Abstract
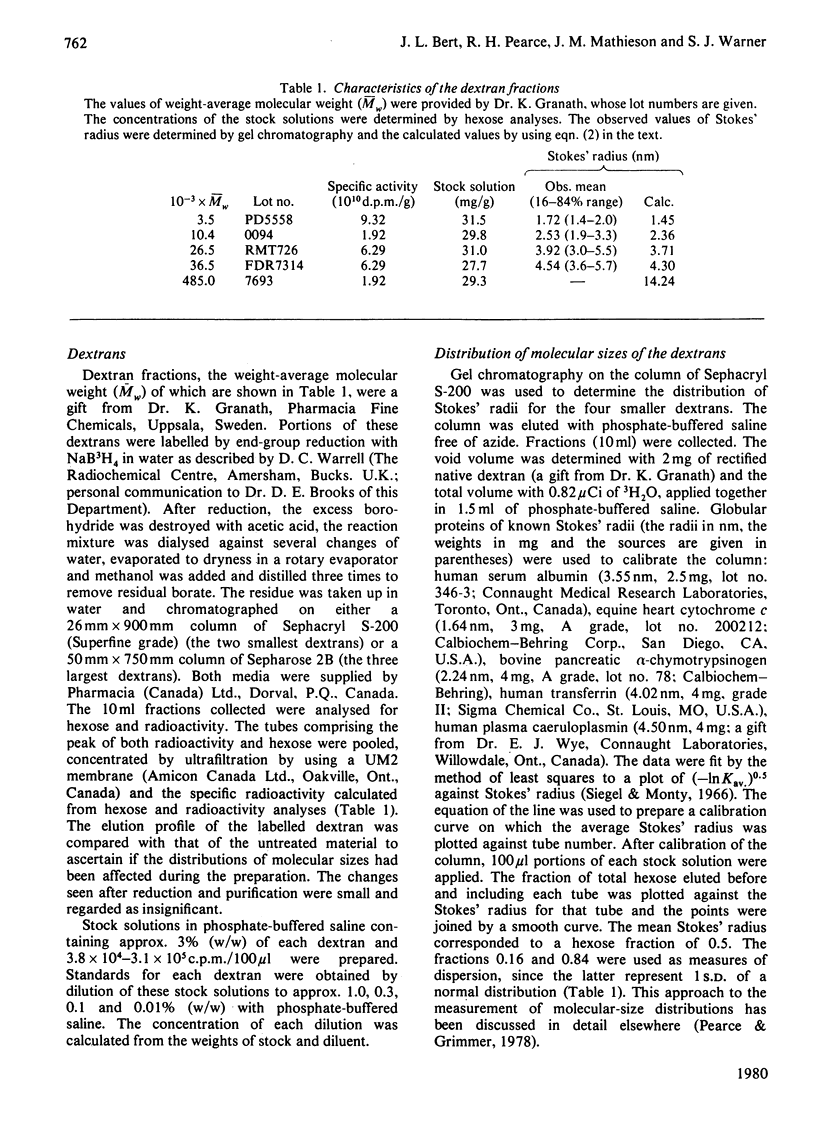
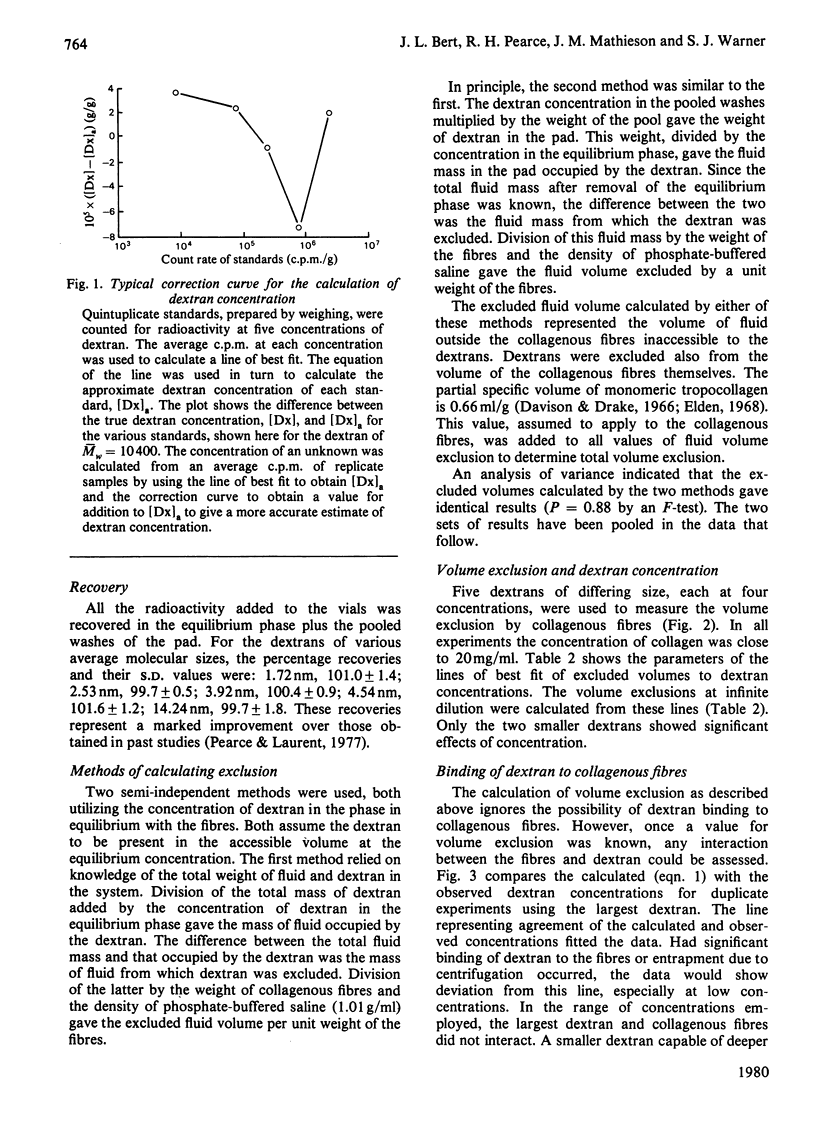
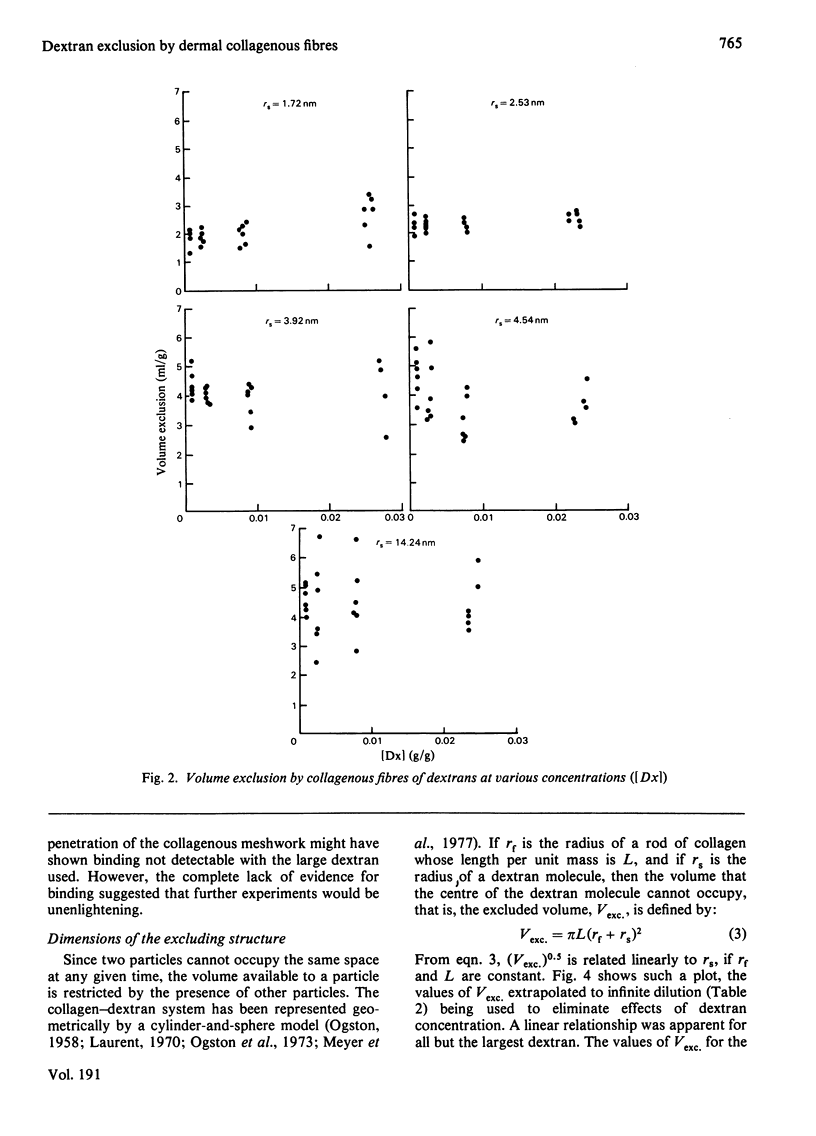
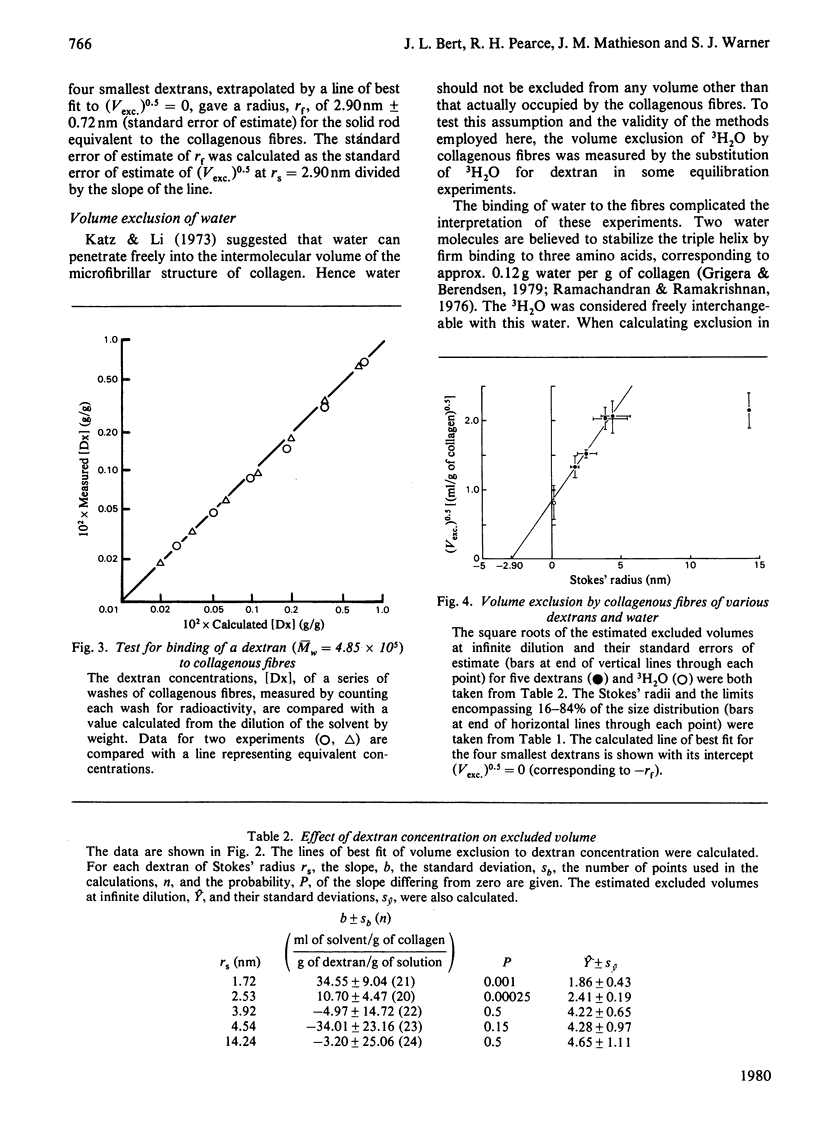
The volumes from which 3H-labelled dextrans are excluded by dermal collagenous fibres were calculated by dilution of dextran probes. Five dextrans, of average Stokes' radii 1.72, 2.53, 3.92, 4.54 and 14.24nm, were investigated at concentrations between 0.1 and 3% (w/w). The excluded volume was dependent on dextran concentration only for the two smaller probes. The largest dextran was shown not to bind to the fibres. A plot of the square root of excluded volume against Stokes' radius was linear for the four smallest dextrans, corresponding to the predictions of Ogston's [(1958) Trans. Faraday Soc. 54, 1754--1757] rod-and-sphere model of fibrous exclusion, and suggesting that dextrans of Stokes' radius between 1.72 and 4.54 nm were excluded by a cylindrical solid fibre of radius 2.90 +/- 0.72 nm. Larger molecules were excluded by a structure of much greater size, since the volume exclusion for the largest dextran was only slightly greater than that of the dextran less than one-third its radius. The excluded volume of 3H2O fell slightly below the line describing the dextran data, indicating that water had access to most of the volume not occupied by the collagenous fibres.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Comper W. D., Laurent T. C. Physiological function of connective tissue polysaccharides. Physiol Rev. 1978 Jan;58(1):255–315. doi: 10.1152/physrev.1978.58.1.255. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. One-step growth curve of Western equine encephalomyelitis virus on chicken embryo cells grown in vitro and analysis of virus yields from single cells. J Exp Med. 1954 Feb;99(2):183–199. doi: 10.1084/jem.99.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison P. F., Drake M. P. The physical characterization of monomeric tropocollagen. Biochemistry. 1966 Jan;5(1):313–321. doi: 10.1021/bi00865a040. [DOI] [PubMed] [Google Scholar]
- Elden H. R. Physical properties of collagen fibers. Int Rev Connect Tissue Res. 1968;4:283–348. doi: 10.1016/b978-1-4831-6754-1.50013-3. [DOI] [PubMed] [Google Scholar]
- Hulmes D. J., Miller A. Quasi-hexagonal molecular packing in collagen fibrils. Nature. 1979 Dec 20;282(5741):878–880. doi: 10.1038/282878a0. [DOI] [PubMed] [Google Scholar]
- Jackson D. S., Cleary E. G. The determination of collagen and elastin. Methods Biochem Anal. 1967;15:25–76. doi: 10.1002/9780470110331.ch2. [DOI] [PubMed] [Google Scholar]
- Kastelic J., Galeski A., Baer E. The multicomposite structure of tendon. Connect Tissue Res. 1978;6(1):11–23. doi: 10.3109/03008207809152283. [DOI] [PubMed] [Google Scholar]
- Meyer F. A., Koblentz M., Silberberg A. Structural investigation of loose connective tissue by using a series of dextran fractions as non-interacting macromolecular probes. Biochem J. 1977 Feb 1;161(2):285–291. doi: 10.1042/bj1610285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogston A. G., Preston B. N. The molecular compression of dextran. Biochem J. 1979 Oct 1;183(1):1–9. doi: 10.1042/bj1830001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry D. A., Craig A. S. Electron microscope evidence for an 80 A unit in collagen fibrils. Nature. 1979 Nov 8;282(5735):213–215. doi: 10.1038/282213a0. [DOI] [PubMed] [Google Scholar]
- Pearce R. H., Grimmer B. J. Calibration of agarose columns for gel chromatography with commercially available dextran fractions. Application to the measurement of distributions of molecular radii of glycosaminoglycans. J Chromatogr. 1978 Mar 21;150(2):548–553. doi: 10.1016/s0021-9673(00)88219-9. [DOI] [PubMed] [Google Scholar]
- Pearce R. H., Laurent T. C. Exclusion of dextrans by meshworks of collagenous fibres. Biochem J. 1977 Jun 1;163(3):617–625. doi: 10.1042/bj1630617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild M. A., Oratz M., Schreiber S. S. Extravascular albumin. N Engl J Med. 1979 Aug 30;301(9):497–498. doi: 10.1056/NEJM197908303010909. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- WOESSNER J. F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Watson P. D., Grodins F. S. An analysis of the effects of the interstitial matrix on plasma--lymph transport. Microvasc Res. 1978 Jul;16(1):19–41. doi: 10.1016/0026-2862(78)90042-0. [DOI] [PubMed] [Google Scholar]
- Wiederhielm C. A., Black L. L. Osmotic interaction of plasma proteins with interstitial macromolecules. Am J Physiol. 1976 Aug;231(2):638–641. doi: 10.1152/ajplegacy.1976.231.2.638. [DOI] [PubMed] [Google Scholar]


