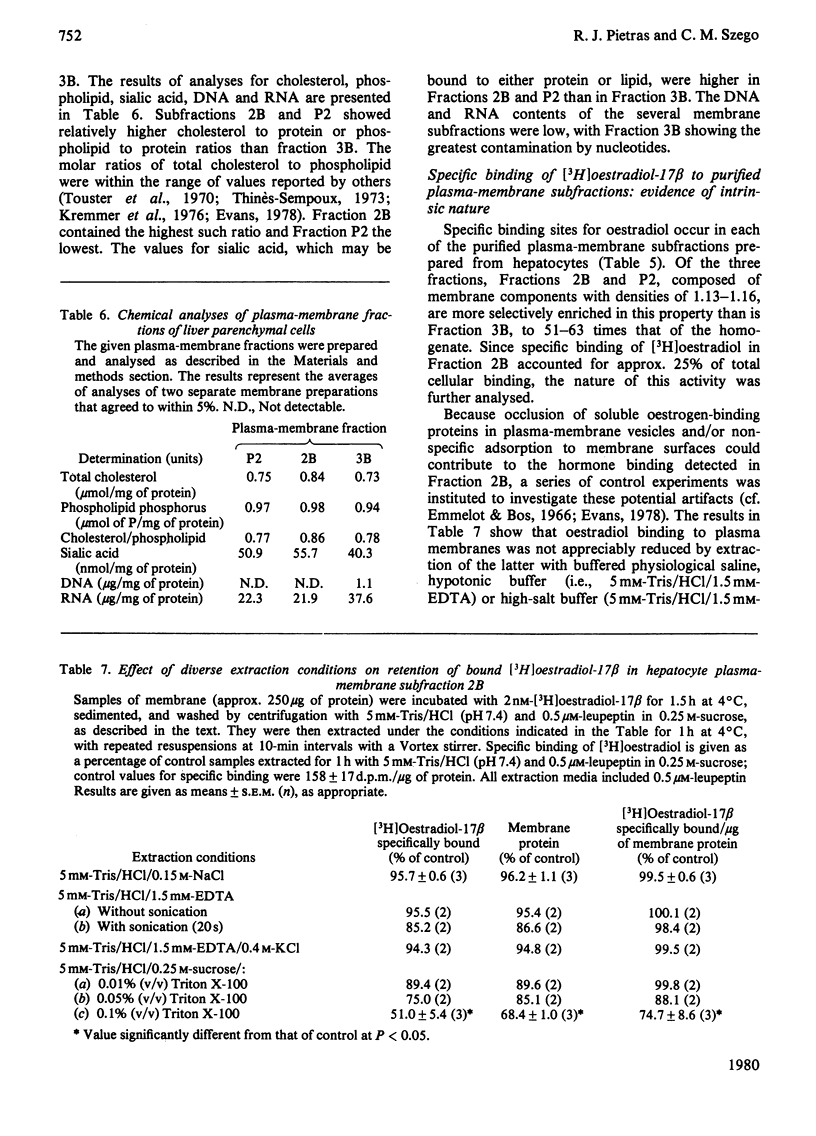
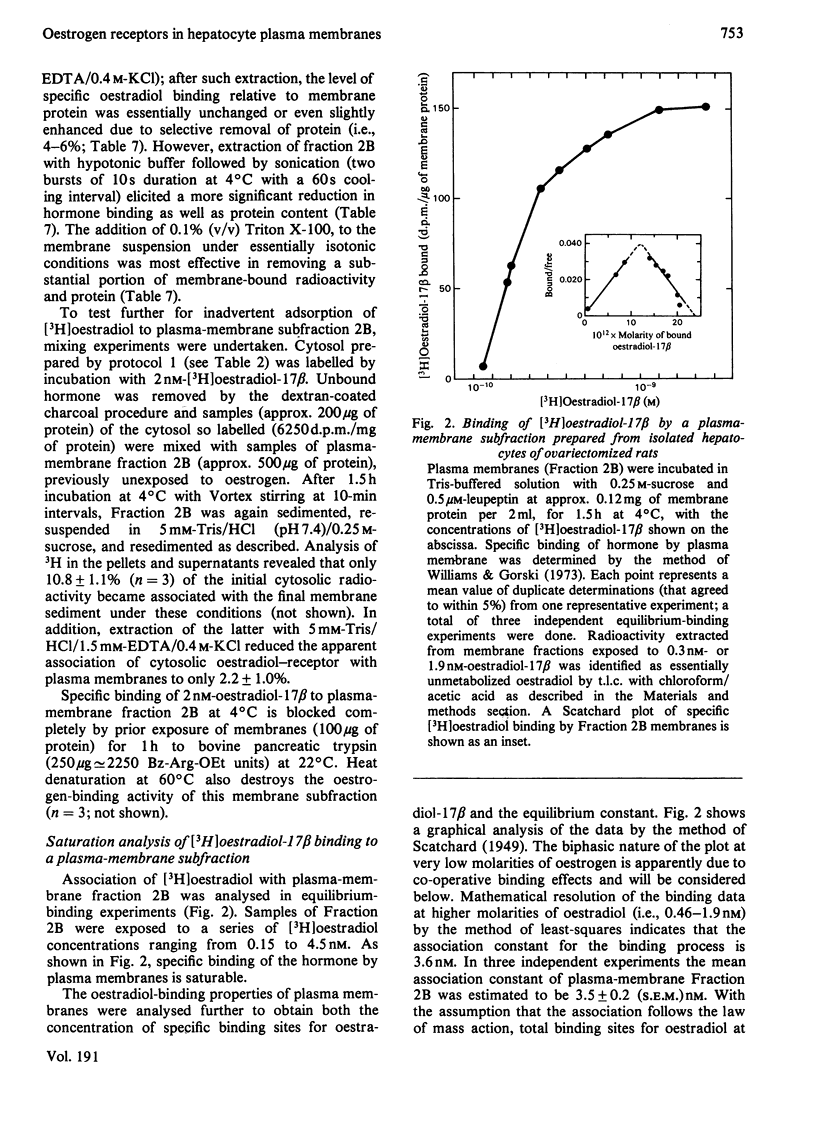
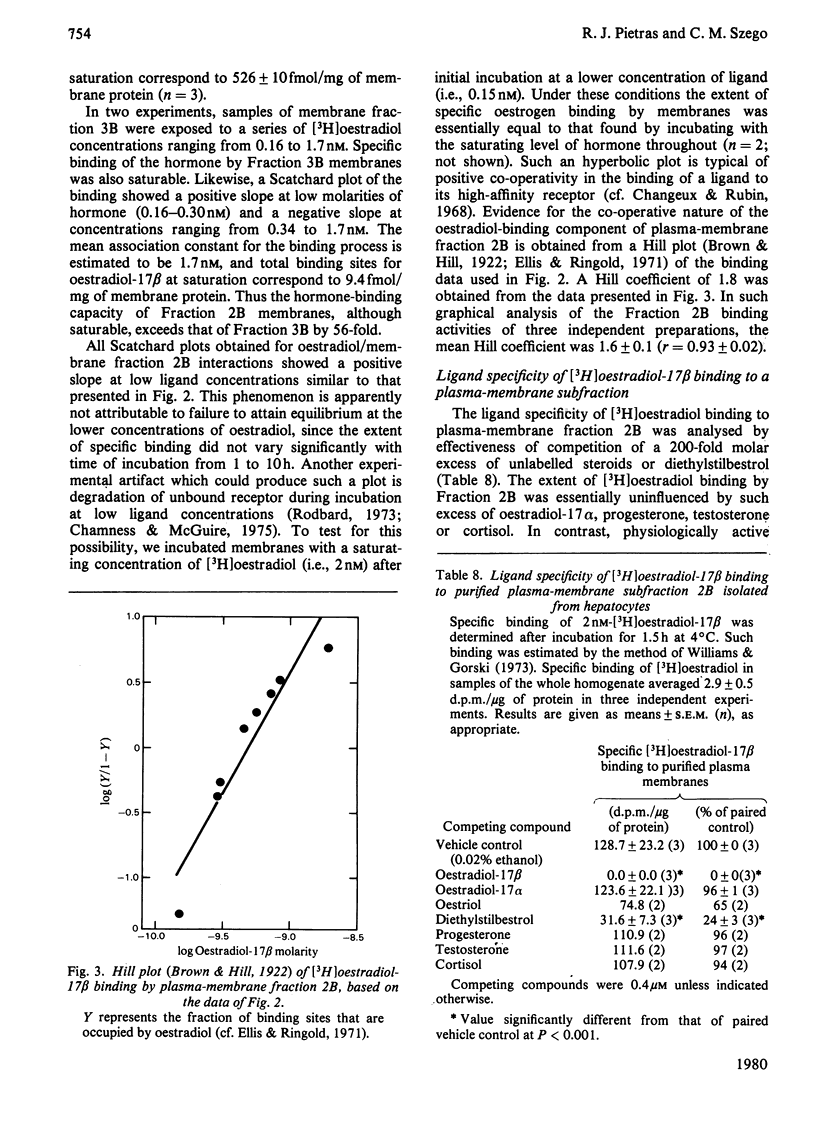
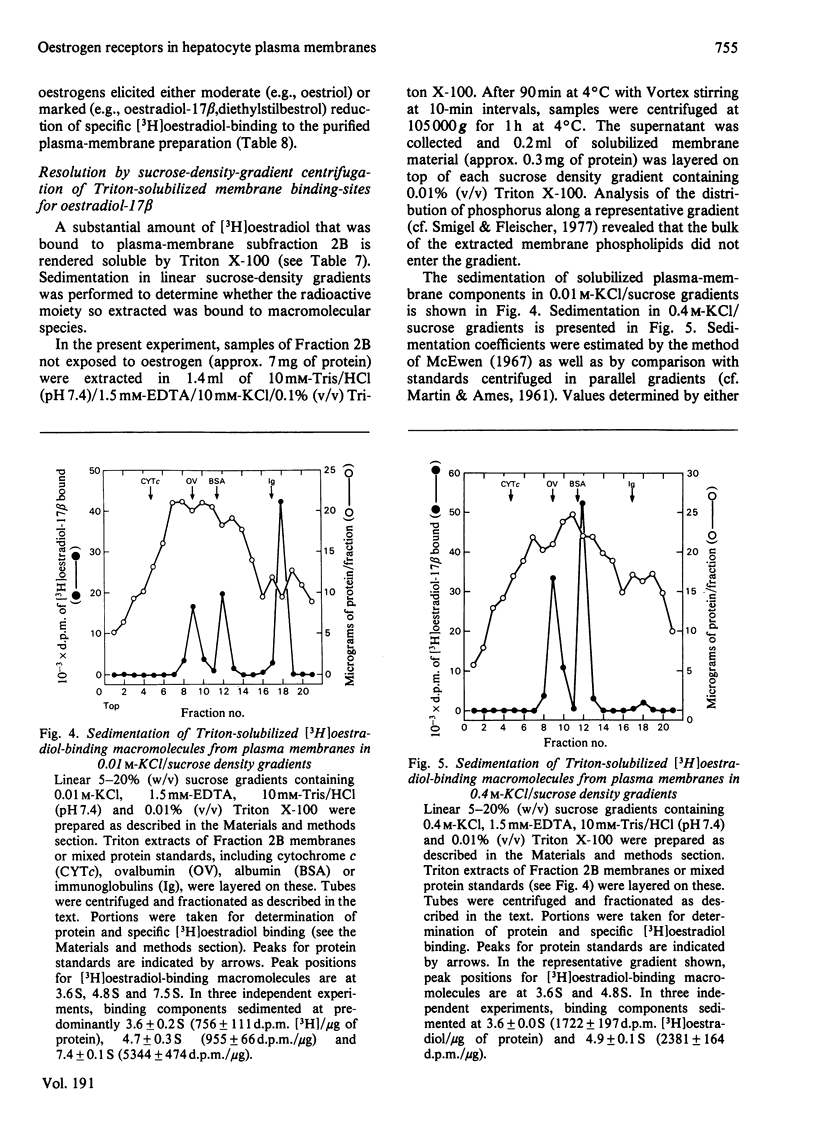
To assess the subcellular distribution of oestrogen-binding components in their native state, plasma membrane and other cell fractions were prepared from hepatocytes in the absence of [3H]oestradiol-17β. Cells from livers of ovariectomized rats were disrupted, with submaximal homogenization in buffered isotonic sucrose with CaCl2 and proteinase inhibitor, and fractionated by using isotonic media. Fractions were characterized by determinations of enzyme activities, biochemical constituents and ligand binding. Specific binding of 2nm-[3H]oestradiol-17β to intact cells and their fractions was detemined after equilibration for 1.5h at 4°C. More than 92% of the radioactivity from representative preparations was verified as authentic oestradiol by thin-layer chromatography. Activities of plasma-membrane marker enzymes as well as binding sites for oestrogen and for wheat germ agglutinin were present principally in particulate fractions, rather than in 105000g-supernatant fractions. However, by using alternative homogenization procedures (i.e. hypotonic media), known to fragment and strip structural components, oestradiol-binding sites and activities of plasma-membrane marker enzymes were distributed predominantly into cytosol. By using the more conservative procedures, plasma membranes of low (ρ=1.13–1.16) and high (ρ=1.16–1.18) density were purified from crude nuclear fractions. A second low-density subfraction of plasma membrane was prepared from microsome-rich fractions. Activities of plasma-membrane marker enzymes were enriched to about 28 and four times that of the homogenate in plasma membranes of low and high density respectively. Binding sites for wheat germ agglutinin and oestradiol were concentrated in low-density plasma membranes to 46–63 times that of the homogenate. Specific binding of oestrogen in low-density plasma membranes purified from crude nuclei was saturable, with an apparent association constant of 3.5nm. At saturation, such oestradiol receptors corresponded to 526fmol/mg of membrane protein. A Hill plot showed a moderate degree of positive co-operativity in the interaction of hormone with plasma membranes. Specific binding of [3H]oestradiol-17β was reduced by a 200-fold molar excess of unlabelled oestradiol-17β, oestriol or diethylstilbestrol, but not by oestradiol-17α, cortisol, testosterone or progesterone. Binding was also blocked by prior exposure of membranes to trypsin or to 60°C, but remained essentially undiminished by extraction of membranes with either hypotonic or high-salt buffers. Extraction with 0.1% (v/v) Triton X-100 partially solubilized the oestrogen-binding component(s) of plasma membranes. Particle-free extracts were resolved on 5–20% (w/v) sucrose density gradients with either 0.01m- or 0.4m-KCl, and the fractions were analysed by adsorption to hydroxyapatite. In low-salt gradients macromolecule-bound oestrogen sedimented at predominantly 7.4S and binding was 1560 times that of the homogenate. Under high-salt conditions oestradiol-binding activity occurred at both 3.6S and 4.9S.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Beers P. C., Rosner W. The binding of estrogens in the liver of the rat: demonstration and endocrine influences. J Steroid Biochem. 1977 Apr;8(4):251–258. doi: 10.1016/0022-4731(77)90017-6. [DOI] [PubMed] [Google Scholar]
- Berman H. M., Gram W., Spirtes M. A. An improved, reproducible method opreparing rat liver plasma cell membranes in buffered isotonic sucrose. Biochim Biophys Acta. 1969 Jun 3;183(1):10–18. doi: 10.1016/0005-2736(69)90124-2. [DOI] [PubMed] [Google Scholar]
- Bishara R. H., Jakovljevic I. M. The separation of some estrogens by thin layer chromatography. J Chromatogr. 1969 Apr 22;41(1):136–138. doi: 10.1016/0021-9673(64)80114-x. [DOI] [PubMed] [Google Scholar]
- Blyth C. A., Clark R. P., Freedman R. B., Hammond J., James D. W., Rabin B. R., Ridge D., Vinter J., Williams D. Steroid-binding properties of a plasma-membrane fraction from rat liver. Eur J Biochem. 1973 Jan 3;32(1):57–62. doi: 10.1111/j.1432-1033.1973.tb02578.x. [DOI] [PubMed] [Google Scholar]
- Blyth C. A., Freedman R. B., Rabin B. R. Sex specific binding of steroid hormones to microsomal embranes of rat liver. Nat New Biol. 1971 Mar 31;230(13):137–139. doi: 10.1038/newbio230137a0. [DOI] [PubMed] [Google Scholar]
- Buller R. E., O'Malley B. W. The biology and mechanism of steroid hormone receptor interaction with the eukaryotic nucleus. Biochem Pharmacol. 1976 Jan;25(1):1–12. doi: 10.1016/0006-2952(76)90164-7. [DOI] [PubMed] [Google Scholar]
- Chamness G. C., Costlow M. E., McGuire W. L. Estrogen receptor in rat liver and its dependence on prolactin. Steroids. 1975 Sep;26(3):363–371. doi: 10.1016/0039-128x(75)90081-1. [DOI] [PubMed] [Google Scholar]
- Chamness G. C., McGuire W. L. Scatchard plots: common errors in correction and interpretation. Steroids. 1975 Oct;26(4):538–542. doi: 10.1016/0039-128x(75)90073-2. [DOI] [PubMed] [Google Scholar]
- Chang K. J., Bennett V., Cuatrecasas P. Membrane receptors as general markers for plasma membrane isolation procedures. The use of 125-I-labeled wheat germ agglutinin, insulin, and cholera toxin. J Biol Chem. 1975 Jan 25;250(2):488–500. [PubMed] [Google Scholar]
- Changeux J. P., Rubin M. M. Allosteric interactions in aspartate transcarbamylase. 3. Interpretation of experimental data in terms of the model of Monod, Wyman, and Changeux. Biochemistry. 1968 Feb;7(2):553–561. doi: 10.1021/bi00842a601. [DOI] [PubMed] [Google Scholar]
- Church R. B., McCarthy B. J. Unstable nuclear RNA synthesis following estrogen stimulation. Biochim Biophys Acta. 1970 Jan 21;199(1):103–114. doi: 10.1016/0005-2787(70)90699-4. [DOI] [PubMed] [Google Scholar]
- Coleman R. Some features of the lipid composition of rat liver surface and cytoplasmic membranes. Chem Phys Lipids. 1968 Feb;2(1):144–146. doi: 10.1016/0009-3084(68)90039-x. [DOI] [PubMed] [Google Scholar]
- Davis R. A., Kern F., Jr, Showalter R., Sutherland E., Sinensky M., Simon F. R. Alterations of hepatic Na+,K+-atpase and bile flow by estrogen: effects on liver surface membrane lipid structure and function. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4130–4134. doi: 10.1073/pnas.75.9.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Plasma membranes of mammalian cells: a review of methods for their characterization and isolation. J Cell Biol. 1973 Feb;56(2):275–303. doi: 10.1083/jcb.56.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMMELOT P., BOS C. J., BENEDETTI E. L., RUEMKE P. STUDIES ON PLASMA MEMBRANES. I. CHEMICAL COMPOSITION AND ENZYME CONTENT OF PLASMA MEMBRANES ISOLATED FROM RAT LIVER. Biochim Biophys Acta. 1964 Jul 15;90:126–145. doi: 10.1016/0304-4165(64)90125-4. [DOI] [PubMed] [Google Scholar]
- Edmondson H. A., Henderson B., Benton B. Liver-cell adenomas associated with use of oral contraceptives. N Engl J Med. 1976 Feb 26;294(9):470–472. doi: 10.1056/NEJM197602262940904. [DOI] [PubMed] [Google Scholar]
- Eisenfeld A. J., Aten R., Weinberger M., Haselbacher G., Halpern K., Krakoff L. Estrogen receptor in the mammalian liver. Science. 1976 Feb 27;191(4229):862–865. doi: 10.1126/science.175442. [DOI] [PubMed] [Google Scholar]
- Emmelot P., Bos C. J. Differences in the association of two glycolytic enzymes with plasma membranes isolated from rat liver and hepatoma. Biochim Biophys Acta. 1966 Jun 29;121(2):434–436. doi: 10.1016/0304-4165(66)90140-1. [DOI] [PubMed] [Google Scholar]
- Erdos T., Best-Belpomme M., Bessada R. A rapid assay for binding estradiol to uterine receptor(s). Anal Biochem. 1970 Oct;37(2):244–252. doi: 10.1016/0003-2697(70)90044-8. [DOI] [PubMed] [Google Scholar]
- Galliard T., Michell R. H., Hawthorne J. N. Incorporation of phosphate into diphosphoinositide by subcellular fractions from liver. Biochim Biophys Acta. 1965 Dec 2;106(3):551–563. doi: 10.1016/0005-2760(65)90071-8. [DOI] [PubMed] [Google Scholar]
- Gorski J., Gannon F. Current models of steroid hormone action: a critique. Annu Rev Physiol. 1976;38:425–450. doi: 10.1146/annurev.ph.38.030176.002233. [DOI] [PubMed] [Google Scholar]
- Gorski J., Toft D., Shyamala G., Smith D., Notides A. Hormone receptors: studies on the interaction of estrogen with the uterus. Recent Prog Horm Res. 1968;24:45–80. doi: 10.1016/b978-1-4831-9827-9.50008-3. [DOI] [PubMed] [Google Scholar]
- HUEBSCHER G., WEST G. R. SPECIFIC ASSAYS OF SOME PHOSPHATASES IN SUBCELLULAR FRACTIONS OF SMALL INTESTINAL MUCOSA. Nature. 1965 Feb 20;205:799–800. doi: 10.1038/205799a0. [DOI] [PubMed] [Google Scholar]
- Hamilton T. H. Control by estrogen of genetic transcription and translation. Binding to chromatin and stimulation of nucleolar RNA synthesis are primary events in the early estrogen action. Science. 1968 Aug 16;161(3842):649–661. doi: 10.1126/science.161.3842.649. [DOI] [PubMed] [Google Scholar]
- Hernández-Pérez O., Ballesteros L. M., Rosado A. Binding of 17-beta-estradiol to the outer surface and nucleus of human spermatozoa. Arch Androl. 1979;3(1):23–29. doi: 10.3109/01485017908985044. [DOI] [PubMed] [Google Scholar]
- Hirsch P. C., Szego C. M. Estradiol receptor functions of soluble proteins from target-specific lysosomes. J Steroid Biochem. 1974 Oct;5(6):533–542. doi: 10.1016/0022-4731(74)90101-0. [DOI] [PubMed] [Google Scholar]
- Howard R. B., Lee J. C., Pesch L. A. The fine structure, potassium content, and respiratory activity of isolated rat liver parenchymal cells prepared by improved enzymatic techniques. J Cell Biol. 1973 Jun;57(3):642–658. doi: 10.1083/jcb.57.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail-Beigi F., Edelman I. S. The mechanism of the calorigenic action of thyroid hormone. Stimulation of Na plus + K plus-activated adenosinetriphosphatase activity. J Gen Physiol. 1971 Jun;57(6):710–722. doi: 10.1085/jgp.57.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson V., Chalkley R. The cytoplasmic estradiol receptors of bovine uterus. Their occurrence, interconversion, and binding properties. J Biol Chem. 1974 Mar 10;249(5):1627–1636. [PubMed] [Google Scholar]
- Jensen E. V., Mohla S., Gorell T. A., De Sombre E. R. The role of estrophilin in estrogen action. Vitam Horm. 1974;32:89–127. doi: 10.1016/s0083-6729(08)60007-2. [DOI] [PubMed] [Google Scholar]
- KING R. J., GORDON J., INMAN D. R. THE INTRACELLULAR LOCALIZATION OF OESTROGEN IN RAT TISSUES. J Endocrinol. 1965 Apr;32:9–15. doi: 10.1677/joe.0.0320009. [DOI] [PubMed] [Google Scholar]
- Korenman S. Use of receptor proteins for steroid-hormone assays. Methods Enzymol. 1975;36:49–52. doi: 10.1016/s0076-6879(75)36006-0. [DOI] [PubMed] [Google Scholar]
- Kremmer T., Wisher M. H., Evans W. H. The lipid composition of plasma membrane subfractions originating from the three major functional domains of the rat hepatocyte cell surface. Biochim Biophys Acta. 1976 Dec 14;455(3):655–664. doi: 10.1016/0005-2736(76)90039-0. [DOI] [PubMed] [Google Scholar]
- Little M., Rosenfeld G. C., Jungblut P. W. Cytoplasmic estradiol "receptors" associated with the "microsomal" fraction of pig uterus. Hoppe Seylers Z Physiol Chem. 1972 Feb;353(2):231–242. doi: 10.1515/bchm2.1972.353.1.231. [DOI] [PubMed] [Google Scholar]
- MAGGIO R., SIEKEVITZ P., PALADE G. E. STUDIES ON ISOLATED NUCLEI. I. ISOLATION AND CHEMICAL CHARACTERIZATION OF A NUCLEAR FRACTION FROM GUINEA PIG LIVER. J Cell Biol. 1963 Aug;18:267–291. doi: 10.1083/jcb.18.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Macartney J. C., Thomas G. H. NADP-linked 17-beta- and 20-alpha-steroid reductase activity in the rabbit uterus. J Endocrinol. 1969 Feb;43(2):247–252. doi: 10.1677/joe.0.0430247. [DOI] [PubMed] [Google Scholar]
- McDOUGAL D. B., Jr, FARMER H. S. A fluorometric method for total serum cholesterol. J Lab Clin Med. 1957 Sep;50(3):485–488. [PubMed] [Google Scholar]
- McEwen C. R. Tables for estimating sedimentation through linear concentration gradients of sucrose solution. Anal Biochem. 1967 Jul;20(1):114–149. doi: 10.1016/0003-2697(67)90271-0. [DOI] [PubMed] [Google Scholar]
- Munthe-Kaas A. C., Berg T., Seljelid R. Distribution of lysosomal enzymes in different types of rat liver cells. Exp Cell Res. 1976 Apr;99(1):146–154. doi: 10.1016/0014-4827(76)90689-3. [DOI] [PubMed] [Google Scholar]
- Müller R. E., Johnston T. C., Wotiz H. H. Binding of estradiol to purified uterine plasma membranes. J Biol Chem. 1979 Aug 25;254(16):7895–7900. [PubMed] [Google Scholar]
- Noteboom W. D., Gorski J. Stereospecific binding of estrogens in the rat uterus. Arch Biochem Biophys. 1965 Sep;111(3):559–568. doi: 10.1016/0003-9861(65)90235-3. [DOI] [PubMed] [Google Scholar]
- Pavlik E. J., Coulson P. B. Hydroxylapatite "batch" assay for estrogen receptors: increased sensitivity over present receptor assays. J Steroid Biochem. 1976 May;7(5):357–368. doi: 10.1016/0022-4731(76)90095-9. [DOI] [PubMed] [Google Scholar]
- Pavlik E. J., Rutledge S., Eckert R. L., Katzenellenbogen B. S. Localization of estrogen receptors in uterine cells. An appraisal of translocation. Exp Cell Res. 1979 Oct 1;123(1):177–189. doi: 10.1016/0014-4827(79)90434-8. [DOI] [PubMed] [Google Scholar]
- Pietras R. J. Heritable membrane alterations and growth associated with enhanced leupeptin-sensitive proteinase activity in epithelial cells exposed to dibutylnitrosamine in vitro. Cancer Res. 1978 Apr;38(4):1019–1030. [PubMed] [Google Scholar]
- Pietras R. J., Szego C. M. Estrogen receptors in uterine plasma membrane. J Steroid Biochem. 1979 Oct;11(4):1471–1483. doi: 10.1016/0022-4731(79)90124-9. [DOI] [PubMed] [Google Scholar]
- Pietras R. J., Szego C. M. Metabolic and proliferative responses to estrogen by hepatocytes selected for plasma membrane binding-sites specific for estradiol-17beta. J Cell Physiol. 1979 Jan;98(1):145–159. doi: 10.1002/jcp.1040980116. [DOI] [PubMed] [Google Scholar]
- Pietras R. J., Szego C. M. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977 Jan 6;265(5589):69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G. RNA synthesis in exponentially growing rat hepatoma cells. I. A caution in equating pulse-labeled polyribosomal RNA with messenger RNA. Biochim Biophys Acta. 1969 May 20;182(1):46–56. doi: 10.1016/0005-2787(69)90519-x. [DOI] [PubMed] [Google Scholar]
- Puca G. A., Nola E., Sica V., Bresciani F. Estrogen-binding proteins of calf uterus. Partial purification and preliminary characterization of two cytoplasmic proteins. Biochemistry. 1971 Sep 28;10(20):3769–3780. doi: 10.1021/bi00796a020. [DOI] [PubMed] [Google Scholar]
- Rodbard D. Mathematics of hormone-receptor interaction. I. Basic principles. Adv Exp Med Biol. 1973;36(0):289–326. doi: 10.1007/978-1-4684-3237-4_14. [DOI] [PubMed] [Google Scholar]
- Rosner A. L., Schwartz A. M., Bray C. L., Burstein N. A. Accelerated dissociation of estrogen receptor--ligand complexes by estradiol. Evidence for negative cooperativity of binding. Arch Biochem Biophys. 1979 Nov;198(1):153–163. doi: 10.1016/0003-9861(79)90406-5. [DOI] [PubMed] [Google Scholar]
- Sanborn B. M., Rao B. R., Korenman S. G. Interaction of 17 -estradiol and its specific uterine receptor. Evidence for complex kinetic and equilibrium behavior. Biochemistry. 1971 Dec 21;10(26):4955–4962. doi: 10.1021/bi00802a019. [DOI] [PubMed] [Google Scholar]
- Sen K. K., Gupta P. D., Talwar G. P. Intracellular localization of estrogens in chick liver: increase of the binding sites for the hormone on repeated treatment of the birds with the hormone. J Steroid Biochem. 1975 Aug;6(8):1223–1227. doi: 10.1016/0022-4731(75)90110-7. [DOI] [PubMed] [Google Scholar]
- Smigel M., Fleischer S. Characterization of triton X-100-solubilized prostaglandin E binding protein of rat liver plasma membranes. J Biol Chem. 1977 Jun 10;252(11):3689–3696. [PubMed] [Google Scholar]
- Solyom A., Trams E. G. Enzyme markers in characterization of isolated plasma membranes. Enzyme. 1972;13(5-6):329–372. doi: 10.1159/000459682. [DOI] [PubMed] [Google Scholar]
- Szego C. M., Seeler B. J., Steadman R. A., Hill D. F., Kimura A. K., Roberts J. A. The lysosomal membrane complex. Focal point of primary steroid hormone action. Biochem J. 1971 Jul;123(4):523–538. doi: 10.1042/bj1230523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szego C. M. The lysosome as a mediator of hormone action. Recent Prog Horm Res. 1974;30(0):171–233. doi: 10.1016/b978-0-12-571130-2.50009-2. [DOI] [PubMed] [Google Scholar]
- Tchernitchin X., Tchernitchin A., Galand P. Dynamics of eosinophils in the uterus after oestrogen administration. Differentiation. 1976 Jun 4;5(2-3):151–154. doi: 10.1111/j.1432-0436.1976.tb00906.x. [DOI] [PubMed] [Google Scholar]
- Touster O., Aronson N. N., Jr, Dulaney J. T., Hendrickson H. Isolation of rat liver plasma membranes. Use of nucleotide pyrophosphatase and phosphodiesterase I as marker enzymes. J Cell Biol. 1970 Dec;47(3):604–618. doi: 10.1083/jcb.47.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viladiu P., Delgado C., Pensky J., Pearson O. H. Estrogen binding protein of rat liver. Endocr Res Commun. 1975;2(3):273–280. doi: 10.3109/07435807509053854. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Williams D., Gorski J. A new assessment of subcellular distribution of bound estrogen in the uterus. Biochem Biophys Res Commun. 1971 Oct 1;45(1):258–264. doi: 10.1016/0006-291x(71)90078-7. [DOI] [PubMed] [Google Scholar]
- Williams D., Gorski J. Equilibrium binding of estradiol by uterine cell suspensions and whole uteri in vitro. Biochemistry. 1974 Dec 31;13(27):5537–5542. doi: 10.1021/bi00724a013. [DOI] [PubMed] [Google Scholar]
- Williams D., Gorski J. Preparation and characterization of free cell suspensions from the immature rat uterus. Biochemistry. 1973 Jan 16;12(2):297–306. doi: 10.1021/bi00726a019. [DOI] [PubMed] [Google Scholar]
- Wisher M. H., Evans W. H. Preparation of plasma-membrane subfractions from isolated rat hepatocytes. Biochem J. 1977 May 15;164(2):415–422. doi: 10.1042/bj1640415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Omata S., Onishi T., Terayama H. In vivo effects of proteases on cell surface architecture and cell proliferation in the liver. Cancer Res. 1973 Mar;33(3):567–572. [PubMed] [Google Scholar]


